Virtual Non-Contrast versus True Native in Photon-Counting CT: Stability of Density of Upper Abdominal Organs and Vessels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. CT Scan and Contrast Protocol
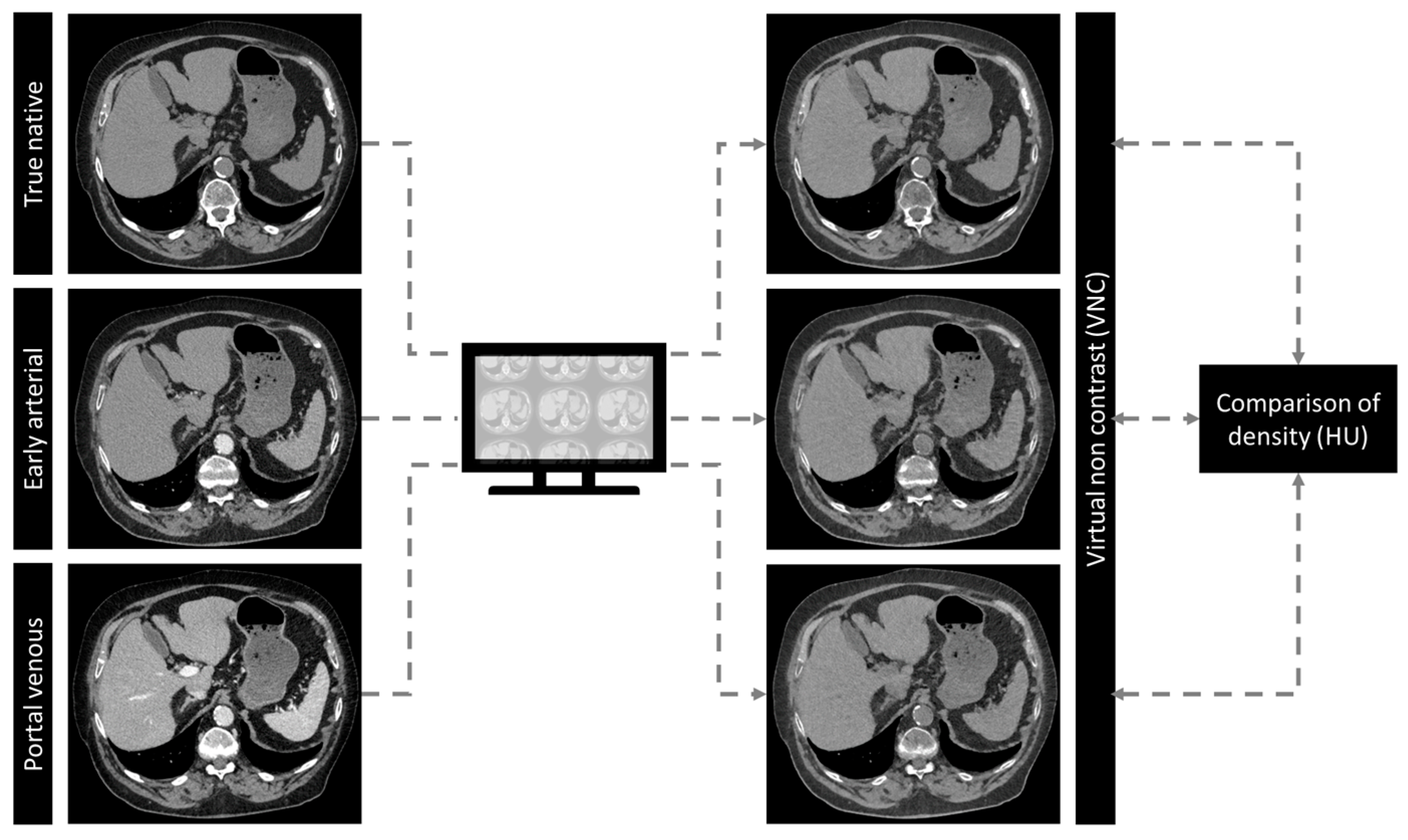
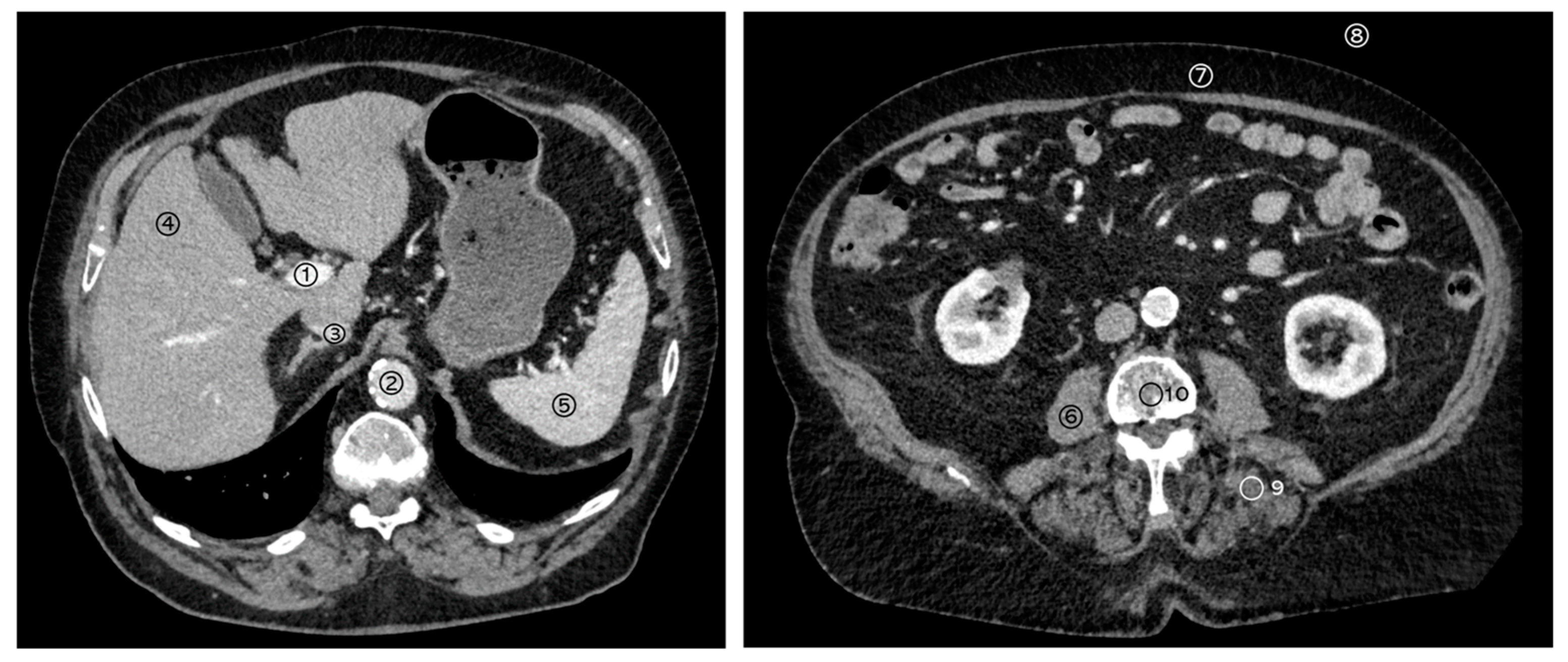
2.3. Structured Measurements
2.4. Statistical Analysis
3. Results
3.1. Patient Cohort
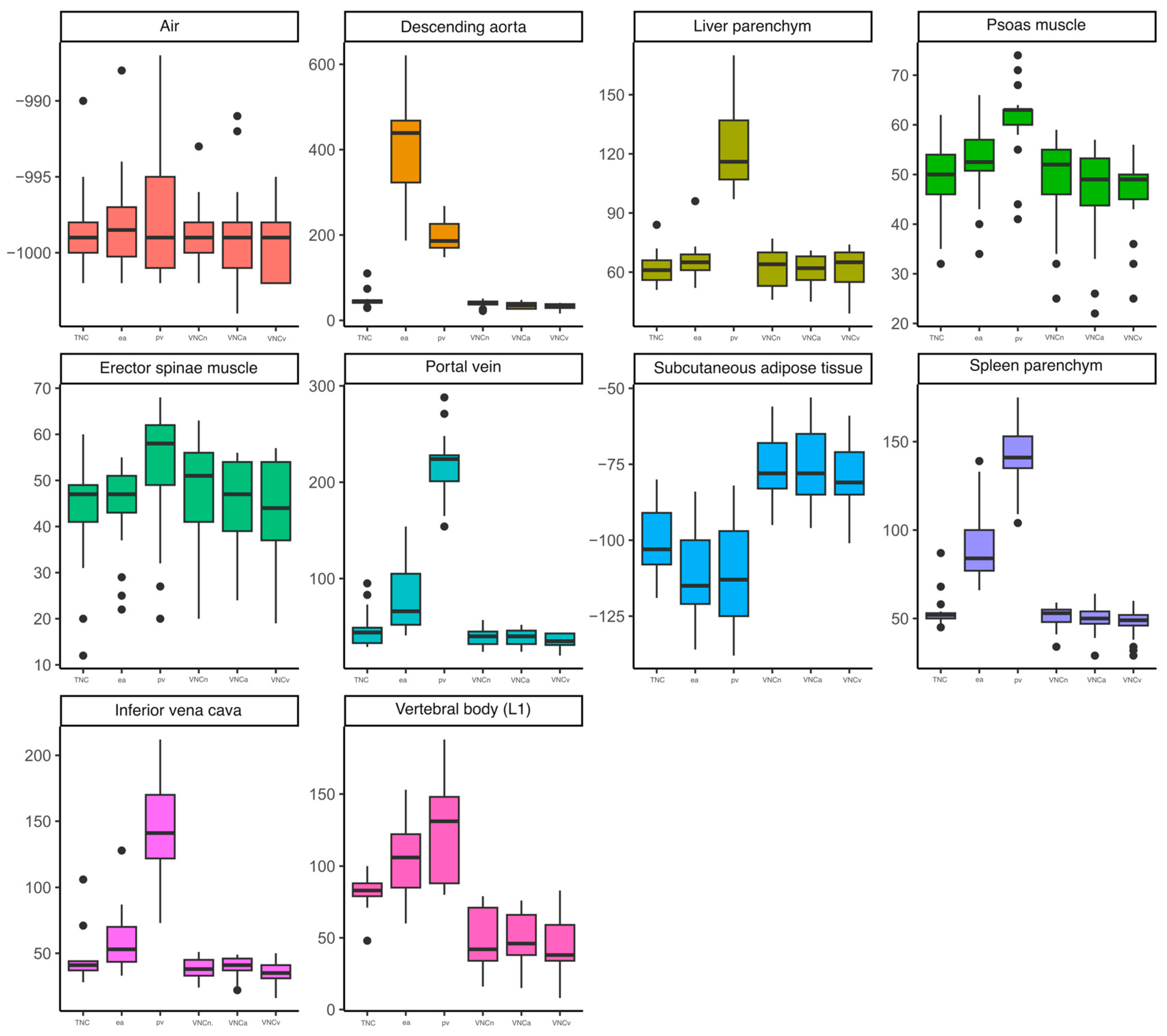
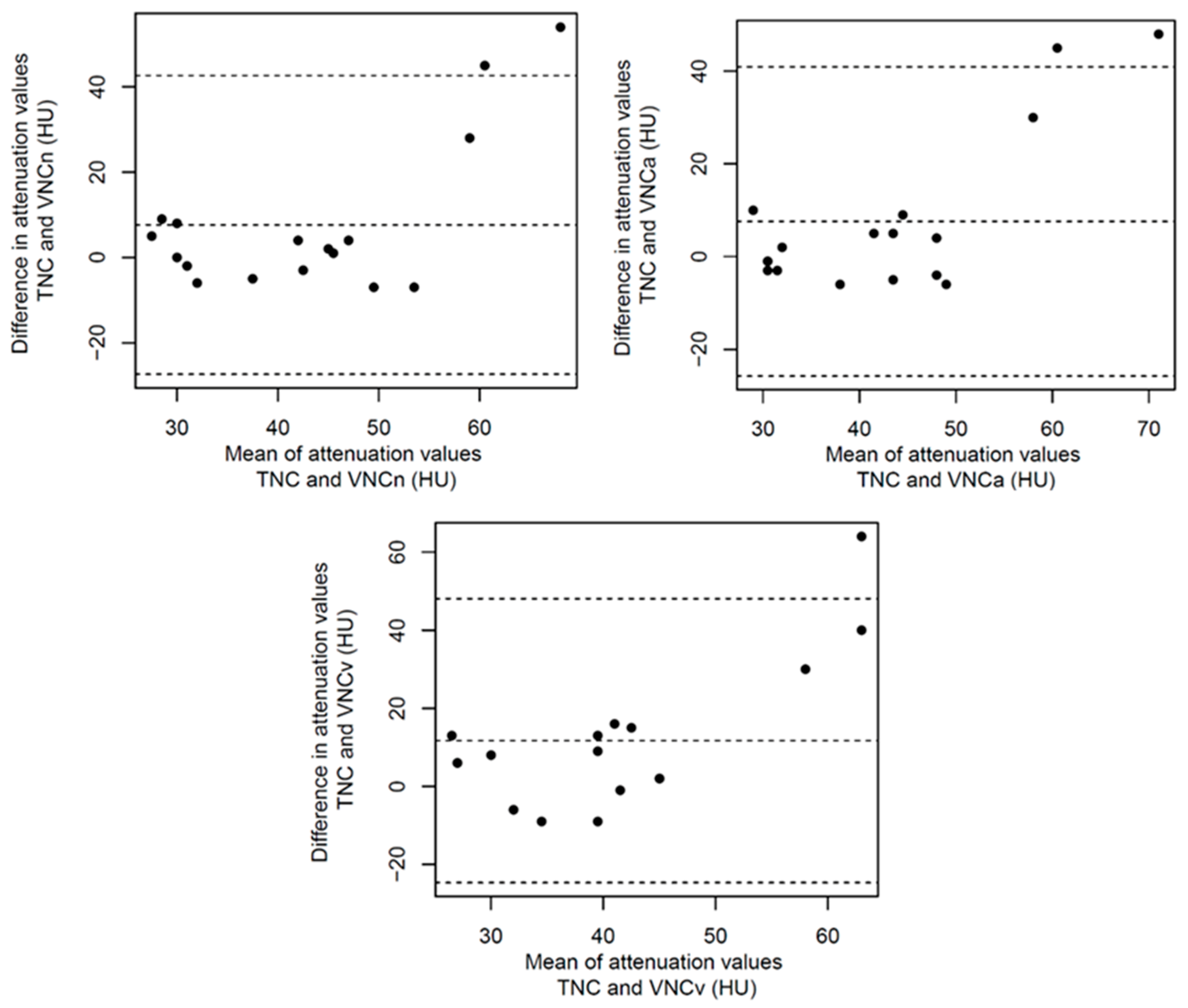
3.2. Structured Measurement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| DA | Descending aorta |
| ESM | Erector spinae muscle |
| HU | Hounsfield units |
| IVC | Inferior vena cava |
| L1 | First lumbar vertebral body |
| PCCT | Photon-counting computed tomography |
| PM | Psoas muscle |
| PV | Portal vein |
| ROI | Regions of interest |
| SAT | Subcutaneous adipose tissue |
| SD | Standard deviation |
| TNC | True non-contrast |
| VNC | Virtual non-contrast |
| VNCa | Virtual non-contrast (derived from early arterial contrast phase) |
| VNCn | Virtual non-contrast (derived from native contrast phase) |
| VNCv | Virtual non-contrast (derived from portal venous contrast phase) |
Appendix A
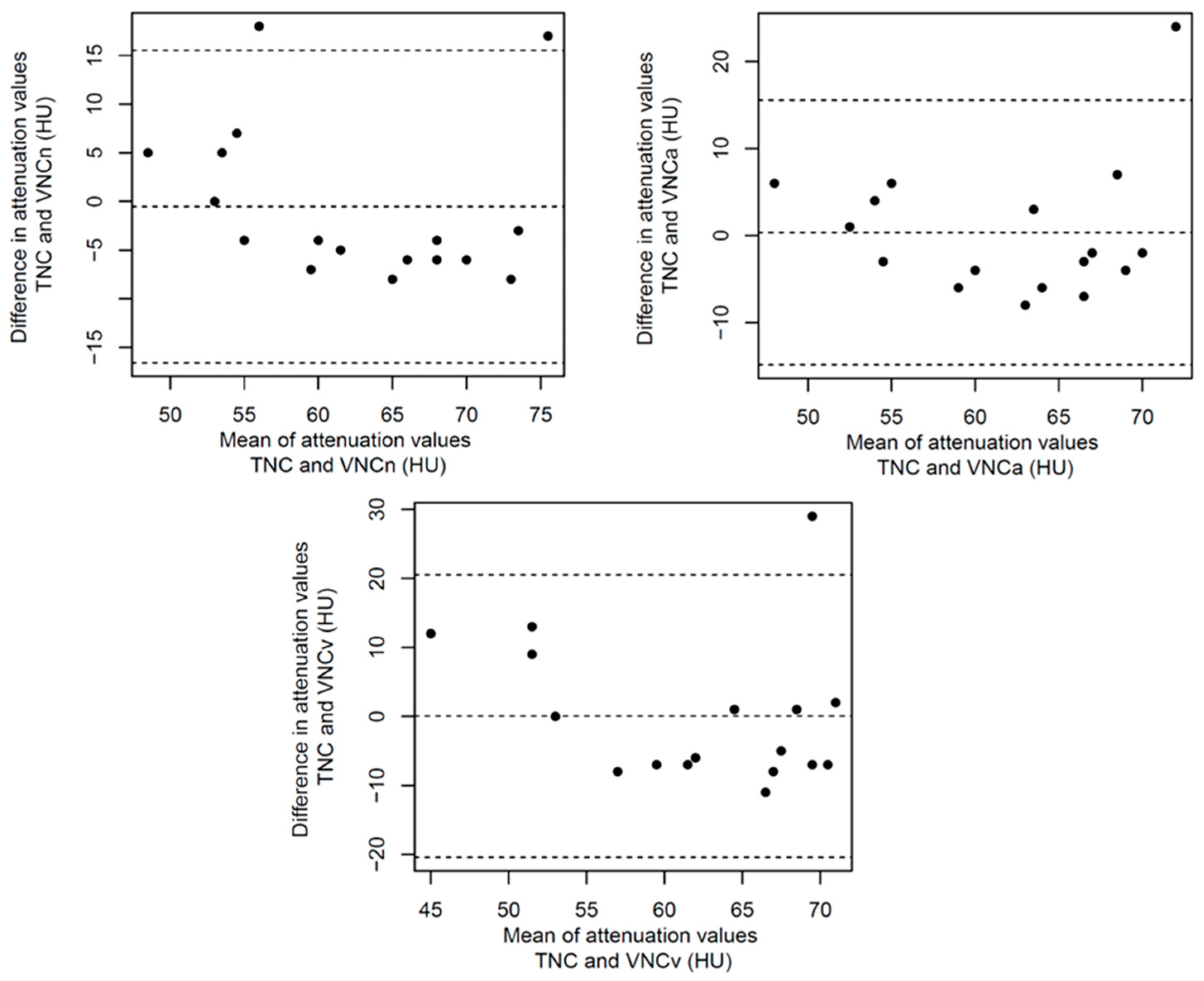
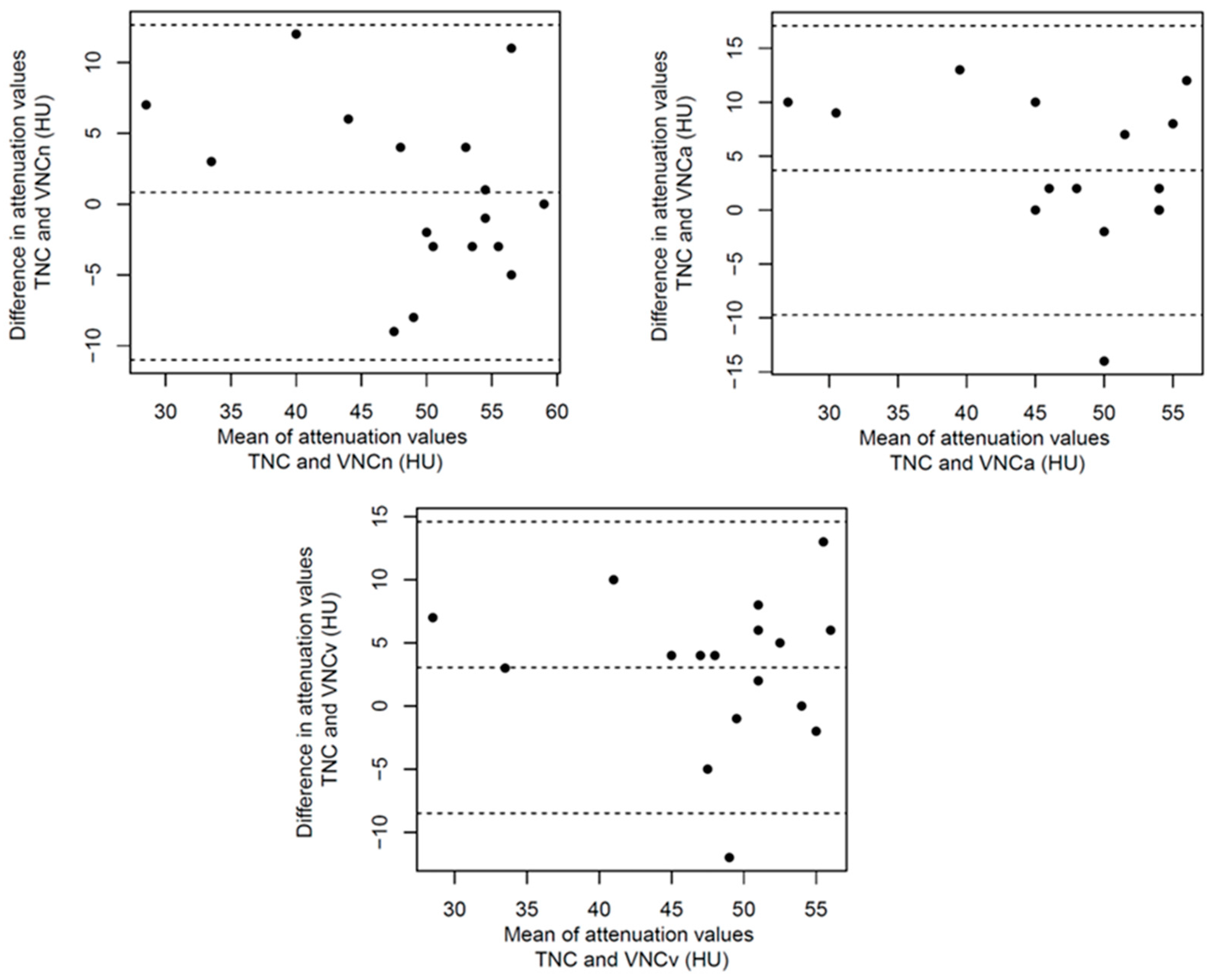
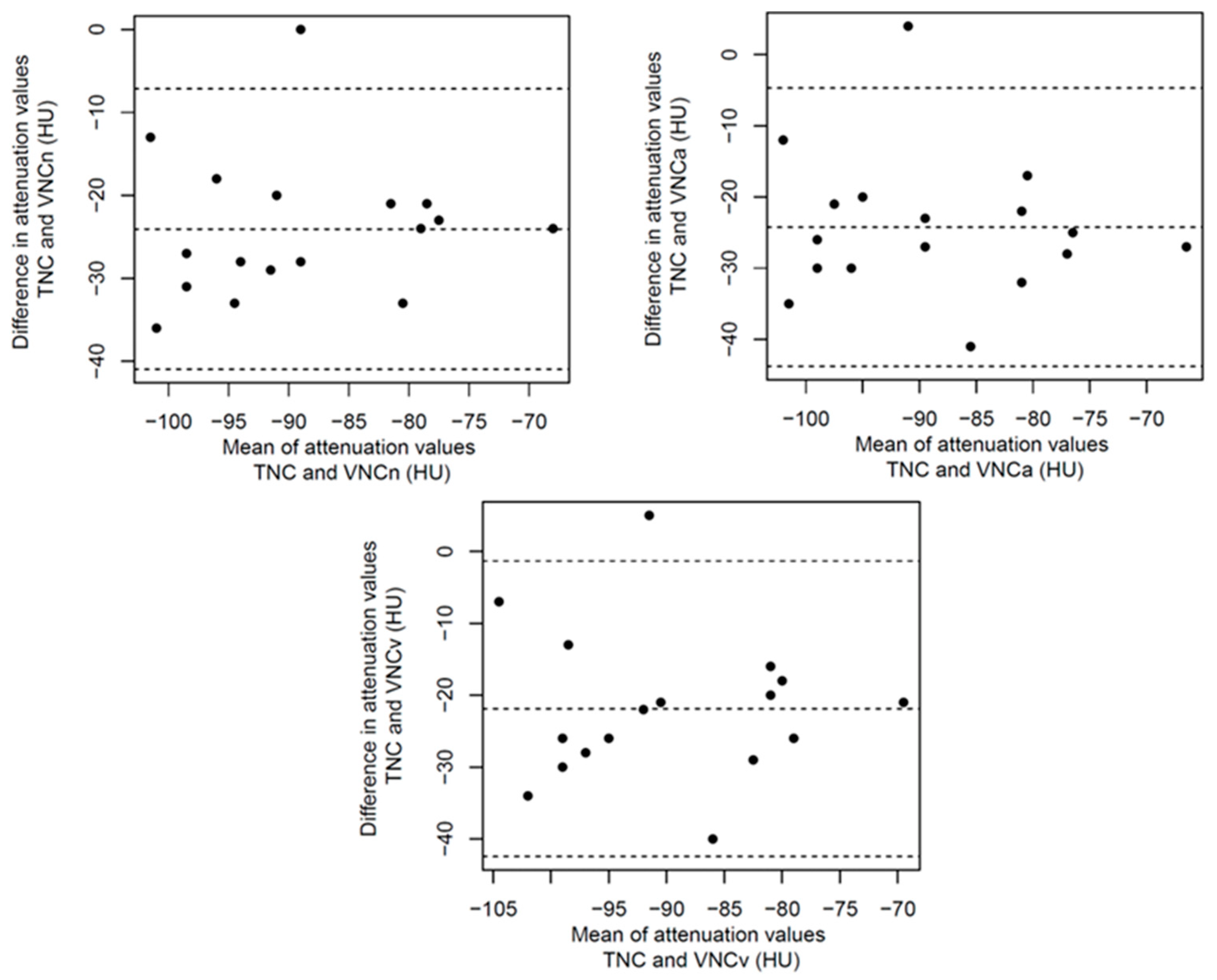
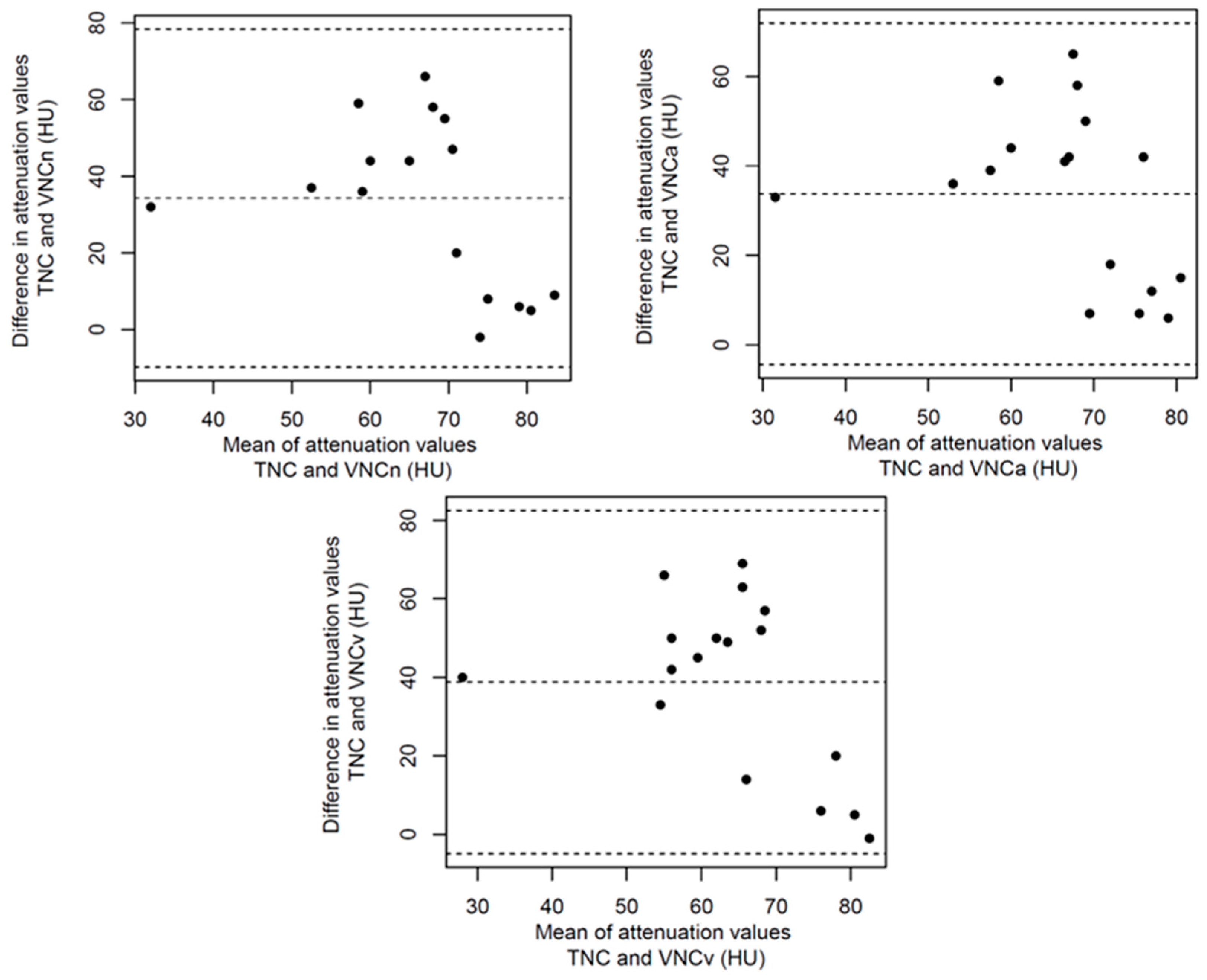
References
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-Counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef]
- Pannenbecker, P.; Huflage, H.; Grunz, J.-P.; Gruschwitz, P.; Patzer, T.S.; Weng, A.M.; Heidenreich, J.F.; Bley, T.A.; Petritsch, B. Photon-counting CT for diagnosis of acute pulmonary embolism: Potential for contrast medium and radiation dose reduction. Eur. Radiol. 2023, 33, 7830–7839. [Google Scholar] [CrossRef]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef]
- Tharmaseelan, H.; Rotkopf, L.T.; Ayx, I.; Hertel, A.; Nörenberg, D.; Schoenberg, S.O.; Froelich, M.F. Evaluation of radiomics feature stability in abdominal monoenergetic photon counting CT reconstructions. Sci. Rep. 2022, 12, 19594. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef]
- Kahn, J.; Fehrenbach, U.; Böning, G.; Feldhaus, F.; Maurer, M.; Renz, D.; Streitparth, F. Spectral CT in patients with acute thoracoabdominal bleeding—A safe technique to improve diagnostic confidence and reduce dose? Medicine 2019, 98, e16101. [Google Scholar] [CrossRef] [PubMed]
- Kessner, R.; Sommer, J.; Große Hokamp, N.; Laukamp, K.R.; Nayate, A. Virtual versus true non-contrast images of the brain from spectral detector CT: Comparison of attenuation values and image quality. Acta Radiol. 2023, 64, 776–783. [Google Scholar] [CrossRef]
- Javadi, S.; Elsherif, S.; Bhosale, P.; Jensen, C.T.; Layman, R.R.; Jacobsen, M.C.; Le, O.; Jia, S.; Parikh, R.J.; Tamm, E.P. Quantitative attenuation accuracy of virtual non-enhanced imaging compared to that of true non-enhanced imaging on dual-source dual-energy CT. Abdom. Radiol. 2020, 45, 1100–1109. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Lennartz, S.; Ho, V.; Große Hokamp, N.; Zopfs, D.; Gupta, A.; Graner, F.P.; Borggrefe, J.; Gilkeson, R.; Ramaiya, N. Evaluation of the liver with virtual non-contrast: Single institution study in 149 patients undergoing TAVR planning. Br. J. Radiol. 2020, 93, 20190701. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.M.; Ascenti, V.; Barbera, S.; Fontana, F.; Aricò, F.M.; Piacentino, F.; Coppola, A.; Cicero, G.; Marino, M.A.; Booz, C.; et al. Virtual Non-Contrast Spectral CT in Renal Masses: Is It Time to Discard Conventional Unenhanced Phase? J. Clin. Med. 2023, 12, 4718. [Google Scholar] [CrossRef]
- Liang, H.; Du, S.; Yan, G.; Zhou, Y.; Yang, T.; Zhang, Z.; Luo, C.; Liao, H.; Li, Y. Dual-energy CT of the pancreas: Comparison between virtual non-contrast images and true non-contrast images in the detection of pancreatic lesion. Abdom. Radiol. 2023, 48, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Park, H.J.; Kim, J.N.; Kim, M.S.; Hong, S.W.; Park, J.H.; Kang, C.H. Virtual non-contrast images from dual-energy CT angiography of the abdominal aorta and femoral arteries: Comparison with true non-contrast CT images. Br. J. Radiol. 2022, 95, 20220378. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Dupuis, N.; Tatard-Leitman, V.; Rotzinger, D.; Boccalini, S.; Dion, M.; Vlassenbroek, A.; Coulon, P.; Yagil, Y.; Shapira, N.; et al. Virtual versus true non-contrast dual-energy CT imaging for the diagnosis of aortic intramural hematoma. Eur. Radiol. 2019, 29, 6762–6771. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Yi, J.G.; Park, J.H.; Kim, S.M.; Lee, K.S.; Chung, M.J. Virtual Non-Contrast CT Using Dual-Energy Spectral CT: Feasibility of Coronary Artery Calcium Scoring. Korean J. Radiol. 2016, 17, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Ghouse, S.; Sartoretti, T.; Manka, R.; Euler, A.; Kasel, A.M.; Alkadhi, H.; Eberhard, M. Cardiac Virtual Noncontrast Images for Calcium Quantification with Photon-Counting Detector CT. Radiol. Cardiothorac. Imaging 2023, 5, e220307. [Google Scholar] [CrossRef] [PubMed]
- Al-Difaie, Z.; Scheepers, M.H.; Bouvy, N.D.; Engelen, S.; Havekes, B.; Postma, A.A. Can virtual non-contrast imaging replace true non-contrast imaging in multiphase scanning of the neck region? Acta Radiol. Open 2023, 12, 20584601231205159. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.P.; Zaytoun, H.; Klein, K.; Sonnabend, K.; Lennartz, S.; Zopfs, D.; Heidenreich, A.; Maintz, D.; Große Hokamp, N. Detection and size measurements of kidney stones on virtual non-contrast reconstructions derived from dual-layer computed tomography in an ex vivo phantom setup. Eur. Radiol. 2023, 33, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Schoenbeck, D.; Pauline Haag, N.; Elias Michael, A.; Michael Woeltjen, M.; Boriesosdick, J.; Saeed, S.; Borggrefe, J.; Robert Kroeger, J.; Henning Niehoff, J. Dedicated virtual non-contrast images adapted for liver tissue in clinical photon counting CT improve virtual non-contrast imaging in various organs beyond the liver. Eur. J. Radiol. 2023, 167, 111031. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, J.H.; Woeltjen, M.M.; Laukamp, K.R.; Borggrefe, J.; Kroeger, J.R. Virtual Non-Contrast versus True Non-Contrast Computed Tomography: Initial Experiences with a Photon Counting Scanner Approved for Clinical Use. Diagnostics 2021, 11, 2377. [Google Scholar] [CrossRef]
- Rickham, P.P. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 20 March 2024).
- Reginelli, A.; Capasso, R.; Ciccone, V.; Croce, M.R.; Di Grezia, G.; Carbone, M.; Maggialetti, N.; Barile, A.; Fonio, P.; Scialpi, M.; et al. Usefulness of triphasic CT aortic angiography in acute and surveillance: Our experience in the assessment of acute aortic dissection and endoleak. Int. J. Surg. 2016, 33 (Suppl. S1), S76–S84. [Google Scholar] [CrossRef] [PubMed]
- Cassagnes, L.; Pérignon, R.; Amokrane, F.; Petermann, A.; Bécaud, T.; Saint-Lebes, B.; Chabrot, P.; Rousseau, H.; Boyer, L. Aortic stent-grafts: Endoleak surveillance. Diagn. Interv. Imaging 2016, 97, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Brisbane, W.; Bailey, M.R.; Sorensen, M.D. An overview of kidney stone imaging techniques. Nat. Rev. Urol. 2016, 13, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Choi, H.H.; Ohliger, M.A. Detection of fatty liver using virtual non-contrast dual-energy CT. Abdom. Radiol. 2022, 47, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, Z.-Q.; Yan, F.-H. Separation of hepatic iron and fat by dual-source dual-energy computed tomography based on material decomposition: An animal study. PLoS ONE 2014, 9, e110964. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, M.T.; Gassenmaier, S.; Walter, S.S.; Artzner, C.; Lades, F.; Faby, S.; Nikolaou, K.; Bongers, M.N. Differentiation of adrenal adenomas from adrenal metastases in single-phased staging dual-energy CT and radiomics. Diagn. Interv. Radiol. 2022, 28, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dodig, D.; Solocki Matić, T.; Žuža, I.; Pavlović, I.; Miletić, D.; Markić, D. Side-by-side evaluation of virtual non-contrast and post-contrast images improves detection of clinically significant urolithiasis on single-phase split bolus dual-energy CT urography. Br. J. Radiol. 2021, 94, 20210013. [Google Scholar] [CrossRef]
- McCoombe, K.; Dobeli, K.; Meikle, S.; Llewellyn, S.; Kench, P. Sensitivity of virtual non-contrast dual-energy CT urogram for detection of urinary calculi: A systematic review and meta-analysis. Eur. Radiol. 2022, 32, 8588–8596. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Hagen, F.; Artzner, K.; Bongers, M.N.; Artzner, C. Dual-Energy CT for Accurate Discrimination of Intraperitoneal Hematoma and Intestinal Structures. Diagnostics 2022, 12, 2542. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Nyman, U.; Gottsäter, A.; Wassélius, J. Reliability of virtual non-contrast computed tomography angiography: Comparing it with the real deal. Acta Radiol. Open 2018, 7, 2058460118790115. [Google Scholar] [CrossRef]
- Yun, S.Y.; Heo, Y.J.; Jeong, H.W.; Baek, J.W.; Choo, H.J.; Shin, G.W.; Kim, S.T.; Jeong, Y.G.; Lee, J.Y.; Jung, H.S. Dual-energy CT angiography-derived virtual non-contrast images for follow-up of patients with surgically clipped aneurysms: A retrospective study. Neuroradiology 2019, 61, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Durieux, P.; Gevenois, P.A.; van Muylem, A.; Howarth, N.; Keyzer, C. Abdominal Attenuation Values on Virtual and True Unenhanced Images Obtained with Third-Generation Dual-Source Dual-Energy CT. AJR Am. J. Roentgenol. 2018, 210, 1042–1058. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, J.H.; Woeltjen, M.M.; Saeed, S.; Michael, A.E.; Boriesosdick, J.; Borggrefe, J.; Kroeger, J.R. Assessment of hepatic steatosis based on virtual non-contrast computed tomography: Initial experiences with a photon counting scanner approved for clinical use. Eur. J. Radiol. 2022, 149, 110185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; van der Bie, J.; van Straten, M.; Hirsch, A.; Bos, D.; Dijkshoorn, M.L.; Booij, R.; Budde, R.P.J. Coronary calcium scoring on virtual non-contrast and virtual non-iodine reconstructions compared to true non-contrast images using photon-counting computed tomography. Eur. Radiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, R.; Deng, W.; An, S.; Li, Y.; Sheng, M.; Chen, X.; Qian, Y.; Yu, Y.; Mu, D.; et al. Feasibility and accuracy of coronary artery calcium score on virtual non-contrast images derived from a dual-layer spectral detector CT: A retrospective multicenter study. Front. Cardiovasc. Med. 2023, 10, 1114058. [Google Scholar] [CrossRef] [PubMed]
- Nadjiri, J.; Kaissis, G.; Meurer, F.; Weis, F.; Laugwitz, K.-L.; Straeter, A.S.; Muenzel, D.; Noël, P.B.; Rummeny, E.J.; Rasper, M. Accuracy of Calcium Scoring calculated from contrast-enhanced Coronary Computed Tomography Angiography using a dual-layer spectral CT: A comparison of Calcium Scoring from real and virtual non-contrast data. PLoS ONE 2018, 13, e0208588. [Google Scholar] [CrossRef]
- Gassert, F.G.; Schacky, C.E.; Müller-Leisse, C.; Gassert, F.T.; Pahn, G.; Laugwitz, K.-L.; Makowski, M.R.; Nadjiri, J. Calcium scoring using virtual non-contrast images from a dual-layer spectral detector CT: Comparison to true non-contrast data and evaluation of proportionality factor in a large patient collective. Eur. Radiol. 2021, 31, 6193–6199. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Lee, Y.J.; Choi, Y.J.; Pak, S. Dual-energy CT of the liver: True noncontrast vs. virtual noncontrast images derived from multiple phases for the diagnosis of fatty liver. Eur. J. Radiol. 2021, 140, 109741. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, C.K.; Park, B.K. Dual-energy CT in assessing therapeutic response to radiofrequency ablation of renal cell carcinomas. Eur. J. Radiol. 2014, 83, e73–e79. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Chiou, Y.-Y.; Wu, M.-H.; Huang, S.-S.; Shen, S.-H. Attenuation values of renal parenchyma in virtual noncontrast images acquired from multiphase renal dual-energy CT: Comparison with standard noncontrast CT. Eur. J. Radiol. 2018, 101, 103–110. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Wassélius, J. Comparing Arterial- and Venous-Phase Acquisition for Optimization of Virtual Noncontrast Images from Dual-Energy Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2019, 43, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Risch, F.; Bette, S.; Sinzinger, A.; Rippel, K.; Scheurig-Muenkler, C.; Kroencke, T.; Decker, J.A. Multiphase photon counting detector CT data sets—Which combination of contrast phase and virtual non-contrast algorithm is best suited to replace true non-contrast series in the assessment of active bleeding? Eur. J. Radiol. 2023, 168, 111125. [Google Scholar] [CrossRef] [PubMed]
- Wildberger, J.E.; Alkadhi, H. New Horizons in Vascular Imaging with Photon-Counting Detector CT. Investig. Radiol. 2023, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]

| n | 17 |
| Age (mean (SD)) | 75.65 (9) |
| Reason for examination | |
| Staging | 9 |
| Vessel examination | 7 |
| Bleeding evaluation | 1 |
| Sex (%) | |
| Male | 11 (65) |
| Female | 6 (35) |
| TNC | VNCn | ea | VNCa | pv | VNCv | |
|---|---|---|---|---|---|---|
| n | 17 | 17 | 17 | 17 | 17 | 17 |
| Portal vein (mean (SD)) | 46.71 (19.42) | 39.06 (9.42) | 76.24 (31.98) | 39.12 (7.83) | 216.35 * (35.87) | 35.00 (7.94) |
| Descending aorta (mean (SD)) | 47.94 (18.69) | 40.18 (8.15) | 411.00 * (126.14) | 34.76 (7.45) | 195.82 (35.77) | 32.18 (7.99) |
| IVC (mean (SD)) | 44.76 (18.30) | 38.59 (8.03) | 59.75 (23.87) | 38.82 (8.44) | 145.47 * (40.08) | 35.06 (8.68) |
| Liver parenchyma (mean (SD)) | 62.12 (8.25) | 62.65 (10.03) | 65.94 (9.74) | 61.76 (7.85) | 123.35 * (22.76) | 62.06 (10.63) |
| Spleen parenchyma (mean (SD)) | 54.12 (9.92) | 50.47 (7.04) | 93.65 * (21.75) | 49.59 (8.37) | 140.12 * (20.72) | 47.35 (9.12) |
| Erector spinae muscle (mean (SD)) | 42.71 (11.78) | 47.47 (11.90) | 43.94 (9.88) | 45.24 (9.78) | 52.65 * (14.14) | 42.82 (11.16) |
| Psoas muscle (mean (SD)) | 49.47 (7.80) | 48.65 (9.90) | 52.00 (7.78) | 45.62 (10.37) | 60.82 * (8.23) | 46.41 (8.35) |
| Subcutaneous adipose tissue (mean (SD)) | −100.82 (10.97) | −76.76 * (10.25) | −111.24 (15.60) | −76.59 * (11.93) | −111.18 (16.98) | −78.94 * (11.29) |
| Vertebral body (L1), (mean (SD)) | 83.24 (12.30) | 48.94 * (20.14) | 104.65 (26.58) | 49.47 * (17.97) | 121.88 (35.73) | 44.41 * (20.61) |
| Air (mean (SD)) | −998.65 (2.91) | −998.88 (2.26) | −998.00 (3.50) | −998.82 (3.40) | −997.47 (4.49) | −999.53 (2.35) |
| Portal Vein | Descending Aorta | IVC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VNCn | ea | VNCa | pv | VNCv | VNCn | ea | VNCa | pv | VNCv | VNCn | ea | VNCa | pv | VNCv | ||
| Kruskal–Wallis Test | p | 0.456 | 0.005 | 0.481 | <0.001 | 0.109 | 0.485 | <0.001 | 0.038 | 0.004 | 0.009 | 0.580 | 0.030 | 0.673 | <0.001 | 0.152 |
| p b | 1 | 0.072 | 1 | <0.001 | 1 | 1 | <0.001 | 0.565 | 0.055 | 0.134 | 1 | 0.455 | 1 | <0.001 | 1 | |
| Liver parenchyma | Spleen parenchyma | |||||||||||||||
| VNCn | ea | VNCa | pv | VNCv | VNCn | ea | VNCa | pv | VNCv | |||||||
| Kruskal–Wallis Test | p | 0.600 | 0.342 | 0.882 | <0.001 | 0.572 | 0.737 | <0.001 | 0.499 | <0.001 | 0.277 | |||||
| p b | 1 | 1 | 1 | <0.001 | 1 | 1 | 0.003 | 1 | <0.001 | 1 | ||||||
| Erector spinae muscle | Psoas muscle | |||||||||||||||
| VNCn | ea | VNCa | pv | VNCv | VNCn | ea | VNCa | pv | VNCv | |||||||
| Kruskal–Wallis Test | p | 0.137 | 0.826 | 0.590 | 0.003 | 0.981 | 0.841 | 0.345 | 0.378 | <0.001 | 0.306 | |||||
| p b | 1 | 1 | 1 | 0.040 | 1 | 1 | 1 | 1 | 0.004 | 1 | ||||||
| Subcutaneous adipose tissue | Vertebral body (L1) | Air | ||||||||||||||
| VNCn | ea | VNCa | pv | VNCv | VNCn | ea | VNCa | pv | VNCv | VNCn | ea | VNCa | pv | VNCv | ||
| Kruskal–Wallis Test | p | <0.001 | 0.245 | <0.001 | 0.263 | <0.001 | <0.001 | 0.244 | <0.001 | 0.042 | <0.001 | a | a | a | a | a |
| p b | <0.001 | 1 | 0.002 | 1 | 0.006 | 0.006 | 1 | 0.006 | 0.623 | <0.001 | a | a | a | a | a | |
| TNC | VNCn | ea | VNCa | pv | VNCv | |
|---|---|---|---|---|---|---|
| n | 17 | 17 | 17 | 17 | 17 | 17 |
| Portal vein (mean (SD)) | 19.29 (7.90) | 15.65 (4.92) | 27.18 (8.46) | 15.06 (2.97) | 28.76 (10.93) | 14.82 (2.88) |
| Descending aorta (mean (SD)) | 15.82 (2.48) | 11.94 (2.19) | 24.35 (4.69) | 15.00 (3.14) | 22.76 (3.78) | 14.12 (2.83) |
| IVC (mean (SD)) | 19.53 (9.09) | 15.65 (7.37) | 24.38 (5.67) | 15.18 (3.78) | 24.88 (5.02) | 13.65 (3.20) |
| Liver parenchyma (mean (SD)) | 13.65 (2.94) | 10.41 (2.18) | 17.76 (3.67) | 11.94 (2.59) | 18.88 (3.53) | 11.18 (2.67) |
| Spleen parenchyma (mean (SD)) | 13.47 (3.08) | 10.65 (2.69) | 23.41 (6.13) | 13.47 (4.36) | 20.29 (4.40) | 12.12 (3.26) |
| Erector spinae muscle (mean (SD)) | 17.71 (5.21) | 13.18 (4.98) | 22.35 (6.41) | 14.18 (4.07) | 21.94 (5.23) | 13.94 (3.33) |
| Psoas muscle (mean (SD)) | 16.12 (2.71) | 13.06 (2.73) | 20.88 (3.54) | 13.31 (2.63) | 22.12 (4.36) | 14.06 (3.01) |
| Subcutaneous adipose tissue (mean (SD)) | 15.94 (6.68) | 12.53 (4.09) | 16.76 (5.14) | 12.53 (2.92) | 16.94 (4.15) | 12.35 (2.03) |
| Vertebral body (L1), (mean (SD)) | 24.29 (6.31) | 15.12 (4.18) | 33.29 (12.89) | 18.35 (6.31) | 31.65 (10.03) | 16.24 (4.91) |
| Air (mean (SD)) | 8.82 (3.59) | 7.12 (2.62) | 12.71 (4.54) | 8.76 (3.73) | 13.12 (5.19) | 8.41 (2.96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haag, F.; Emmrich, S.S.; Hertel, A.; Rink, J.S.; Nörenberg, D.; Schoenberg, S.O.; Froelich, M.F. Virtual Non-Contrast versus True Native in Photon-Counting CT: Stability of Density of Upper Abdominal Organs and Vessels. Diagnostics 2024, 14, 1130. https://doi.org/10.3390/diagnostics14111130
Haag F, Emmrich SS, Hertel A, Rink JS, Nörenberg D, Schoenberg SO, Froelich MF. Virtual Non-Contrast versus True Native in Photon-Counting CT: Stability of Density of Upper Abdominal Organs and Vessels. Diagnostics. 2024; 14(11):1130. https://doi.org/10.3390/diagnostics14111130
Chicago/Turabian StyleHaag, Florian, Shanice S. Emmrich, Alexander Hertel, Johann S. Rink, Dominik Nörenberg, Stefan O. Schoenberg, and Matthias F. Froelich. 2024. "Virtual Non-Contrast versus True Native in Photon-Counting CT: Stability of Density of Upper Abdominal Organs and Vessels" Diagnostics 14, no. 11: 1130. https://doi.org/10.3390/diagnostics14111130





