ABCD2-I Score Predicts Unplanned Emergency Department Revisits within 72 Hours Due to Recurrent Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
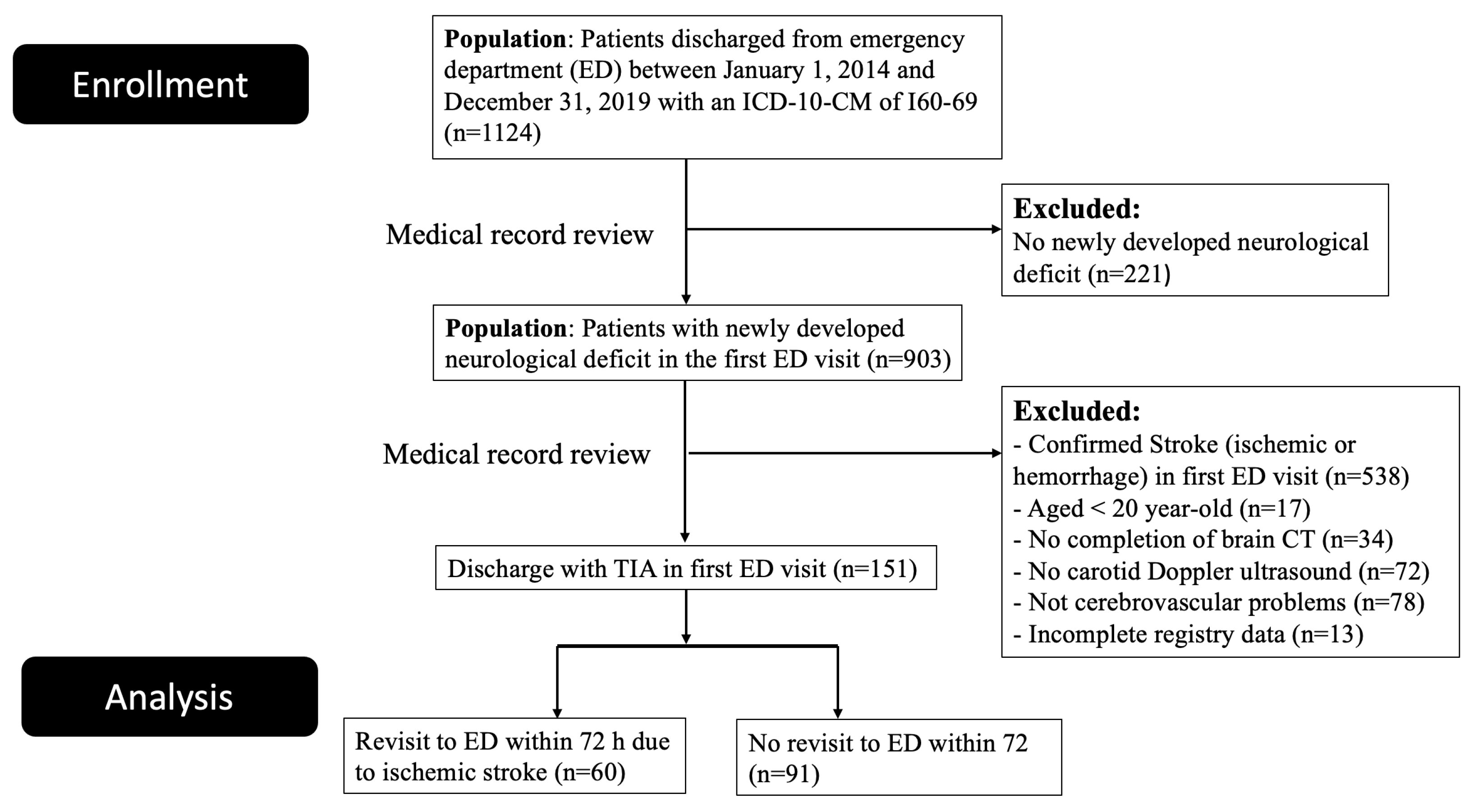
2.1. Design and Study Population
2.2. Sonographic Measurement
2.3. ABCD2 Scoring Systems and Risk Group Stratification
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Sonographic Features
3.3. Risk Group Stratification Analyses
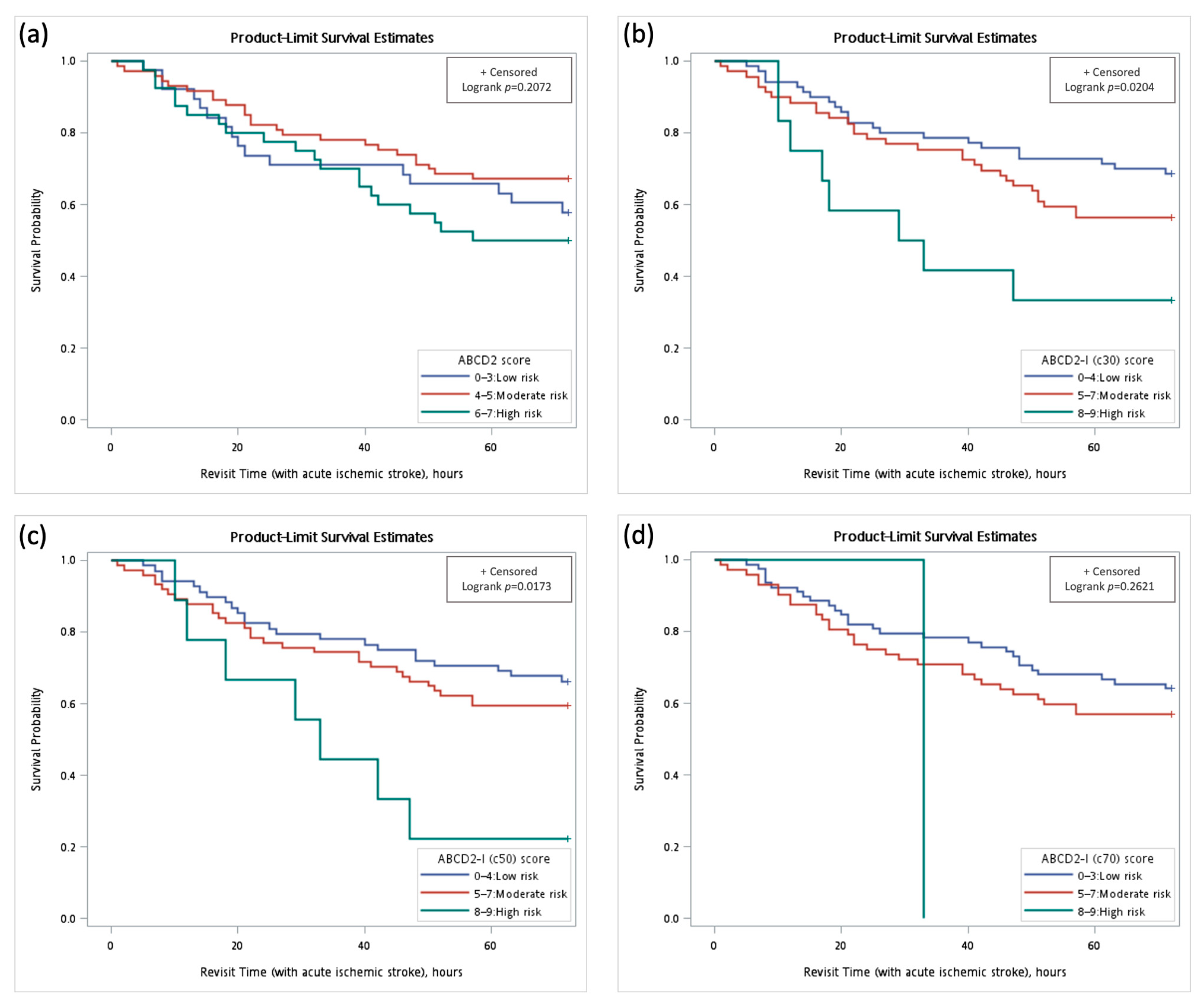
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, H.P.; Madsen, T.E.; Bravata, D.M.; Wira, C.R.; Johnston, S.C.; Ashcraft, S.; Burrus, T.M.; Panagos, P.D.; Wintermark, M.; Esenwa, C.; et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: A scientific statement from the american heart association. Stroke 2023, 54, e109–e121. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009, 40, 2276–2293. [Google Scholar] [PubMed]
- Hurford, R.; Li, L.; Lovett, N.; Kubiak, M.; Kuker, W.; Rothwell, P.M.; Oxford Vascular Study. Prognostic value of “tissue-based” definitions of TIA and minor stroke. Neurology 2019, 92, e2455–e2461. [Google Scholar] [CrossRef] [PubMed]
- Douglas, V.C.; Johnston, C.M.; Elkins, J.; Sidney, S.; Gress, D.R.; Johnston, S.C. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke 2003, 34, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, M.; Chappell, F.M.; Miranda, H.; Shuler, K.; Dennis, M.; Sandercock, P.A.; Muir, K.; Wardlaw, J.M. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann. Neurol. 2014, 75, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.L.; Silvestrini, M.; Topakian, R.; Golledge, J.; Brunser, A.M.; de Borst, G.J.; Harbaugh, R.E.; Doubal, F.N.; Rundek, T.; Thapar, A.; et al. Optimizing the definitions of stroke, transient ischemic attack, and infarction for research and application in clinical practice. Front. Neurol. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Lövblad, K.-O.; Bouchez, L.; Altrichter, S.; Ratib, O.; Machi, P.; Vargas, M.I.; Sztajzel, R. The role of advanced neuroimaging techniques in ischemic stroke prevention. Clin. Transl. Neurosci. 2019, 3, 18. [Google Scholar] [CrossRef]
- Degan, D.; Ornello, R.; Tiseo, C.; De Santis, F.; Pistoia, F.; Carolei, A.; Sacco, S. Epidemiology of transient ischemic attacks using time- or tissue-based definitions: A population-based study. Stroke 2017, 48, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Sanelli, P.C.; Albers, G.W.; Bello, J.A.; Derdeyn, C.P.; Hetts, S.W.; Johnson, M.H.; Kidwell, C.S.; Lev, M.H.; Liebeskind, D.S.; et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology and the Society of NeuroInterventional Surgery. J. Am. Coll. Radiol. 2013, 10, 828–832. [Google Scholar] [CrossRef]
- Brinjikji, W.; Demchuk, A.M.; Murad, M.H.; Rabinstein, A.A.; McDonald, R.J.; McDonald, J.S.; Kallmes, D.F. Neurons over nephrons: Systematic review and meta-analysis of contrast-induced nephropathy in patients with acute stroke. Stroke 2017, 48, 1862–1868. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Chappell, F.M.; Best, J.J.; Wartolowska, K.; Berry, E.; NHS Research and Development Health Technology Assessment Carotid Stenosis Imaging Group. Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: A meta-analysis. Lancet 2006, 367, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Rothwell, P.M.; Nguyen-Huynh, M.N.; Giles, M.F.; Elkins, J.S.; Bernstein, A.L.; Sidney, S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007, 369, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Giles, M.F.; Flossmann, E.; Lovelock, C.E.; Redgrave, J.N.; Warlow, C.P.; Mehta, Z. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005, 366, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, T.; Kamouchi, M.; Kumai, Y.; Ninomiya, T.; Hata, J.; Yoshimura, S.; Ago, T.; Okada, Y.; Kitazono, T.; Fukuoka Stroke Registry Investigators. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke 2014, 45, 418–425. [Google Scholar] [CrossRef]
- Knoflach, M.; Lang, W.; Seyfang, L.; Fertl, E.; Oberndorfer, S.; Daniel, G.; Seifert-Held, T.; Brainin, M.; Krebs, S.; Matosevic, B.; et al. Predictive value of ABCD2 and ABCD3-I scores in TIA and minor stroke in the stroke unit setting. Neurology 2016, 87, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. The natural history of transient ischaemic cerebro-vascular attacks. QJM Int. J. Med. 1964, 33, 309–324. [Google Scholar]
- Anonymous. A classification and outline of cerebrovascular diseases. II. Stroke 1975, 6, 564–616. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Srivastava, M.; Stone, P.A.; Mousa, A.Y.; Jain, A.; Dean, L.S.; Keiffer, T.; Emmett, M. Critical appraisal of the carotid duplex consensus criteria in the diagnosis of carotid artery stenosis. J. Vasc. Surg. 2011, 53, 53–59, discussion 59–60. [Google Scholar] [CrossRef]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid artery stenosis: Grayscale and Doppler ultrasound diagnosis—Society of Radiologists in Ultrasound consensus conference. Ultrasound Q. 2003, 19, 190–198. [Google Scholar] [CrossRef]
- Baumgartner, R.W.; Mattle, H.P.; Schroth, G. Assessment of >/=50% and <50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke 1999, 30, 87–92. [Google Scholar] [PubMed]
- Warlow, C.P. Symptomatic patients: The European Carotid Surgery Trial (ECST). J. Mal. Vasc. 1993, 18, 198–201. [Google Scholar]
- Eliasziw, M.; Kennedy, J.; Hill, M.D.; Buchan, A.M.; Barnett, H.J.; The North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ 2004, 170, 1105–1109. [Google Scholar] [CrossRef]
- Coutts, S.B.; Eliasziw, M.; Hill, M.D.; Scott, J.N.; Subramaniam, S.; Buchan, A.M.; Demchuk, A.M.; VISION Study Group. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int. J. Stroke 2008, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.S.; Lev, M.H.; English, J.D.; Camargo, E.C.; Chou, M.; Johnston, S.C.; Gonzalez, G.; Schaefer, P.W.; Dillon, W.P.; Koroshetz, W.J.; et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009, 40, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.; Cullinane, M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001, 124, 457–467. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Naylor, A.R. Delay may reduce procedural risk, but at what price to the patient? Eur. J. Vasc. Endovasc. Surg. 2008, 35, 383–391. [Google Scholar] [CrossRef]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s choice—Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Nordanstig, A.; Rosengren, L.; Stromberg, S.; Osterberg, K.; Karlsson, L.; Bergstrom, G.; Fekete, Z.; Jood, K. Editor’s choice—Very urgent carotid endarterectomy is associated with an increased procedural risk: The carotid alarm study. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 278–286. [Google Scholar] [CrossRef]
- Merwick, A.; Albers, G.W.; Amarenco, P.; Arsava, E.M.; Ay, H.; Calvet, D.; Coutts, S.B.; Cucchiara, B.L.; Demchuk, A.M.; Furie, K.L.; et al. Addition of brain and carotid imaging to the ABCD(2) score to identify patients at early risk of stroke after transient ischaemic attack: A multicentre observational study. Lancet Neurol. 2010, 9, 1060–1069. [Google Scholar] [CrossRef]
- Bots, M.L.; van der Wilk, E.C.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Transient neurological attacks in the general population. Prevalence, risk factors, and clinical relevance. Stroke 1997, 28, 768–773. [Google Scholar] [CrossRef]
- Hsieh, F.I.; Lien, L.M.; Chen, S.T.; Bai, C.H.; Sun, M.C.; Tseng, H.P.; Chen, Y.W.; Chen, C.H.; Jeng, J.S.; Tsai, S.Y.; et al. Get with the Guidelines-Stroke performance indicators: Surveillance of stroke care in the Taiwan stroke registry: Get with the guidelines-stroke in Taiwan. Circulation 2010, 122, 1116–1123. [Google Scholar] [CrossRef]
- Chiang, C.E.; Wang, T.D.; Ueng, K.C.; Lin, T.H.; Yeh, H.I.; Chen, C.Y.; Wu, Y.J.; Tsai, W.C.; Chao, T.H.; Chen, C.H.; et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan hypertension society for the management of hypertension. J. Chin. Med. Assoc. 2015, 78, 1–47. [Google Scholar] [CrossRef]
- Chang, Y.J.; Ryu, S.J.; Chen, J.R.; Hu, H.H.; Yip, P.K.; Chiu, T.F.; Society, C.G.o.T.S. [Guidelines for the general management of patients with acute ischemic stroke]. Acta Neurol Taiwan 2008, 17, 275–294. [Google Scholar]
- Ntaios, G.; Egli, M.; Faouzi, M.; Michel, P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 2010, 41, 2366–2370. [Google Scholar] [CrossRef]
- Fuentes, B.; Castillo, J.; San José, B.; Leira, R.; Serena, J.; Vivancos, J.; Dávalos, A.; Nuñez, A.G.; Egido, J.; Díez-Tejedor, E.; et al. The prognostic value of capillary glucose levels in acute stroke: The Glycemia in Acute Stroke (GLIAS) study. Stroke 2009, 40, 562–568. [Google Scholar] [CrossRef]
- Tian, D.; Yang, Q.; Dong, Q.; Li, N.; Yan, B.; Fan, D. Trends in stroke subtypes and vascular risk factors in a stroke center in China over 10 years. Sci. Rep. 2018, 8, 5037. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, S.H.; Kim, B.J.; Yu, K.H.; Hong, K.S.; Lee, B.C.; Roh, J.K.; Korean Stroke Registry Study, G. Secular trends in ischemic stroke characteristics in a rapidly developed country: Results from the Korean Stroke Registry Study (secular trends in Korean stroke). Circ. Cardiovasc. Qual. Outcomes 2012, 5, 327–334. [Google Scholar] [CrossRef]
- Tsai, C.F.; Sudlow, C.L.M.; Anderson, N.; Jeng, J.S. Variations of risk factors for ischemic stroke and its subtypes in Chinese patients in Taiwan. Sci. Rep. 2021, 11, 9700. [Google Scholar] [CrossRef]
- Song, B.; Fang, H.; Zhao, L.; Gao, Y.; Tan, S.; Lu, J.; Sun, S.; Chandra, A.; Wang, R.; Xu, Y. Validation of the ABCD3-I score to predict stroke risk after transient ischemic attack. Stroke 2013, 44, 1244–1248. [Google Scholar] [CrossRef]
- Ildstad, F.; Ellekjaer, H.; Wethal, T.; Lydersen, S.; Fjaertoft, H.; Indredavik, B. ABCD3-I and ABCD2 scores in a TIA population with low stroke risk. Stroke Res. Treat. 2021, 2021, 8845898. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Liu, L.; Wang, D.; Wang, C.; Li, H.; Meng, X.; Cui, L.; Jia, J.; Dong, Q.; et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N. Engl. J. Med. 2013, 369, 11–19. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Zhao, X.; Li, H.; Wang, D.; Johnston, S.C.; Liu, L.; Meng, X.; Wang, A.; Wang, C.; et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (chance) Trial: One-year outcomes. Circulation 2015, 132, 40–46. [Google Scholar] [CrossRef]

| Scoring Systems | ABCD2 | ABCD2-I (c30) | ABCD2-I (c50) | ABCD2-I (c70) |
|---|---|---|---|---|
| Age ≥ 60 y | 1 | 1 | 1 | 1 |
| BP ≥ 140/90 mmHg | 1 | 1 | 1 | 1 |
| Clinical symptoms | ||||
| Slurred speech without weakness | 1 | 1 | 1 | 1 |
| Unilateral weakness | 2 | 2 | 2 | 2 |
| Duration of symptoms | ||||
| 10–59 min | 1 | 1 | 1 | 1 |
| ≥60 min | 2 | 2 | 2 | 2 |
| Diabetes mellitus | 1 | 1 | 1 | 1 |
| Carotid duplex | ||||
| Any side of ICA stenosis > 30% | NA | 2 | NA | NA |
| Any side of ICA stenosis > 50% | NA | NA | 2 | NA |
| Any side of ICA stenosis > 70% | NA | NA | NA | 2 |
| Total points (maximum) | 7 | 9 | 9 | 9 |
| Characteristic Variables | Total | Revisit (with Acute Ischemic Stroke) | Discharge (without Acute Ischemic Stroke) | p Value |
|---|---|---|---|---|
| (n = 151) | (n = 60) | (n = 91) | ||
| Age | 68.2 ± 13.4 | 69.8 ± 12.4 | 67.2 ± 14.0 | 0.2488 |
| Female sex (%) | 42.4% (64/151) | 40.0% (24/60) | 44.0% (40/91) | 0.6302 |
| First visit | ||||
| Systolic BP | 157.3 ± 28.3 | 164.4 ± 31.9 | 152.6 ± 24.6 | 0.0174 * |
| Diastolic BP | 87.1 ± 16.6 | 89.3 ± 18.4 | 85.7 ± 15.3 | 0.1941 |
| ED revisit | ||||
| Systolic BP | - | 155.7 ± 27.8 | - | - |
| Diastolic BP | - | 88.1 ± 19.7 | - | - |
| Symptoms and comorbidity | ||||
| Slurred speech (%) | 21.9% (33/151) | 13.3% (8/60) | 27.5% (25/91) | 0.0397 * |
| Unilateral weakness (%) | 50.3% (76/151) | 43.3% (26/60) | 55.0% (50/91) | 0.1626 |
| Hypertension (%) | 67.6% (102/151) | 76.7% (46/60) | 61.5% (56/91) | 0.0520 |
| Diabetes (%) | 23.8% (36/151) | 26.7% (16/60) | 22.0% (20/91) | 0.5082 |
| Ischemic stroke classification | ||||
| Large artery (%) | - | 46.7% (28/60) | - | - |
| Small artery (%) | - | 30.0% (18/60) | - | - |
| Embolic stroke (%) | - | 3.3% (2/60) | - | - |
| Undetermined (%) | - | 20.0% (12/60) | - | - |
| Sonographic findings | ||||
| Intima thickness | 0.90 ± 0.31 | 0.89 ± 0.26 | 0.91 ± 0.35 | 0.7870 |
| Intima thickness > 1 mm | 18.5% (28/151) | 20.0% (12/60) | 17.6% (16/91) | 0.7084 |
| CCA stenosis > 30% | 37.1% (56/151) | 48.3% (29/60) | 29.7% (27/91) | 0.0202 * |
| CCA stenosis > 50% | 17.9% (27/151) | 25.0% (15/60) | 13.2% (12/91) | 0.0638 |
| CCA stenosis > 70% | 2.7% (4/151) | 2.2% (2/60) | 3.3% (2/91) | 0.6707 |
| ICA stenosis > 30% | 14.6% (22/151) | 23.3% (14/60) | 8.8% (8/91) | 0.0132 * |
| ICA stenosis > 50% | 6.6% (10/151) | 24.2% (8/60) | 2.2% (2/91) | 0.0071 * |
| ICA stenosis > 70% | 0.7% (1/151) | 1.7% (1/60) | 0% (0/91) | 0.3974 |
| CCA PSV (cm/s) | 70.2 ± 29.0 | 68.8 ± 38.8 | 71.3 ± 18.3 | 0.6200 |
| CCA RI | 0.72 ± 0.08 | 0.74 ± 0.07 | 0.71 ± 0.08 | 0.0140 * |
| CCA flow (mL/min) | 380.9 ± 105.3 | 362.6 ± 111.0 | 394.9 ± 99.1 | 0.0604 |
| ICA PSV (cm/s) | 62.2 ± 26.7 | 64.8 ± 35.6 | 60.3 ± 17.5 | 0.3448 |
| ICA RI | 0.63 ± 0.09 | 0.65 ± 0.09 | 0.61 ± 0.09 | 0.0032 * |
| ICA Flow (mL/min) | 219.0 ± 75.4 | 201.0 ± 70.4 | 232.6 ± 76.6 | 0.0104 * |
| Bilateral VA flow | 129.2 ± 48.6 | 124.5 ± 42.1 | 132.6 ± 53.1 | 0.4789 |
| Bilateral VA flow < 100 mL/min | 65.6% (99/151) | 61.7% (37/60) | 68.1% (62/91) | 0.8340 |
| Intracranial vessel stenosis > 50% | 26.2% (17/65) | 29.0% (9/31) | 23.5% (8/34) | 0.6141 |
| Day 1 | Day 2 | Day 3 | p Value | |
|---|---|---|---|---|
| Number | 32 (53.3%) | 20 (33.3%) | 8 (13.3%) | |
| Age | 67.8 ± 13.8 | 72.2 ± 11.3 | 71.6 ± 8.0 | 0.4239 |
| Female sex (%) | 34.4% (11/32) | 50.0% (10/20) | 37.5% (3/8) | 0.5753 |
| First visit | ||||
| Systolic BP | 166.3 ± 27.7 | 168.9 ± 39.1 | 143.6 ± 24.6 | 0.1831 |
| Diastolic BP | 91.2 ± 18.4 | 88.6 ± 19.8 | 85.4 ± 15.7 | 0.7269 |
| ED revisit | ||||
| Systolic BP | 158.7 ± 30.8 | 153.5 ± 27.3 | 147.2 ± 14.8 | 0.6147 |
| Diastolic BP | 92.7 ± 19.5 | 85.4 ± 19.3 | 79.8 ± 16.9 | 0.2243 |
| Symptoms and comorbidity | ||||
| Slurred speech (%) | 12.9% (4/31) | 20.0% (4/20) | 0 (0/8) | 0.5127 |
| Unilateral weakness (%) | 45.2% (6/31) | 45.0% (7/20) | 37.5% (3/8) | 0.9221 |
| Hypertension (%) | 71.0% (22/31) | 80.0% (16/20) | 87.5% (7/8) | 0.5508 |
| Diabetes (%) | 19.4% (6/31) | 35.0% (7/20) | 37.5% (3/5) | 0.3894 |
| Stroke classification | 0.8172 | |||
| Large artery (%) | 46.9% (15/32) | 40.0% (8/20) | 62.5% (5/8) | |
| Small artery (%) | 25.0% (8/32) | 40.0% (8/20) | 25.0% (2/8) | |
| Embolic stroke (%) | 3.1% (1/32) | 5.0% (1/20) | 0% (0/8) | |
| Undetermined (%) | 25.0% (8/32) | 15.0% (3/20) | 12.5% (1/8) | |
| Point score | ||||
| ABCD2 score | 4.4 ± 1.2 | 4.9 ± 1.3 | 4.5 ± 1.4 | 0.4140 |
| ABCD2-I (c30) | 4.9 ± 1.7 | 5.4 ± 1.5 | 4.8 ± 1.5 | 0.5175 |
| ABCD2-I (c50) | 4.7 ± 1.6 | 5.2 ± 1.5 | 4.8 ± 1.5 | 0.4876 |
| ABCD2-I (c70) | 4.4 ± 1.2 | 5.0 ± 1.4 | 4.5 ± 1.4 | 0.3214 |
| (n = 151) | Total | Revisit (with Acute Ischemic Stroke) | Discharge (without Acute Ischemic Stroke) | p Value |
|---|---|---|---|---|
| Number | 151 | 60 (39.7%) | 91 (60.3%) | |
| Point score | ||||
| ABCD2 score, mean | 4.45 ± 1.34 | 4.60 ± 1.26 | 4.35 ± 1.39 | 0.2665 |
| ABCD2 score, distribution | 0.1937 | |||
| 0–3: Low risk (%) | 25.2% (38/151) | 26.7% (16/60) | 24.2% (22/91) | |
| 4–5: Moderate risk (%) | 48.3% (73/151) | 40.0% (24/60) | 53.9% (49/91) | |
| 6–7: High risk (%) | 26.5% (40/151) | 33.3% (20/60) | 22.0% (20/91) | |
| ABCD2-I (c30) score, mean | 4.74 ± 1.63 | 5.07 ± 1.62 | 4.53 ± 1.60 | 0.0450 * |
| ABCD2-I (c30) score, distribution | 0.0484 * | |||
| 0–4: Low risk (%) | 46.4% (70/151) | 36.7% (22/60) | 52.8% (48/91) | |
| 5–7: Moderate risk (%) | 45.7% (69/151) | 50.0% (30/60) | 42.9% (39/91) | |
| 8–9: High risk (%) | 8.0% (12/151) | 13.3% (8/60) | 4.4% (4/91) | |
| ABCD2-I (c50) score, mean | 4.79 ± 1.59 | 5.13 ± 1.60 | 4.57 ± 1.56 | 0.0335 * |
| ABCD2-I (c50) score, distribution | 0.0397 * | |||
| 0–4: Low risk (%) | 45.0% (68/151) | 38.3% (23/60) | 49.5% (45/91) | |
| 5–7: Moderate risk (%) | 49.0% (74/151) | 50.0% (30/60) | 48.4% (44/91) | |
| 8–9: High risk (%) | 6.0% (9/151) | 11.7% (7/60) | 2.2% (2/91) | |
| ABCD2-I (c70) score, mean | 4.6 ± 1.5 | 4.9 ± 1.5 | 4.4 ± 1.5 | 0.0569 |
| ABCD2-I (c70) score, distribution | 0.3123 | |||
| 0–4: Low risk (%) | 51.7% (78/151) | 46.7% (28/60) | 55.0% (50/91) | |
| 5–7: Moderate risk (%) | 47.7% (72/151) | 51.7% (31/60) | 45.1% (41/91) | |
| 8–9: High risk (%) | 0.7% (1/151) | 1.7% (1/60) | 0 (0/91) | |
| (n = 151) | HR (95% CI) | p Value | Adjusted HR | p Value |
|---|---|---|---|---|
| Model 1 of ABCD2 score | ||||
| ABCD2 score, low risk | Reference | - | ||
| ABCD2 score, moderate risk | 0.74 (0.39–1.39) | 0.3498 | 0.72 (0.38–1.36) | 0.3081 |
| ABCD2 score, high risk | 1.21 (0.63–2.34) | 0.5675 | 1.12 (0.57–2.22) | 0.7427 |
| Model 2 of ABCD2-I (c30) score | ||||
| ABCD2-I (c30), low risk | Reference | |||
| ABCD2-I (c30), moderate risk | 1.49 (0.86–2.58) | 0.1578 | 1.46 (0.83–2.55) | 0.1855 |
| ABCD2-I (c30), high risk | 3.02 (1.34–6.80) | 0.0077 * | 2.79 (1.20–6.46) | 0.0170 * |
| Model 3 of ABCD2-I (c50) score | ||||
| ABCD2-I (c50) score, low risk | Reference | |||
| ABCD2-I (c50), moderate risk | 1.28 (0.74–2.20) | 0.3770 | 1.24 (0.72–2.15) | 0.4364 |
| ABCD2-I (c50), high risk | 3.25 (1.39–7.62) | 0.0066 * | 3.12 (1.31–7.41) | 0.0102 * |
| Model 4 of ABCD2-I (c70) score | ||||
| ABCD2-I (c70), low risk | Reference | |||
| ABCD2-I (c70), moderate risk | 1.30 (0.78–2.16) | 0.3208 | 1.26 (0.74–2.12) | 0.3923 |
| ABCD2-I (c70), high risk | 3.91 (0.53–29.02) | 0.1825 | 3.64 (0.49–27.23) | 0.2084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-Z.; Lin, H.-A.; Hou, S.-K.; Lin, S.-F. ABCD2-I Score Predicts Unplanned Emergency Department Revisits within 72 Hours Due to Recurrent Acute Ischemic Stroke. Diagnostics 2024, 14, 1118. https://doi.org/10.3390/diagnostics14111118
Lu W-Z, Lin H-A, Hou S-K, Lin S-F. ABCD2-I Score Predicts Unplanned Emergency Department Revisits within 72 Hours Due to Recurrent Acute Ischemic Stroke. Diagnostics. 2024; 14(11):1118. https://doi.org/10.3390/diagnostics14111118
Chicago/Turabian StyleLu, Wei-Zhen, Hui-An Lin, Sen-Kuang Hou, and Sheng-Feng Lin. 2024. "ABCD2-I Score Predicts Unplanned Emergency Department Revisits within 72 Hours Due to Recurrent Acute Ischemic Stroke" Diagnostics 14, no. 11: 1118. https://doi.org/10.3390/diagnostics14111118
APA StyleLu, W.-Z., Lin, H.-A., Hou, S.-K., & Lin, S.-F. (2024). ABCD2-I Score Predicts Unplanned Emergency Department Revisits within 72 Hours Due to Recurrent Acute Ischemic Stroke. Diagnostics, 14(11), 1118. https://doi.org/10.3390/diagnostics14111118







