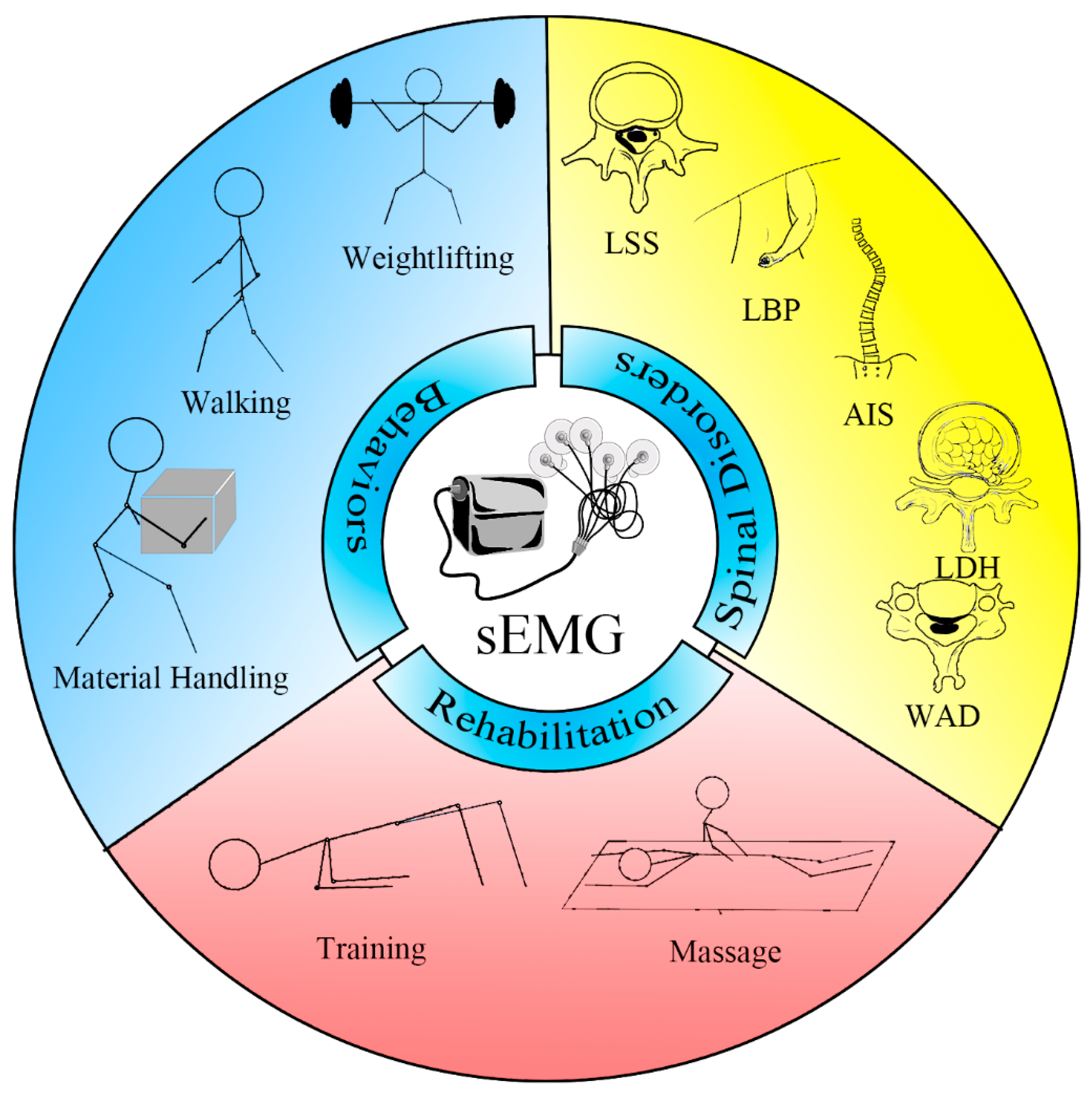
The Application of Surface Electromyography Technology in Evaluating Paraspinal Muscle Function
Abstract
1. Introduction
2. Paraspinal Muscles
3. sEMG
4. Evaluate Paraspinal Muscles with sEMG
4.1. sEMG in Health and Behaviors
4.2. sEMG in Lumbar Disorders
4.2.1. LBP
4.2.2. LDH
4.2.3. Lumbar Spinal Stenosis (LSS)
4.3. sEMG in Cervical and Thoracic Disorders
4.3.1. Adolescent Idiopathic Scoliosis (AIS)
4.3.2. Spinal Cord Injury (SCI)
4.3.3. Neck Pain (NP)
4.3.4. Whiplash-Associated Disorder (WAD)
5. sEMG in Massage Therapy and Rehabilitation Training
6. Limitations and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.A.; Cross, M.; Hill, C.; Smith, E.; Carson-Chahhoud, K.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Kaufman, J.; et al. Prevalence, Deaths, and Disability-Adjusted Life Years Due to Musculoskeletal Disorders for 195 Countries and Territories 1990–2017. Arthritis Rheumatol. 2021, 73, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P. Low back pain: A call for action. Lancet 2018, 391, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Eriksson Crommert, M.; Tucker, K.; Holford, C.; Wight, A.; McCook, D.; Hodges, P. Directional preference of activation of abdominal and paraspinal muscles during position-control tasks in sitting. J. Electromyogr. Kinesiol. 2017, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cram, J.R.; Steger, J.C. EMG scanning in the diagnosis of chronic pain. Biofeedback Self-Regul. 1983, 8, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, S.L.; Zhang, J.; Feng, D. A study on the paraspinal muscle surface electromyography in acute nonspecific lower back pain. Medicine 2019, 98, e16904. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, T.; Ishida, H.; Kobara, K.; Osaka, H.; Kurozumi, C.; Watanabe, S. Immediate changes in trunk muscle activation patterns during a lifting task following an abdominal drawing-in exercise in subjects with recurrent low back pain. J. Back Musculoskelet. Rehabil. 2021, 34, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.; Arsenault, A.B.; Larivière, C.; DeSerres, S.J.; Rivard, C.H. Assessment of the paraspinal muscles of subjects presenting an idiopathic scoliosis: An EMG pilot study. BMC Musculoskelet. Disord. 2005, 6, 14. [Google Scholar] [CrossRef]
- Mengiardi, B.; Schmid, M.R.; Boos, N.; Pfirrmann, C.W.; Brunner, F.; Elfering, A.; Hodler, J. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: Quantification with MR spectroscopy. Radiology 2006, 240, 786–792. [Google Scholar] [CrossRef]
- Ogon, I.; Takebayashi, T.; Takashima, H.; Morita, T.; Yoshimoto, M.; Terashima, Y.; Yamashita, T. Magnetic resonance spectroscopic analysis of multifidus muscles lipid content and association with spinopelvic malalignment in chronic low back pain. Br. J. Radiol. 2017, 90, 20160753. [Google Scholar] [CrossRef]
- Olson, M.W. Trunk muscle activation during sub-maximal extension efforts. Man. Ther. 2010, 15, 105–110. [Google Scholar] [CrossRef]
- Kankaanpää, M.; Laaksonen, D.; Taimela, S.; Kokko, S.M.; Airaksinen, O.; Hänninen, O. Age, sex, and body mass index as determinants of back and hip extensor fatigue in the isometric Sørensen back endurance test. Arch. Phys. Med. Rehabil. 1998, 79, 1069–1075. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, J.S.; An, D.H.; Yoo, W.G.; Kim, J.M.; Kim, T.H.; Kang, M.H. Difference in selective muscle activity of thoracic erector spinae during prone trunk extension exercise in subjects with slouched thoracic posture. PMR J. Inj. Funct. Rehabil. 2015, 7, 479–484. [Google Scholar] [CrossRef]
- Hashemirad, F.; Talebian, S.; Hatef, B.; Kahlaee, A.H. The relationship between flexibility and EMG activity pattern of the erector spinae muscles during trunk flexion-extension. J. Electromyogr. Kinesiol. 2009, 19, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Azar, N.R.; Kallakuri, S.; Chen, C.; Cavanaugh, J.M. Muscular response to physiologic tensile stretch of the caprine c5/6 facet joint capsule: Dynamic recruitment thresholds and latencies. Stapp Car Crash J. 2011, 55, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Anton, D.; Mizner, R.L.; Hess, J.A. The effect of lift teams on kinematics and muscle activity of the upper extremity and trunk in bricklayers. J. Orthop. Sports Phys. Ther. 2013, 43, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, D.; Urabe, Y.; Maeda, N.; Sasadai, J.; Fujii, E.; Moriyama, N.; Yamamoto, T.; Iwata, S. The effect of different working postures while felling a tree with a chain-saw on trunk muscles’ activity. Sangyo Eiseigaku Zasshi J. Occup. Health 2015, 57, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Shim, J.S.; Lee, S.T.; Kim, M.; Ryu, J.S. Facilitating effects of fast and slope walking on paraspinal muscles. Ann. Rehabil. Med. 2014, 38, 514–522. [Google Scholar] [CrossRef]
- du Rose, A.; Breen, A. Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion. Healthcare 2016, 4, 4. [Google Scholar] [CrossRef]
- Becker, S.; Bergamo, F.; Schnake, K.J.; Schreyer, S.; Rembitzki, I.V.; Disselhorst-Klug, C. The relationship between functionality and erector spinae activity in patients with specific low back pain during dynamic and static movements. Gait Posture 2018, 66, 208–213. [Google Scholar] [CrossRef]
- Siegmund, G.P.; Sanderson, D.J.; Myers, B.S.; Inglis, J.T. Awareness affects the response of human subjects exposed to a single whiplash-like perturbation. Spine 2003, 28, 671–679. [Google Scholar] [CrossRef]
- Zou, C.J.; Li, J.H.; Wu, F.C.; Li, Y.Z.; Pan, H.Y.; Wu, T. The effects of core stability training in nurses with nonspecific low back pain. Medicine 2021, 100, e26357. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yoon, S.H.; Kim, M.; Lee, S.H.; Kim, D.H.; Kim, K.H. Effect of direct vibration on the activity of deep trunk muscles of patients with non-specific chronic low back pain. J. Back Musculoskelet. Rehabil. 2022, 35, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, L.S.; Zhang, C.Z.; Zhang, M.; Zhan, H.S.; Yuan, W.A. A comparative study on the surface electromyography of lumbosacral multifidus muscle in patients with lumbar disc herniation. Zhongguo Gu Shang China J. Orthop. Traumatol. 2020, 33, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Borzelli, D.; Burdet, E.; Pastorelli, S.; d’Avella, A.; Gastaldi, L. Identification of the best strategy to command variable stiffness using electromyographic signals. J. Neural Eng. 2020, 17, 016058. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.M.; Chatterji, S.; Kumar, A.J.I.J.S.E.R. Trends and challenges in EMG based control scheme of exoskeleton robots—A review. Int. J. Sci. Eng. Res. 2012, 3, 933–940. [Google Scholar]
- Gertken, J.T.; Hunt, C.H.; Chinea, N.I.; Morris, J.M.; Sorenson, E.J.; Boon, A.J. Risk of hematoma following needle electromyography of the paraspinal muscles. Muscle Nerve 2011, 44, 439–440. [Google Scholar] [CrossRef] [PubMed]
- London, Z.; Quint, D.J.; Haig, A.J.; Yamakawa, K.S. The risk of hematoma following extensive electromyography of the lumbar paraspinal muscles. Muscle Nerve 2012, 46, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Arokoski, J.P.; Kankaanpää, M.; Valta, T.; Juvonen, I.; Partanen, J.; Taimela, S.; Lindgren, K.A.; Airaksinen, O. Back and hip extensor muscle function during therapeutic exercises. Arch. Phys. Med. Rehabil. 1999, 80, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Zoffoli, L.; Ditroilo, M.; Federici, A.; Lucertini, F. Patterns of trunk muscle activation during walking and pole walking using statistical non-parametric mapping. J. Electromyogr. Kinesiol. 2017, 37, 52–60. [Google Scholar] [CrossRef]
- Rimmele, P.; Steinhilber, B.; Rieger, M.A.; Luger, T. Motor variability during a repetitive lifting task is impaired by wearing a passive back-support exoskeleton. J. Electromyogr. Kinesiol. 2023, 68, 102739. [Google Scholar] [CrossRef]
- Veiskarami, M.; Aminian, G.; Bahramizadeh, M.; Gholami, M.; Ebrahimzadeh, F.; Arazpour, M. The Efficacy of “Anatomical Posture Control Orthosis” on the Activity of Erector spinae Muscle, Risk of Falling, Balance Confidence, and Walking Speed in Osteoporotic Hyperkyphotic Subjects. Arch. Bone Jt. Surg. 2022, 10, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Saeterbakken, A.H.; Stien, N.; Pedersen, H.; Andersen, V. Core Muscle Activation in Three Lower Extremity Exercises with Different Stability Requirements. J. Strength Cond. Res. 2022, 36, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Hanafusa, M.; Hashimoto, J.; Inada, R.; Koda, H. Effects of arm and leg positions on lumbar multifidus muscle activity while on hands and knees or while standing. J. Physiol. Anthropol. 2022, 41, 6. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M.; Udermann, B.E.; Verna, J.L. Electromyographic Analysis of the Lumbar Extensor Muscles during Dynamic Exercise on a Home Exercise Device. J. Funct. Morphol. Kinesiol. 2022, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Creze, M.; Soubeyrand, M.; Gagey, O. The paraspinal muscle-tendon system: Its paradoxical anatomy. PLoS ONE 2019, 14, e0214812. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Hsu, A.W.; Vasavada, A.N. Morphology, architecture, and biomechanics of human cervical multifidus. Spine 2005, 30, E86–E91. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, J.E.; Valencia, F.; Bogduk, N.; Munro, R.R. The morphology of the human lumbar multifidus. Clin. Biomech. 1986, 1, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, J.E.; Bogduk, N. The attachments of the lumbar erector spinae. Spine 1991, 16, 783–792. [Google Scholar] [CrossRef]
- Daggfeldt, K.; Huang, Q.M.; Thorstensson, A. The visible human anatomy of the lumbar erector spinae. Spine 2000, 25, 2719–2725. [Google Scholar] [CrossRef]
- Lippold, O.C. The relation between integrated action potentials in a human muscle and its isometric tension. J. Physiol. 1952, 117, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.G.; Lippold, O.C. The relation between force and integrated electrical activity in fatigued muscle. J. Physiol. 1956, 132, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Yagi, R.; Akasaka, K.; Momose, K.; Ihashi, K.; Handa, Y. Relationship between EMG signals and force in human vastus lateralis muscle using multiple bipolar wire electrodes. J. Electromyogr. Kinesiol. 2000, 10, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.J.; Bigland-Ritchie, B. Linear and non-linear surface EMG/force relationships in human muscles. An anatomical/functional argument for the existence of both. Am. J. Phys. Med. 1983, 62, 287–299. [Google Scholar] [PubMed]
- Lee, D.J.; Stokes, M.J.; Taylor, R.J.; Cooper, R.G. Electro and acoustic myography for noninvasive assessment of lumbar paraspinal muscle function. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 199–203. [Google Scholar] [CrossRef]
- Ng, J.K.; Richardson, C.A. Reliability of electromyographic power spectral analysis of back muscle endurance in healthy subjects. Arch. Phys. Med. Rehabil. 1996, 77, 259–264. [Google Scholar] [CrossRef]
- Nargol, A.V.; Jones, A.P.; Kelly, P.J.; Greenough, C.G. Factors in the reproducibility of electromyographic power spectrum analysis of lumbar paraspinal muscle fatigue. Spine 1999, 24, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Solomonow, M.; Baten, C.; Smit, J.; Baratta, R.; Hermens, H.; D’Ambrosia, R.; Shoji, H. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J. Appl. Physiol. 1990, 68, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Weber, B.R.; Dvorak, J.; Grob, D.; Müntener, M. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J. Orthop. Res. 1997, 15, 881–887. [Google Scholar] [CrossRef]
- Mannion, A.F.; Dolan, P. Electromyographic median frequency changes during isometric contraction of the back extensors to fatigue. Spine 1994, 19, 1223–1229. [Google Scholar] [CrossRef]
- Kankaanpää, M.; Taimela, S.; Webber, C.L., Jr.; Airaksinen, O.; Hänninen, O. Lumbar paraspinal muscle fatigability in repetitive isoinertial loading: EMG spectral indices, Borg scale and endurance time. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ebenbichler, G.; Habenicht, R.; Ziegelbecker, S.; Kollmitzer, J.; Mair, P.; Kienbacher, T. Age- and sex-specific effects in paravertebral surface electromyographic back extensor muscle fatigue in chronic low back pain. GeroScience 2020, 42, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.C.; Mayo, J.J.; Tucker, W.S.; Wax, B.; Hendrix, R.C. Electromyographical Comparison of Muscle Activation Patterns Across Three Commonly Performed Kettlebell Exercises. J. Strength Cond. Res. 2017, 31, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.D.; Sundstrup, E.; Brandt, M.; Persson, R.; Andersen, L.L. Estimation of physical workload of the low-back based on exposure variation analysis during a full working day among male blue-collar workers. Cross-sectional workplace study. Appl. Ergon. 2018, 70, 127–133. [Google Scholar] [CrossRef]
- Lee, S.P.; Gillis, C.B.; Ibarra, J.J.; Oldroyd, D.F.; Zane, R.S. Heel-Raised Foot Posture Does Not Affect Trunk and Lower Extremity Biomechanics During a Barbell Back Squat in Recreational Weight lifters. J. Strength Cond. Res. 2019, 33, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.; Hecimovich, M.; Dempsey, A. Lumbopelvic muscle activation patterns in adolescent fast bowlers. Eur. J. Sport Sci. 2016, 16, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.G.; Yoo, W.G. Effects of different lifting and lowering heights on upper arm, shoulder and back muscle activity during a manual material handling task. Work 2015, 53, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.G.; Kim, M.H. Effect of different seat support characteristics on the neck and trunk muscles and forward head posture of visual display terminal workers. Work 2010, 36, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Stauffer, M.; Jäger, J.; List, R.; Lorenzetti, S. Sling-based infant carrying affects lumbar and thoracic spine neuromechanics during standing and walking. Gait Posture 2019, 67, 172–180. [Google Scholar] [CrossRef]
- Arokoski, J.P.; Valta, T.; Airaksinen, O.; Kankaanpää, M. Back and abdominal muscle function during stabilization exercises. Arch. Phys. Med. Rehabil. 2001, 82, 1089–1098. [Google Scholar] [CrossRef]
- Arokoski, J.P.; Valta, T.; Kankaanpää, M.; Airaksinen, O. Activation of paraspinal and abdominal muscles during manually assisted and nonassisted therapeutic exercise. Am. J. Phys. Med. Rehabil. 2002, 81, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Khosrokiani, Z.; Letafatkar, A.; Sheikhi, B.; Thomas, A.C.; Aghaie-Ataabadi, P.; Hedayati, M.T. Hip and Core Muscle Activation During High-Load Core Stabilization Exercises. Sports Health 2022, 14, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mello, R.G.; Carriço, I.R.; da Matta, T.T.; Nadal, J.; Oliveira, L.F. Lumbar multifidus and erector spinae electromyograms during back bridge exercise in time and frequency domains. J. Back Musculoskelet. Rehabil. 2016, 29, 123–133. [Google Scholar] [CrossRef]
- Masumoto, K.; Takasugi, S.; Hotta, N.; Fujishima, K.; Iwamoto, Y. A comparison of muscle activity and heart rate response during backward and forward walking on an underwater treadmill. Gait Posture 2007, 25, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Pablos, C.; Chulvi-Medrano, I.; Garcia-Masso, X.; Flandez, J.; Behm, D.G. The progression of paraspinal muscle recruitment intensity in localized and global strength training exercises is not based on instability alone. Arch. Phys. Med. Rehabil. 2011, 92, 1875–1883. [Google Scholar] [CrossRef]
- Matthijs, O.C.; Dedrick, G.S.; James, C.R.; Brismée, J.M.; Hooper, T.L.; McGalliard, M.K.; Sizer, P.S., Jr. Co-contractive activation of the superficial multifidus during volitional preemptive abdominal contraction. PMR J. Inj. Funct. Rehabil. 2014, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yoo, W.G. Effects of hand and knee positions on muscular activity during trunk extension exercise with the Roman chair. J. Electromyogr. Kinesiol. 2014, 24, 972–976. [Google Scholar] [CrossRef]
- Wagner, H.; Rehmes, U.; Kohle, D.; Puta, C. Laughing: A demanding exercise for trunk muscles. J. Mot. Behav. 2014, 46, 33–37. [Google Scholar] [CrossRef]
- Andrade, L.S.; Mochizuki, L.; Pires, F.O.; da Silva, R.A.; Mota, Y.L. Application of Pilates principles increases paraspinal muscle activation. J. Bodyw. Mov. Ther. 2015, 19, 62–66. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, M.H.; Jang, J.H.; Oh, J.S. Comparison of selective electromyographic activity of the superficial lumbar multifidus between prone trunk extension and four-point kneeling arm and leg lift exercises. J. Phys. Ther. Sci. 2015, 27, 1037–1039. [Google Scholar] [CrossRef]
- Masaki, M.; Tateuchi, H.; Tsukagoshi, R.; Ibuki, S.; Ichihashi, N. Electromyographic analysis of training to selectively strengthen the lumbar multifidus muscle: Effects of different lifting directions and weight loading of the extremities during quadruped upper and lower extremity lifts. J. Manip. Physiol. Ther. 2015, 38, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Youdas, J.W.; Coleman, K.C.; Holstad, E.E.; Long, S.D.; Veldkamp, N.L.; Hollman, J.H. Magnitudes of muscle activation of spine stabilizers in healthy adults during prone on elbow planking exercises with and without a fitness ball. Physiother. Theory Pract. 2018, 34, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, R.F.; Lewis, C.; Pecson, A.; Imamura, R.; Andrews, J.R. Muscle Activation Among Supine, Prone, and Side Position Exercises With and Without a Swiss Ball. Sports Health 2016, 8, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, J.; Casaña, J.; Martín, F.; Jakobsen, M.D.; Colado, J.C.; Gargallo, P.; Juesas, Á.; Muñoz, V.; Andersen, L.L. Trunk muscle activity during different variations of the supine plank exercise. Musculoskelet. Sci. Pract. 2017, 28, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Nijem, R.M.; Coburn, J.W.; Brown, L.E.; Lynn, S.K.; Ciccone, A.B. Electromyographic and Force Plate Analysis of the Deadlift Performed With and Without Chains. J. Strength Cond. Res. 2016, 30, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Donath, L.; Faude, O.; Cresswell, A.G. Trunk muscle activity during different types of low weighted squat exercises in normal and forefoot standing conditions. J. Sports Sci. 2020, 38, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shan, X. Spasm and flexion-relaxation phenomenon response to large lifting load during the performance of a trunk flexion-extension exercise. BMC Musculoskelet. Disord. 2017, 18, 505. [Google Scholar] [CrossRef] [PubMed]
- Seroussi, R.E.; Wilder, D.G.; Pope, M.H. Trunk muscle electromyography and whole body vibration. J. Biomech. 1989, 22, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Perchthaler, D.; Hauser, S.; Heitkamp, H.C.; Hein, T.; Grau, S. Acute effects of whole-body vibration on trunk and neck muscle activity in consideration of different vibration loads. J. Sports Sci. Med. 2015, 14, 155–162. [Google Scholar]
- Nolan, A.J.; Govers, M.E.; Oliver, M.L. Effect of fatigue on muscle latency, muscle activation and perceived discomfort when exposed to whole-body vibration. Ergonomics 2021, 64, 1281–1296. [Google Scholar] [CrossRef]
- Lu, W.W.; Luk, K.D.; Cheung, K.M.; Wong, Y.W.; Leong, J.C. Back muscle contraction patterns of patients with low back pain before and after rehabilitation treatment: An electromyographic evaluation. J. Spinal Disord. 2001, 14, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.G.; Stokes, M.J.; Sweet, C.; Taylor, R.J.; Jayson, M.I. Increased central drive during fatiguing contractions of the paraspinal muscles in patients with chronic low back pain. Spine 1993, 18, 610–616. [Google Scholar] [CrossRef]
- Djordjevic, O.; Konstantinovic, L.; Miljkovic, N.; Bijelic, G. Relationship Between Electromyographic Signal Amplitude and Thickness Change of the Trunk Muscles in Patients With and Without Low Back Pain. Clin. J. Pain 2015, 31, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Crossman, K.; Mahon, M.; Watson, P.J.; Oldham, J.A.; Cooper, R.G. Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally determined “adverse” fiber-type composition. Spine 2004, 29, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.Y.; Koutsos, E.; Georgiou, P.; Strutton, P.H. Association between spectral characteristics of paraspinal muscles and functional disability in patients with low back pain: A cohort study. BMJ Open 2018, 8, e017091. [Google Scholar] [CrossRef] [PubMed]
- Jalovaara, P.; Niinimäki, T.; Vanharanta, H. Pocket-size, portable surface EMG device in the differentiation of low back pain patients. Eur. Spine J. 1995, 4, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S. Difference of the thickness and activation of trunk muscles during static stoop lift at different loads between subjects with and without low back pain. J. Back Musculoskelet. Rehabil. 2018, 31, 481–488. [Google Scholar] [CrossRef]
- da Silva, R.A.; Vieira, E.R.; Léonard, G.; Beaulieu, L.D.; Ngomo, S.; Nowotny, A.H.; Amorim, C.F. Age- and low back pain-related differences in trunk muscle activation during one-legged stance balance task. Gait Posture 2019, 69, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, A.; Martinez-Valdes, E.; Heneghan, N.R.; Murillo, C.; Rushton, A.; Falla, D. Variation in the spatial distribution of erector spinae activity during a lumbar endurance task in people with low back pain. J. Anat. 2019, 234, 532–542. [Google Scholar] [CrossRef]
- Shah, J.; Tanwar, T.; Iram, I.; Aldabbas, M.; Veqar, Z. Effect of Increased Lumbar Lordosis on Lumbar Multifidus and Longissimus Thoracis Activation During Quadruped Exercise in Patients with Chronic Low Back Pain: An EMG Study. J. Appl. Biomech. 2020, 36, 436–443. [Google Scholar] [CrossRef]
- Varrecchia, T.; Conforto, S.; De Nunzio, A.M.; Draicchio, F.; Falla, D.; Ranavolo, A. Trunk Muscle Coactivation in People with and without Low Back Pain during Fatiguing Frequency-Dependent Lifting Activities. Sensors 2022, 22, 1417. [Google Scholar] [CrossRef]
- Sánchez-Zuriaga, D.; López-Pascual, J.; Garrido-Jaén, D.; García-Mas, M.A. A comparison of lumbopelvic motion patterns and erector spinae behavior between asymptomatic subjects and patients with recurrent low back pain during pain-free periods. J. Manip. Physiol. Ther. 2015, 38, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, N.; Ito, H. Electromyographic functional analysis of the lumbar spinal muscles with low back pain. J. Nippon Med. Sch. Nippon Ika Daigaku Zasshi 2005, 72, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Arvanitidis, M.; Jiménez-Grande, D.; Haouidji-Javaux, N.; Falla, D.; Martinez-Valdes, E. People with chronic low back pain display spatial alterations in high-density surface EMG-torque oscillations. Sci. Rep. 2022, 12, 15178. [Google Scholar] [CrossRef]
- Balasch-Bernat, M.; Willems, T.; Danneels, L.; Meeus, M.; Goubert, D. Differences in myoelectric activity of the lumbar muscles between recurrent and chronic low back pain: A cross-sectional study. BMC Musculoskelet. Disord. 2021, 22, 756. [Google Scholar] [CrossRef]
- Suehiro, T.; Ishida, H.; Kobara, K.; Osaka, H.; Kurozumi, C. Trunk muscle activation patterns during active hip abduction test during remission from recurrent low back pain: An observational study. BMC Musculoskelet. Disord. 2021, 22, 671. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, A.R.; Nargol, A.V.; Jones, A.P.; Ratcliffe, A.A.; Greenough, C.G. The value of electromyography of the lumbar paraspinal muscles in discriminating between chronic-low-back-pain sufferers and normal subjects. Eur. Spine J. 2005, 14, 175–184. [Google Scholar] [CrossRef]
- Cassisi, J.E.; Robinson, M.E.; O’Conner, P.; MacMillan, M. Trunk strength and lumbar paraspinal muscle activity during isometric exercise in chronic low-back pain patients and controls. Spine 1993, 18, 245–251. [Google Scholar] [CrossRef]
- Adeyemi, A.J.; Rohani, J.M.; Rani, M.R. Interaction of body mass index and age in muscular activities among backpack carrying male schoolchildren. Work 2015, 52, 677–686. [Google Scholar] [CrossRef]
- Martinez-Valdes, E.; Wilson, F.; Fleming, N.; McDonnell, S.J.; Horgan, A.; Falla, D. Rowers with a recent history of low back pain engage different regions of the lumbar erector spinae during rowing. J. Sci. Med. Sport 2019, 22, 1206–1212. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, L.; Wang, J.; Hou, Z. Spatial Distribution and Asymmetry of Surface Electromyography on Lumbar Muscles of Soldiers with Chronic Low Back Pain. Pain Res. Manag. 2020, 2020, 6946294. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.J.; Booker, C.K.; Main, C.J.; Chen, A.C. Surface electromyography in the identification of chronic low back pain patients: The development of the flexion relaxation ratio. Clin. Biomech. 1997, 12, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gouteron, A.; Tabard-Fougère, A.; Moissenet, F.; Bourredjem, A.; Rose-Dulcina, K.; Genevay, S.; Laroche, D.; Armand, S. Sensitivity and specificity of the flexion and extension relaxation ratios to identify altered paraspinal muscles’ flexion relaxation phenomenon in nonspecific chronic low back pain patients. J. Electromyogr. Kinesiol. 2023, 68, 102740. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.F., Jr.; Gudavalli, M.R.; Wilder, D.G. Paraspinal muscle function assessed with the flexion-relaxation ratio at baseline in a population of patients with back-related leg pain. J. Manip. Physiol. Ther. 2011, 34, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.N.; Hu, Y.; Cheng, A.C.; Kwok, H.Y.; Chen, Y.H.; Luk, K.D. Flexion-relaxation ratio in sitting: Application in low back pain rehabilitation. Spine 2010, 35, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Kankaanpää, M.; Taimela, S.; Laaksonen, D.; Hänninen, O.; Airaksinen, O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch. Phys. Med. Rehabil. 1998, 79, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Shigetoh, H.; Nishi, Y.; Osumi, M.; Morioka, S. Combined abnormal muscle activity and pain-related factors affect disability in patients with chronic low back pain: An association rule analysis. PLoS ONE 2020, 15, e0244111. [Google Scholar] [CrossRef]
- Rose-Dulcina, K.; Genevay, S.; Dominguez, D.; Armand, S.; Vuillerme, N. Flexion-Relaxation Ratio Asymmetry and Its Relation With Trunk Lateral ROM in Individuals With and Without Chronic Nonspecific Low Back Pain. Spine 2020, 45, E1–E9. [Google Scholar] [CrossRef]
- Ramos, L.A.V.; França, F.J.R.; Callegari, B.; Burke, T.N.; Magalhães, M.O.; Marques, A.P. Are lumbar multifidus fatigue and transversus abdominis activation similar in patients with lumbar disc herniation and healthy controls? A case control study. Eur. Spine J. 2016, 25, 1435–1442. [Google Scholar] [CrossRef]
- Leinonen, V.; Kankaanpää, M.; Luukkonen, M.; Hänninen, O.; Airaksinen, O.; Taimela, S. Disc herniation-related back pain impairs feed-forward control of paraspinal muscles. Spine 2001, 26, E367–E372. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Dai, J.; Wang, J.; Wu, H.; Liu, J.; Chen, J.; Zhu, Y.; Zhao, F. Changes in the Flexion-Relaxation Response After Percutaneous Endoscopic Lumbar Discectomy in Patients with Disc Herniation. World Neurosurg. 2019, 125, e1042–e1049. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, C.; Mandelli, F.; Przybilla, P.; Schären, S.; Mündermann, A.; Netzer, C. Kinematics and paraspinal muscle activation patterns during walking differ between patients with lumbar spinal stenosis and controls. Gait Posture 2023, 99, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, V.; Määttä, S.; Taimela, S.; Herno, A.; Kankaanpää, M.; Partanen, J.; Hänninen, O.; Airaksinen, O. Paraspinal muscle denervation, paradoxically good lumbar endurance, and an abnormal flexion-extension cycle in lumbar spinal stenosis. Spine 2003, 28, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, T.; Taimela, S.; Aalto, T.; Kröger, H.; Herno, A.; Turunen, V.; Savolainen, S.; Kankaanpää, M.; Airaksinen, O.; Leinonen, V. The effect of decompressive surgery on lumbar paraspinal and biceps brachii muscle function and movement perception in lumbar spinal stenosis: A 2-year follow-up. Eur. Spine J. 2016, 25, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, T.; Leinonen, V.; Taimela, S.; Aalto, T.; Kröger, H.; Herno, A.; Turunen, V.; Savolainen, S.; Kankaanpää, M.; Airaksinen, O. Lumbar paraspinal and biceps brachii muscle function and movement perception in lumbar spinal stenosis. Eur. Spine J. 2013, 22, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Shen, J.; Chen, L.; Wang, H.; Yu, K.; Cong, H.; Zhou, J.; Lin, Y. Differences in Nonspecific Low Back Pain between Young Adult Females with and without Lumbar Scoliosis. Pain Res. Manag. 2019, 2019, 9758273. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ko, J.Y.; Jang, J.Y.; Lee, S.; Beom, J.; Ryu, J.S. Asymmetrical activation and asymmetrical weakness as two different mechanisms of adolescent idiopathic scoliosis. Sci. Rep. 2021, 11, 17582. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.W.; Hu, Y.; Luk, K.D.; Cheung, K.M.; Leong, J.C. Paraspinal muscle activities of patients with scoliosis after spine fusion: An electromyographic study. Spine 2002, 27, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Veldhuizen, A.G.; Halbertsma, J.P.; Maurits, N.M.; Sluiter, W.J.; Cool, J.C.; Van Horn, J.R. The relation between electromyography and growth velocity of the spine in the evaluation of curve progression in idiopathic scoliosis. Spine 2004, 29, 1011–1016. [Google Scholar] [CrossRef]
- Chan, R.Y.H.; Ma, A.C.F.; Cheung, T.S.K.; Chan, J.C.L.; Kwok, R.W.Y.; Fu, A.C.L.; Tsang, S.M.H. Effect of muscle fatigue of the thoracic erector spinae on neuromuscular control when performing the upper extremity functional tasks in people with adolescent idiopathic scoliosis. PLoS ONE 2023, 18, e0281001. [Google Scholar] [CrossRef]
- Cheung, J.; Halbertsma, J.P.; Veldhuizen, A.G.; Sluiter, W.J.; Maurits, N.M.; Cool, J.C.; van Horn, J.R. A preliminary study on electromyographic analysis of the paraspinal musculature in idiopathic scoliosis. Eur. Spine J. 2005, 14, 130–137. [Google Scholar] [CrossRef]
- Liang, R.; Yip, J.; Fan, Y.; Cheung, J.P.Y.; To, K.M. Electromyographic Analysis of Paraspinal Muscles of Scoliosis Patients Using Machine Learning Approaches. Int. J. Environ. Res. Public Health 2022, 19, 1177. [Google Scholar] [CrossRef]
- Cheung, M.C.; Yip, J.; Lai, J.S.K. Biofeedback Posture Training for Adolescents with Mild Scoliosis. BioMed Res. Int. 2022, 2022, 5918698. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Leong, C.P.; Huang, Y.C.; Kuo, S.H.; Wang, H.C.; Yeh, H.C.; Lau, Y.C. The electromyographic responses of paraspinal muscles during isokinetic exercise in adolescents with idiopathic scoliosis with a Cobb’s angle less than fifty degrees. Chang Gung Med. J. 2010, 33, 540–550. [Google Scholar]
- Ko, J.Y.; Suh, J.H.; Kim, H.; Ryu, J.S. Proposal of a new exercise protocol for idiopathic scoliosis: A preliminary study. Medicine 2018, 97, e13336. [Google Scholar] [CrossRef] [PubMed]
- Mooney, V.; Brigham, A. The role of measured resistance exercises in adolescent scoliosis. Orthopedics 2003, 26, 167–171; discussion 171. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yang, J.T.; Zheng, Q.; Mei, Z.; Ma, C.Z. How do Paraspinal Muscles Contract during the Schroth Exercise Treatment in Patients with Adolescent Idiopathic Scoliosis (AIS)? Bioengineering 2022, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Farahpour, N.; Ghasemi, S.; Allard, P.; Saba, M.S. Electromyographic responses of erector spinae and lower limb’s muscles to dynamic postural perturbations in patients with adolescent idiopathic scoliosis. J. Electromyogr. Kinesiol. 2014, 24, 645–651. [Google Scholar] [CrossRef]
- Perret, C.; Robert, J. Electromyographic responses of paraspinal muscles to postural disturbance with special reference to scoliotic children. J. Manip. Physiol. Ther. 2004, 27, 375–380. [Google Scholar] [CrossRef]
- Singh, G.; Aslan, S.; Ugiliweneza, B.; Behrman, A. Contribution of Trunk Muscles to Upright Sitting with Segmental Support in Children with Spinal Cord Injury. Children 2020, 7, 278. [Google Scholar] [CrossRef]
- Hoglund, B.K.; Zurn, C.A.; Madden, L.R.; Hoover, C.; Slopsema, J.P.; Balser, D.; Parr, A.; Samadani, U.; Johnson, M.D.; Netoff, T.I.; et al. Mapping Spinal Cord Stimulation-Evoked Muscle Responses in Patients with Chronic Spinal Cord Injury. Neuromodul. J. Int. Neuromodul. Soc. 2022, 26, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Sremakaew, M.; Treleaven, J.; Jull, G.; Vongvaivanichakul, P.; Uthaikhup, S. Altered neuromuscular activity and postural stability during standing balance tasks in persons with non-specific neck pain. J. Electromyogr. Kinesiol. 2021, 61, 102608. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, J.; Maisetti, O.; Guillaume, A.; Skalli, W.; Portero, P. Neck strength and EMG activity in fighter pilots with episodic neck pain. Aviat. Space Environ. Med. 2008, 79, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Park, K.N.; Kwon, O.Y.; Kim, S.J.; Kim, S.H. Asymmetry of neck motion and activation of the cervical paraspinal muscles during prone neck extension in subjects with unilateral posterior neck pain. J. Back Musculoskelet. Rehabil. 2017, 30, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Nanno, M. Effects of intermittent cervical traction on muscle pain. Flowmetric and electromyographic studies of the cervical paraspinal muscles. Nihon Ika Daigaku Zasshi 1994, 61, 137–147. [Google Scholar] [CrossRef]
- Siegmund, G.P.; Sanderson, D.J.; Myers, B.S.; Inglis, J.T. Rapid neck muscle adaptation alters the head kinematics of aware and unaware subjects undergoing multiple whiplash-like perturbations. J. Biomech. 2003, 36, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Mang, D.W.; Siegmund, G.P.; Brown, H.J.; Goonetilleke, S.C.; Blouin, J.S. Loud preimpact tones reduce the cervical multifidus muscle response during rear-end collisions: A potential method for reducing whiplash injuries. Spine J. 2015, 15, 153–161. [Google Scholar] [CrossRef]
- Descarreaux, M.; Mayrand, N.; Raymond, J. Neuromuscular control of the head in an isometric force reproduction task: Comparison of whiplash subjects and healthy controls. Spine J. 2007, 7, 647–653. [Google Scholar] [CrossRef]
- Colloca, C.J.; Keller, T.S. Electromyographic reflex responses to mechanical force, manually assisted spinal manipulative therapy. Spine 2001, 26, 1117–1124. [Google Scholar] [CrossRef]
- Daneau, C.; Cantin, V.; Descarreaux, M. Effect of Massage on Clinical and Physiological Variables During Muscle Fatigue Task in Participants With Chronic Low Back Pain: A Crossover Study. J. Manip. Physiol. Ther. 2019, 42, 55–65. [Google Scholar] [CrossRef]
- Bicalho, E.; Setti, J.A.; Macagnan, J.; Cano, J.L.; Manffra, E.F. Immediate effects of a high-velocity spine manipulation in paraspinal muscles activity of nonspecific chronic low-back pain subjects. Man. Ther. 2010, 15, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.; Tateuchi, H.; Koyama, Y.; Sakuma, K.; Otsuka, N.; Ichihashi, N. Back muscle activity and sagittal spinal alignment during quadruped upper and lower extremity lift in young men with low back pain history. Gait Posture 2018, 66, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Haładaj, R.; Topol, M. Multiple Impulse Therapy in the Assessment of Paraspinal Muscle Tone in Patients with Low Back Pain. Ortop. Traumatol. Rehabil. 2016, 18, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kahlaee, A.H.; Ghamkhar, L.; Arab, A.M. Effect of the Abdominal Hollowing and Bracing Maneuvers on Activity Pattern of the Lumbopelvic Muscles During Prone Hip Extension in Subjects With or Without Chronic Low Back Pain: A Preliminary Study. J. Manip. Physiol. Ther. 2017, 40, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Holmes, P.; Woby, S.; Hindle, J.; Fowler, N. Changes in muscle activity and stature recovery after active rehabilitation for chronic low back pain. Man. Ther. 2014, 19, 178–183. [Google Scholar] [CrossRef] [PubMed]
- de Souza Júnior, J.R.; Lemos, T.V.; Hamu, T.; Calaça, F.I.R.; Dos Santos, M.G.R.; Faria, A.M.; Silva, A.T.; Matheus, J.P.C. Effects of Kinesio Taping on peak torque and muscle activity in women with low back pain presenting fears and beliefs related to physical activity. J. Bodyw. Mov. Ther. 2020, 24, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Arokoski, J.P.; Valta, T.; Kankaanpää, M.; Airaksinen, O. Activation of lumbar paraspinal and abdominal muscles during therapeutic exercises in chronic low back pain patients. Arch. Phys. Med. Rehabil. 2004, 85, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Jiménez-Grande, D.; Atashzar, S.F.; Martinez-Valdes, E.; Falla, D. Muscle network topology analysis for the classification of chronic neck pain based on EMG biomarkers extracted during walking. PLoS ONE 2021, 16, e0252657. [Google Scholar] [CrossRef]
- Vergari, C.; Skalli, W.; Gajny, L. A convolutional neural network to detect scoliosis treatment in radiographs. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1069–1074. [Google Scholar] [CrossRef]
- Liew, B.X.W.; Rugamer, D.; De Nunzio, A.M.; Falla, D. Interpretable machine learning models for classifying low back pain status using functional physiological variables. Eur. Spine J. 2020, 29, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, K.; Fan, H.; Huang, Z.; Xiang, Y.; Yang, J.; He, L.; Zhang, L.; Yang, Y.; Li, R.; et al. Development and validation of deep learning algorithms for scoliosis screening using back images. Commun. Biol. 2019, 2, 390. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yip, J.; Cheung, M.C.; Yick, K.L. Evaluation of Myoelectric Activity of Paraspinal Muscles in Adolescents with Idiopathic Scoliosis during Habitual Standing and Sitting. BioMed Res. Int. 2015, 2015, 958450. [Google Scholar] [CrossRef] [PubMed]
| Years | First Author | Samples | Conclusions |
|---|---|---|---|
| 1983 | Cram [4] | 66 | The differences between both sides’ sEMG activity in the paraspinal muscle groups were important to distinguish LBP patients. |
| 1992 | Lee [45] | 39 | There was a good reproducibility for evaluating patients with LBP by sEMG. |
| 1993 | Cassisi [98] | 21 | Maximum surface integrated electromyography could be helpful for the classification of chronic LBP during isometric exercise. |
| 1993 | Cooper [82] | 39 | The muscle excess fatigue might be peripheral in origin. |
| 1995 | Jalovaara [86] | 43 | sEMG was a valid tool for indirectly assessing pain in LBP patients. |
| 2001 | Lu [81] | 40 | There was a difference in muscle activity patterns between healthy persons and patients with LBP. |
| 2004 | Crossman [84] | 67 | There was non-existent histomorphometric discrepancy between the subjects with and without CLBP. |
| 2005 | Humphrey [97] | 350 | Half-width, initial median frequency, and peak amplitude were great parameters to distinguish the individuals with and without CLBP. |
| 2005 | Kuriyama [93] | 44 | The paraspinal muscles played a role in spinal stabilization. |
| 2015 | Adeyemi [99] | 47 | The activity of paraspinal muscles in the lower back was more influenced by backpack carriage than the upper back among schoolchildren. |
| 2015 | Djordjevic [83] | 73 | There was a significant relationship between the sEMG signal and relative thickness change in the transversal abdominal. |
| 2015 | Sánchez-Zuriaga [92] | 30 | Patients with LBP during pain-free periods displayed changes in EMG activity and reduced maximum lumbar flexion ranges, distinguishing them from non-LBP subjects. |
| 2018 | Becker [19] | 30 | There was a high relationship in lumbar erector spinae between the changes of activity and the degree of function. |
| 2018 | Chiou [85] | 15 | Spectral characteristics of sEMG reflected muscle function. |
| 2018 | Yang [87] | 56 | Less activity of lumbar multifidus muscles observed in the subjects with LBP might contribute to decrease the lumbar stabilization during stoop lift. |
| 2019 | da Silva [88] | 40 | Less lumbar muscle activity and more co-activation between rectus adominis and multifidus muscles in the patients with LBP during one-legged stance task. |
| 2019 | Martinez-Valdes [100] | 18 | Magnitude of activation and the distribution of erector spinae activity were observed as changed in LBP patients. |
| 2019 | Sanderson [80] | 26 | Individuals without symptoms exhibited a spatial redistribution of lumbar erector spinae muscle activity during an endurance task, whereas this adaptation was diminished in the subjects with LBP. |
| 2020 | Hao [101] | 40 | The uneven spatial distribution and asymmetry of lumbar muscle activity were significant factors in CLBP patients. |
| 2020 | Shah [90] | 23 | There was a significant increase in the recruitment of the lumbar multifidus muscle with increased lumbar lordosis in patients with CLBP during quadruped exercise. |
| 2021 | Balasch-Bernat [95] | 75 | Patients with continuous CLBP exhibited elevated EMG activity in the erector spinae and multifidus muscles during the isometric and concentric phases of back extension exercises, as compared to healthy individuals. This difference in muscle activity was also present to a lesser degree when compared to patients with RLBP. |
| 2021 | Suehiro [96] | 34 | In individuals with recurrent LBP, the activation of contralateral erector spinae occurred with a delay compared to those without recurrent LBP during both right and left active hip abduction tests. |
| 2021 | Zou [21] | 40 | Core stability training had the potential to ameliorate symptoms, enhance the fatigue resistance of core muscles, and promote the balance of bilateral multifidus muscle function in patients suffering from nonspecific LBP. |
| 2022 | Arvanitidis [94] | 30 | Patients with CLBP were unable to increase the common fluctuations in torque and high-density sEMG activity during exertion of higher lumbar extension forces. |
| 2022 | Varrecchia [91] | 23 | The individuals with LBP engaged in greater co-activation of their trunk muscles than healthy controls, via the implementation of a fatiguing trunk-stiffening strategy. |
| Disorders | Years | First Author | Samples | Conclusions |
|---|---|---|---|---|
| LDH | 2001 | Leinonen [110] | 35 | Chronic pain could impair lumbar feedforward control in CLBP patients. |
| 2016 | Ramos [109] | 60 | Increased fatigue of the lumbar multifidus compared with the healthy controls. | |
| 2019 | Li [111] | 30 | Percutaneous endoscopic lumbar discectomy for individuals with LDH could normalize paraspinal muscle activation during lumbar flexion–extension movement. | |
| 2020 | Zhao [23] | 70 | There was an imbalance in myoelectric activity in the individuals with chronic LDH, and the muscle strength on the affected side was significantly reduced. | |
| LSS | 2003 | Leinonen [113] | 25 | The endurance of paraspinal muscle in LSS patients was good. |
| 2013 | Kääriäinen [115] | 60 | The activation of paraspinal muscles could be observed in the patients with LSS, which indicated an extensive loss of motor functions in the patients with LSS. | |
| 2016 | Kääriäinen [114] | 30 | During two years of follow-up after decompressive surgery, muscle activation profiles tended to further deteriorate. | |
| 2023 | Nüesch [112] | 39 | During midstance, higher activation of multifidus and erector spinae showed in the patients with LSS compared with the healthy controls. |
| Years | First Author | Samples | Conclusions |
|---|---|---|---|
| 2002 | Lu [118] | 34 | Unbalanced sEMG activity in the paravertebral muscles could be found in the patients with AIS, which could be decreased after spine fusion. |
| 2004 | Perret [129] | 16 | The presence of short-latency responses and later activities in sEMG after a postural perturbation. |
| 2005 | Cheung [121] | 23 | sEMG of the paraspinal muscles could forecast the progression in idiopathic scoliosis. |
| 2005 | Gaudreault [7] | 16 | There might be a potentially difference at the lower level of the spine |
| 2010 | Tsai [124] | 74 | During isokinetic flexion and extension exercises, the sEMG activities of the thoracic muscle were significantly higher on the concave side than on the convex side in AIS patients with larger curves, which was different from the healthy control group and those with AIS with smaller curves. |
| 2014 | Farahpour [128] | 20 | Asymmetry of muscle activity in AIS patients relied on the direction of the perturbation during postural perturbation. |
| 2018 | Ko [125] | 25 | Asymmetric spinal stabilization exercise could improve the severity of scoliosis, especially at the concave side of paraspinal muscles. |
| 2019 | Yuan [116] | 90 | The ratios of RMS on paraspinal muscles and relaxation time in the scoliotic patients were found greater than non-scoliotic patients. |
| 2021 | Park [117] | 101 | There was a different characteristic of sEMG in different types of adolescent idiopathic scoliosis curves. |
| 2022 | Cheung [123] | 7 | sEMG biofeedback posture training could reduce the asymmetric paraspinal muscle activities and the curve progression. |
| 2022 | He [127] | 21 | The asymmetric and symmetric exercises elicited greater sEMG activity on the convex and concave side, respectively. |
| 2022 | Liang [122] | 106 | The dynamic asymmetric of the erector spinae group of muscles could predict scoliosis aside. |
| 2023 | Chan [120] | 30 | Imbalance in paraspinal muscles could play a potential role in the rehabilitation for AIS patients. |
| Disorders | Years | First Author | Samples | Conclusions |
|---|---|---|---|---|
| SCI | 2020 | Singh [130] | 36 | Children with SCI exhibited compromised trunk control, which affected their ability to activate trunk muscles both above and below the level of injury. |
| 2022 | Hoglund [131] | 15 | Different stimulations resulted in different responses of different paraspinal muscles in the patients with SCI. | |
| NP | 1994 | Nanno [135] | 96 | The cervical intermittent traction could contribute to relieve pain, increase the frequency of electromyographic signals, and improve blood flow in affected muscles. |
| 2008 | Lecompte [133] | 27 | There was a difference in the muscles’ function between the fighter pilots with and without NP. | |
| 2017 | Park [134] | 40 | Significantly altered neck motion and muscle activation during active neck extension were observed in the patients with unilateral posterior NP. | |
| 2021 | Sremakaew [132] | 50 | Except for the upper trapezius, there was higher activity in all muscles in NP patients. | |
| WAD | 2003 | Siegmund [136] | 44 | Habituation might be a potential confounder of whiplash injury studies using repeated perturbations. |
| 2007 | Descarreaux [138] | 31 | The patients with WAD were able to produce isometric forces with spatial precision in which the time to peak force was increased. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suo, M.; Zhou, L.; Wang, J.; Huang, H.; Zhang, J.; Sun, T.; Liu, X.; Chen, X.; Song, C.; Li, Z. The Application of Surface Electromyography Technology in Evaluating Paraspinal Muscle Function. Diagnostics 2024, 14, 1086. https://doi.org/10.3390/diagnostics14111086
Suo M, Zhou L, Wang J, Huang H, Zhang J, Sun T, Liu X, Chen X, Song C, Li Z. The Application of Surface Electromyography Technology in Evaluating Paraspinal Muscle Function. Diagnostics. 2024; 14(11):1086. https://doi.org/10.3390/diagnostics14111086
Chicago/Turabian StyleSuo, Moran, Lina Zhou, Jinzuo Wang, Huagui Huang, Jing Zhang, Tianze Sun, Xin Liu, Xin Chen, Chunli Song, and Zhonghai Li. 2024. "The Application of Surface Electromyography Technology in Evaluating Paraspinal Muscle Function" Diagnostics 14, no. 11: 1086. https://doi.org/10.3390/diagnostics14111086
APA StyleSuo, M., Zhou, L., Wang, J., Huang, H., Zhang, J., Sun, T., Liu, X., Chen, X., Song, C., & Li, Z. (2024). The Application of Surface Electromyography Technology in Evaluating Paraspinal Muscle Function. Diagnostics, 14(11), 1086. https://doi.org/10.3390/diagnostics14111086







