Autoimmune Diseases and Plasma Cells Dyscrasias: Pathogenetic, Molecular and Prognostic Correlations
Abstract
:1. Introduction
2. Physiopathology of Autoimmune Diseases and Multiple Myeloma
2.1. Hematological Conditions
2.2. Rheumatological Conditions
3. A Closer Look: Molecular Aspects
3.1. IL-6
3.2. Differentiation of Plasma Cells
3.3. APRIL: A Proliferation Inducing Ligand
3.4. The Role of Innate Lymphoid Cells in ADs and MM
3.5. The Role of Multi-Omics Analysis in MGUS and ADs
4. Impact of Autoimmune Disease on Multiple Myeloma Prognosis
5. Psoriasis, Multiple Myeloma and Impaired Immune Tolerance
Regulatory B Cells
6. Role of Multiple Myeloma Therapy in Autoimmune Diseases
6.1. Daratumumab
6.2. Bortezomib
6.3. Lenalidomide
6.4. Effect of Transplantation for Multiple Myeloma on Autoimmune Disease
7. Conclusions and Future Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caserta, S.; Innao, V.; Musolino, C.; Allegra, A. Immune checkpoint inhibitors in multiple myeloma: A review of the literature. Pathol. Res. Pract. 2020, 216, 153114. [Google Scholar] [CrossRef] [PubMed]
- Pydi, V.R.; Bala, S.C.; Kuruva, S.P.; Chennamaneni, R.; Konatam, M.L.; Gundeti, S. Multiple Myeloma in Young Adults: A Single Centre Real World Experience. Indian. J. Hematol. Blood Transfus. 2021, 37, 679–683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pál, I.; Illés, Á.; Váróczy, L. Multiple Myeloma of the Young—A Single Center Experience Highlights Future Directions. Pathol. Oncol. Res. 2020, 26, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Merlini, G.; Bridoux, F.; Leung, N.; Mikhael, J.; Harrison, S.J.; Kastritis, E.; Garderet, L.; Gozzetti, A.; van de Donk, N.W.C.J.; et al. Management of multiple myeloma-related renal impairment: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2023, 24, e293–e311. [Google Scholar] [CrossRef] [PubMed]
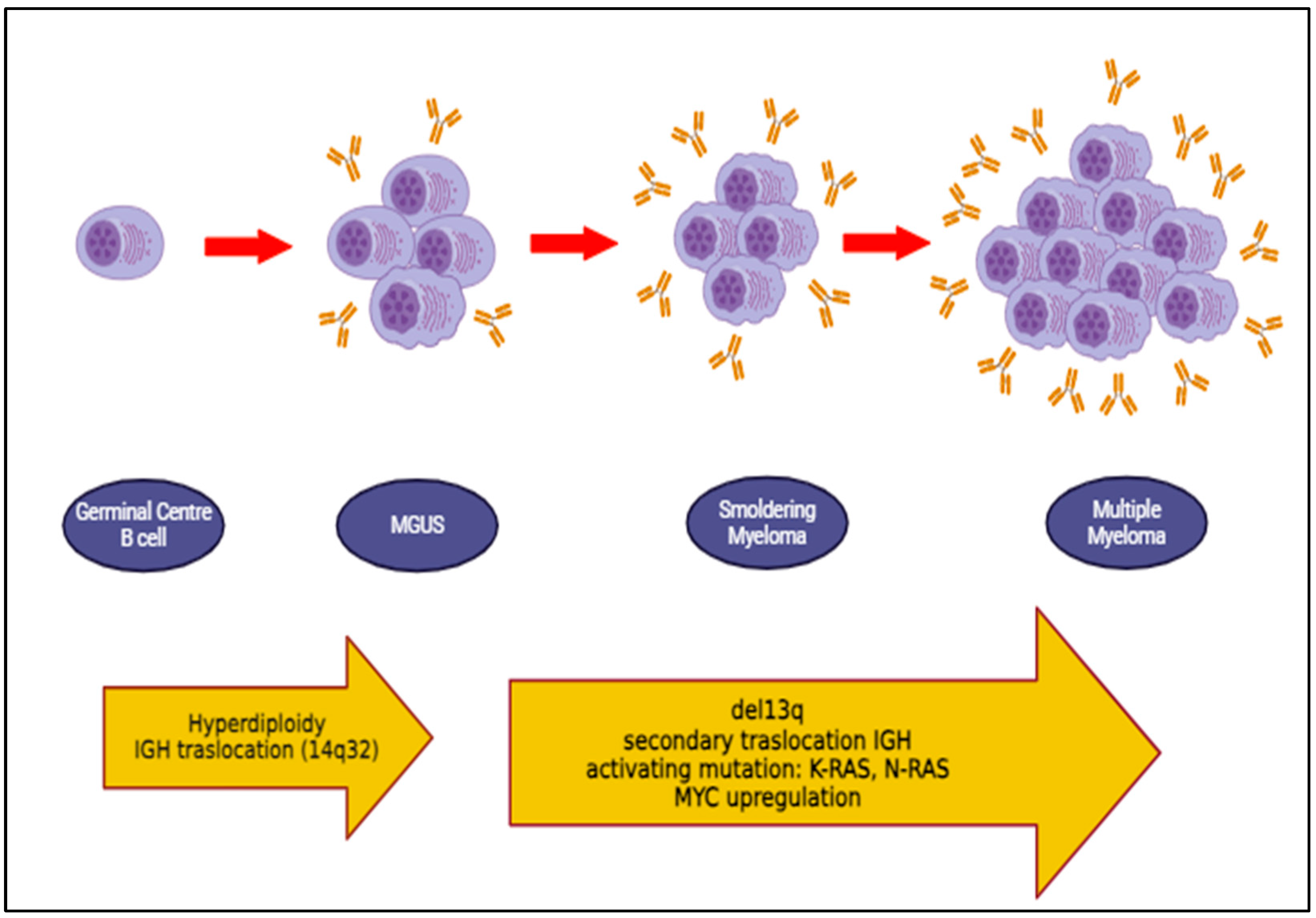
- van Nieuwenhuijzen, N.; Spaan, I.; Raymakers, R.; Peperzak, V. From MGUS to Multiple Myeloma, a Paradigm for Clonal Evolution of Premalignant Cells. Cancer Res. 2018, 78, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Thornton, J.; Sunderland, K.; Pham, K.; DeStefano, C. Multiple Myeloma in Adolescent and Young Adults: An ASCO CancerLinQ and SEER Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 23, e335–e340. [Google Scholar] [CrossRef] [PubMed]
- Brigle, K.; Rogers, B. Pathobiology and Diagnosis of Multiple Myeloma. Semin. Oncol. Nurs. 2017, 33, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Yang, H.S.; Jeon, Y.L.; You, E.; Lee, H.J.; Yoon, H.J.; Park, T.S. A case series of autoimmune diseases accompanied by incidentally diagnosed monoclonal gammopathy: Is there a link between the two diseases? Int. J. Rheum. Dis. 2014, 17, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Durie, B.G.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kröger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozet, A.; Güran, S.; Beksac, M. Familial multiple myeloma associated with disorders of chronic inflammation: First report from Turkey. Clin. Lymphoma Myeloma 2008, 8, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Bladé, J.; Rosiñol, L. Renal, hematologic and infectious complications in multiple myeloma. Best Pract. Res. Clin. Haematol. 2005, 18, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Cavo, M.; Offidani, M.; Scaglione, F.; Corso, A.; Di Raimondo, F.; Musto, P.; Petrucci, M.T.; Barosi, G. Management of infectious complications in multiple myeloma patients: Expert panel consensus-based recommendations. Blood Rev. 2019, 34, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Pruchniewski, Ł.; Kwiatek, M.; Gil, L. Powikłaniainfekcyjne w przebieguszpiczakaplazmocytowego [Infectious complications in a course of multiple myeloma]. Pol. Merkur. Lek. 2018, 45, 251–255. [Google Scholar] [PubMed]
- Khalesi, N.; Korani, S.; Korani, M.; Johnston, T.P.; Sahebkar, A. Bortezomib: A proteasome inhibitor for the treatment of autoimmune diseases. Inflammopharmacology 2021, 29, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
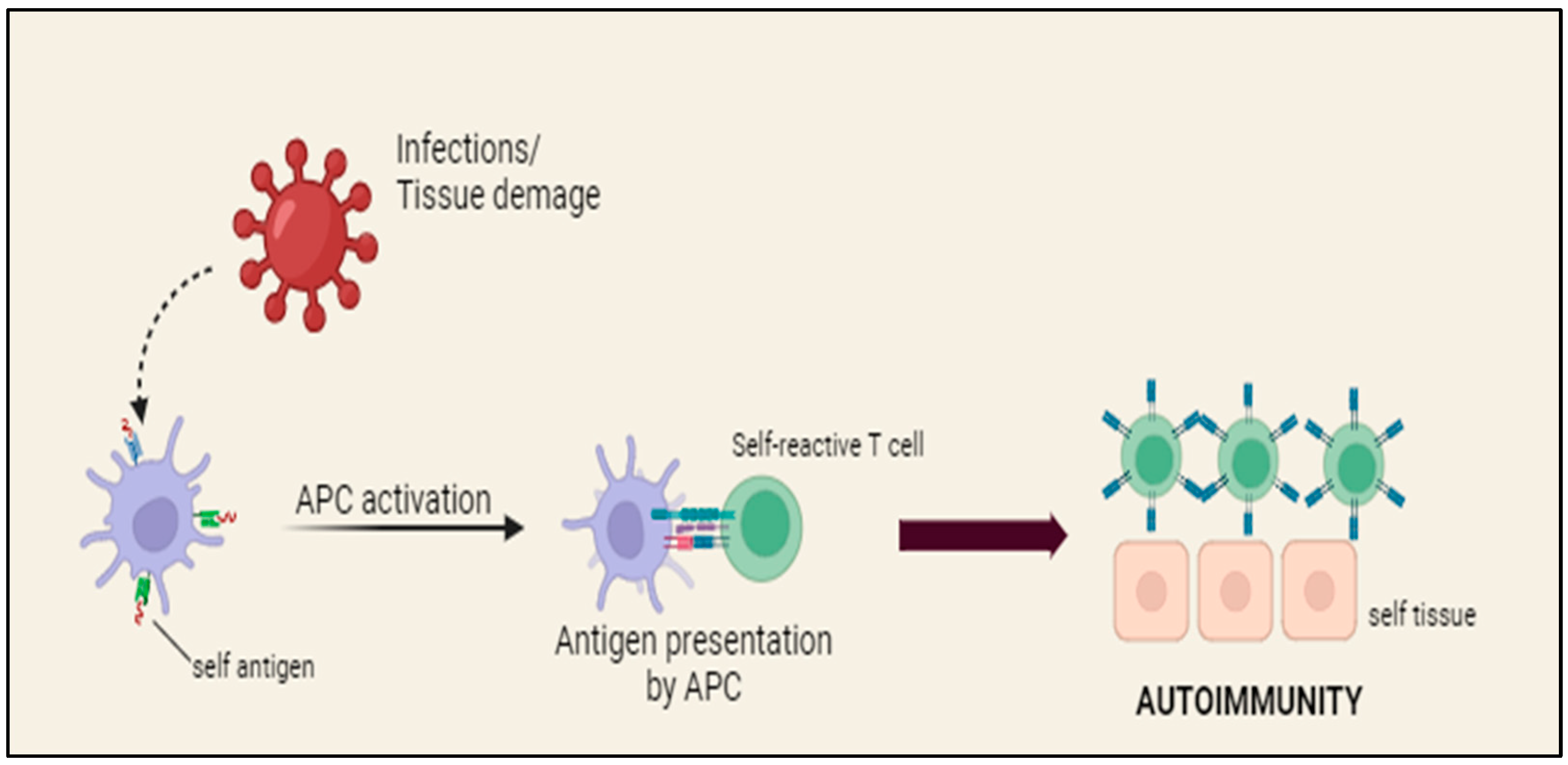
- Zhernakova, A.; van Diemen, C.C.; Wijmenga, C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat. Rev. Genet. 2009, 10, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Goldin, L.R.; Landgren, O. Autoimmunity and lymphomagenesis. Int. J. Cancer 2009, 124, 1497–1502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimanovsky, A.; Alvarez Argote, J.; Murali, S.; Dasanu, C.A. Autoimmune manifestations in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. BBA Clin. 2016, 6, 12–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerard, E.J.; Tuchman, S.A. Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma in Older Adults. Clin. Geriatr. Med. 2016, 32, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutta, A.K.; Fink, J.L.; Grady, J.P.; Morgan, G.J.; Mullighan, C.G.; To, L.B.; Hewett, D.R.; Zannettino, A.C.W. Subclonal evolution in disease progression from MGUS/SMM to multiple myeloma is characterised by clonal stability. Leukemia 2019, 33, 457–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hemminki, K.; Försti, A.; Sundquist, K.; Sundquist, J.; Li, X. Familial associations of lymphoma and myeloma with autoimmune diseases. Blood Cancer J. 2017, 7, e515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hemminki, K.; Försti, A.; Sundquist, K.; Sundquist, J.; Li, X. Familial associations of monoclonal gammopathy of unknown significance with autoimmune diseases. Leukemia 2016, 30, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- McShane, C.M.; Murray, L.J.; Landgren, O.; O’Rorke, M.A.; Korde, N.; Kunzmann, A.T.; Ismail, M.R.; Anderson, L.A. Prior autoimmune disease and risk of monoclonal gammopathy of undetermined significance and multiple myeloma: A systematic review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Kyle, R.A.; Therneau, T.M.; Foreman, B.J.; Larson, D.R.; Colby, C.L.; Phelps, T.K.; Dispenzieri, A.; Kumar, S.K.; Katzmann, J.A.; et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood 2009, 114, 785–790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grass, S.; Preuss, K.D.; Wikowicz, A.; Terpos, E.; Ziepert, M.; Nikolaus, D.; Yang, Y.; Fadle, N.; Regitz, E.; Dimopoulos, M.A.; et al. Hyperphosphorylated paratarg-7: A new molecularly defined risk factor for monoclonal gammopathy of undetermined significance of the IgM type and Waldenstrom macroglobulinemia. Blood 2011, 117, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Grass, S.; Preuss, K.D.; Pfreundschuh, M. Autosomal-dominant inheritance of hyperphosphorylated paratarg-7. Lancet Oncol. 2010, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Grass, S.; Preuss, K.D.; Ahlgrimm, M.; Fadle, N.; Regitz, E.; Pfoehler, C.; Murawski, N.; Pfreundschuh, M. Association of a dominantly inherited hyperphosphorylated paraprotein target with sporadic and familial multiple myeloma and monoclonal gammopathy of undetermined significance: A case-control study. Lancet Oncol. 2009, 10, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.R.; Bhatt, V.R.; Tandra, P.; Krishnamurthy, J.; Yuan, J.; Greiner, T.C.; Akhtari, M. Autoimmune neutropenia in multiple myeloma and the role of clonal T-cell expansion: Evidence of cross-talk between B-cell and T-cell lineages? Clin. Lymphoma Myeloma Leuk. 2014, 14, e19–e23. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Singh, A.; Kumar, P. Prevalence of autoimmune hemolytic anemia in multiple myeloma: A prospective study. Asia Pac. J. Clin. Oncol. 2016, 12, e319–e322. [Google Scholar] [CrossRef] [PubMed]
- Naithani, R.; Dayal, N. Autoimmune Hemolytic Anemia as Presenting Manifestation of Multiple Myeloma. Indian. J. Hematol. Blood Transfus. 2020, 36, 578–579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacca, R.L.; Silva, J.B.C.B.D.; Souza, K.C.E.; Carbinatto, R.B. Autoimmune hemolytic anemia and hyperglobulinemia leading to the diagnosis of multiple myeloma. Rev. Bras. Hematol. Hemoter. 2017, 39, 357–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barcellini, W.; Giannotta, J.A.; Fattizzo, B. Autoimmune Complications in HematologicNeoplasms. Cancers 2021, 13, 1532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forehand, W., III; Ajebo, G.; Toscano, M.; Jillella, A.; Dainer, P. Lenalidomide-Associated Immune Thrombocytopenia: A Case Report and Review of the Literature. Case Rep. Hematol. 2020, 2020, 8825618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alliot, C.; Barrios, M.; Tabuteau, S.; Desablens, B. Autoimmune cytopenias associated with malignancies and successfully treated with intravenous immune globulins: About two cases. Therapie 2000, 55, 371–374. [Google Scholar] [PubMed]
- Jalowiec, K.A.; Andres, M.; Taleghani, B.M.; Musa, A.; Dickenmann, M.; Angelillo-Scherrer, A.; Rovó, A.; Kremer Hovinga, J.A. Acquired hemophilia A and plasma cell neoplasms: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franchini, M.; Vaglio, S.; Marano, G.; Mengoli, C.; Gentili, S.; Pupella, S.; Liumbruno, G.M. Acquired hemophilia A: A review of recent data and new therapeutic options. Hematology 2017, 22, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Innao, V.; Allegra, A.; Morreale, R.; Russo, S.; Musolino, C. Disappearance of Acquired Hemophilia A after Complete Remission in a Multiple Myeloma Patient. Turk. J. Haematol. 2017, 34, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Siragusa, S.; Mancuso, S.; Kessler, C.M. Acquired haemophilia in cancer: A systematic and critical literature review. Haemophilia 2018, 24, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Afeltra, A.; Amoroso, A.; Garzia, P.; Addessi, M.A.; Pulsoni, A.; Bonomo, L. Systemic lupus erythematosus and multiple myeloma: A rare association. Semin. Arthritis Rheum. 1997, 26, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Wang, K.; Xu, S. Systemic lupus erythematosus associated with multiple myeloma: Two case reports and a literature review. Immun. Inflamm. Dis. 2023, 11, e755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Wang, Y.; Wang, Y.; Bai, Y.; Gu, D. Association Between Systemic Lupus Erythematosus and Cancer Morbidity and Mortality: Findings From Cohort Studies. Front. Oncol. 2022, 12, 860794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Connor, B.P.; Gleeson, M.W.; Noelle, R.J.; Erickson, L.D. The rise and fall of long-lived humoral immunity: Terminal differentiation of plasma cells in health and disease. Immunol. Rev. 2003, 194, 61–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brito-Zerón, P.; Kostov, B.; Fraile, G.; Caravia-Durán, D.; Maure, B.; Rascón, F.J.; Zamora, M.; Casanovas, A.; Lopez-Dupla, M.; Ripoll, M.; et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J. Hematol. Oncol. 2017, 10, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terpos, E.; Angelopoulou, M.K.; Variami, E.; Meletis, J.C.; Vaiopoulos, G. Sjögren’s syndrome associated with multiple myeloma. Ann. Hematol. 2000, 79, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Saka, B.; Kalayoglu-Besisik, S.; Ozturk, G.B.; Dogan, O.; Erten, N. Primary biliary cirrhosis and IgG-kappa type multiple myeloma both respond well to vincristine, adriamycin and dexamethasone: Is there a pathogenic relationship? J. Formos. Med. Assoc. 2008, 107, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.; Cappell, M.S. Primary biliary cirrhosis: Pathophysiology, clinical presentation and therapy. World J. Hepatol. 2015, 7, 926–941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindor, K.D.; Gershwin, M.E.; Poupon, R.; Kaplan, M.; Bergasa, N.V.; Heathcote, E.J.; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology 2009, 50, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Endo, T.; Saitoh, H.; Katsuta, Y.; Aramaki, T.; Hayakawa, H. Primary biliary cirrhosis associated with multiple myeloma. Intern. Med. 1993, 32, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Kumagi, T.; Heathcote, E.J. Primary biliary cirrhosis. Orphanet J. Rare Dis. 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silberman, J.; Lonial, S. Review of peripheral neuropathy in plasma cell disorders. Hematol. Oncol. 2008, 26, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Simmons, Z.; Albers, J.W.; Bromberg, M.B.; Feldman, E.L. Long-term follow-up of patients with chronic inflammatory demyelinating polyradiculoneuropathy, without and with monoclonal gammopathy. Brain 1995, 118 Pt 2, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ramchandren, S.; Lewis, R.A. Monoclonal gammopathy and neuropathy. Curr. Opin. Neurol. 2009, 22, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Fasanya, A.A.; Loncharich, M.F.; Gandhi, V.; Rana, S.; Balaan, M. Multiple Myeloma Associated Chronic Inflammatory Demyelinating Polyradiculoneuropathy: The Importance of Continued Surveillance. Cureus 2016, 8, e899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osório, R.M.; Pina, S.; Salero, T.; Coelho, M.V.; Sousa, D.; Mendonça, C. Association of Multiple Myeloma and Giant Cell Arteritis—A Case Report. Eur. J. Case Rep. Intern. Med. 2020, 7, 001360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frøiland, M.; Thompson, K.M.; Thorpe, S.J.; Sahota, S.S.; Gedde-Dahl, T.; Bogen, B. A VH4-34+ myeloma protein with weak autoreactivity. Haematologica 2007, 92, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor. Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Planelles, L.; Medema, J.P.; Hahne, M.; Hardenberg, G. The expanding role of APRIL in cancer and immunity. Curr. Mol. Med. 2008, 8, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yanaba, K.; Umezawa, Y.; Yoshihara, Y.; Kikuchi, S.; Ishiuji, Y.; Saeki, H.; Nakagawa, H. IL-10-producing regulatory B cells are decreased in patients with psoriasis. J. Dermatol. Sci. 2016, 81, 93–100, Erratum in: J. Dermatol. Sci. 2017, 86, 79. [Google Scholar] [CrossRef] [PubMed]
- Jego, G.; Bataille, R.; Pellat-Deceunynck, C. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood 2001, 97, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.; Zhang, X.G.; Lu, Z.Y.; Bataille, R. Interleukin-6 in human multiple myeloma. Blood 1995, 85, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Gadó, K.; Domján, G.; Hegyesi, H.; Falus, A. Role of INTERLEUKIN-6 in the pathogenesis of multiple myeloma. Cell Biol. Int. 2000, 24, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, S.; Hibi, M.; Sugita, T.; Saito, M.; Murakami, M.; Matsusaka, T.; Matsuda, T.; Hirano, T.; Taga, T.; Kishimoto, T. Interleukin 6 (IL-6) and its receptor (IL-6R) in myeloma/plasmacytoma. Curr. Top. Microbiol. Immunol. 1990, 166, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Z.; Wang, X.; Wang, G.; Li, W. Association of IL-6 Promoter and Receptor Polymorphisms with Multiple Myeloma Risk: A Systematic Review and Meta-Analysis. Genet. Test. Mol. Biomark. 2016, 20, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Manz, R.A.; Löhning, M.; Cassese, G.; Thiel, A.; Radbruch, A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 1998, 10, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Suan, D.; Sundling, C.; Brink, R. Plasma cell and memory B cell differentiation from the germinal center. Curr. Opin. Immunol. 2017, 45, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kometani, K.; Kurosaki, T. Differentiation and maintenance of long-lived plasma cells. Curr. Opin. Immunol. 2015, 33, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Manz, R.A.; Arce, S.; Cassese, G.; Hauser, A.E.; Hiepe, F.; Radbruch, A. Humoral immunity and long-lived plasma cells. Curr. Opin. Immunol. 2002, 14, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.P.; Kim, C.H.; Butcher, E.C. Cytokine control of memory B cell homing machinery. J. Immunol. 2002, 169, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Cascalho, M.; Noelle, R.J. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 2002, 195, 737–745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Epstein, J.; Yaccoby, S. Consequences of interactions between the bone marrow stroma and myeloma. Hematol. J. 2003, 4, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Teoh, G.; Anderson, K.C. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol. Oncol. Clin. N. Am. 1997, 11, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Casciaro, M.; Barone, P.; Musolino, C.; Gangemi, S. Epigenetic Crosstalk between Malignant Plasma Cells and the Tumour Microenvironment in Multiple Myeloma. Cancers 2022, 14, 2597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mackay, F.; Schneider, P.; Rennert, P.; Browning, J. BAFF and APRIL: A tutorial on B cell survival. Annu. Rev. Immunol. 2003, 21, 231–264. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, R.; González, M.; Orfão, A.; Moro, M.J.; Hernández, J.M.; Borrego, D.; Carnero, M.; Casanova, F.; Bárez, A.; Jiménez, R.; et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br. J. Haematol. 1996, 93, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Ruiz-Cabello, F.; Garrido, F. IFN inducibility of major histocompatibility antigens in tumors. Adv. Cancer Res. 2008, 101, 249–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kini Bailur, J.; Mehta, S.; Zhang, L.; Neparidze, N.; Parker, T.; Bar, N.; Anderson, T.; Xu, M.L.; Dhodapkar, K.M.; Dhodapkar, M.V. Changes in bone marrow innate lymphoid cell subsets in monoclonal gammopathy: Target for IMiD therapy. Blood Adv. 2017, 1, 2343–2347, Erratum in: Blood Adv. 2018, 2, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szudy-Szczyrek, A.; Ahern, S.; Kozioł, M.; Majowicz, D.; Szczyrek, M.; Krawczyk, J.; Hus, M. Therapeutic Potential of Innate Lymphoid Cells for Multiple Myeloma Therapy. Cancers 2021, 13, 4806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, T.; Turner, J.E. Innate lymphoid cells in autoimmunity and chronic inflammatory diseases. Semin. Immunopathol. 2018, 40, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Villanova, F.; Flutter, B.; Tosi, I.; Grys, K.; Sreeneebus, H.; Perera, G.K.; Chapman, A.; Smith, C.H.; Di Meglio, P.; Nestle, F.O. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Investig. Dermatol. 2014, 134, 984–991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teunissen, M.B.M.; Munneke, J.M.; Bernink, J.H.; Spuls, P.I.; Res, P.C.M.; Te Velde, A.; Cheuk, S.; Brouwer, M.W.D.; Menting, S.P.; Eidsmo, L.; et al. Composition of innate lymphoid cell subsets in the human skin: Enrichment of NCR+ ILC3 in lesional skin and blood of psoriasis patients. J. Investig. Dermatol. 2014, 134, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tang, D.; Chen, D.; Zheng, F.; Huang, S.; Xu, Y.; Yu, H.; He, J.; Hong, X.; Yin, L.; et al. Advances in applying of multi-omics approaches in the research of systemic lupus erythematosus. Int. Rev. Immunol. 2020, 39, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K. Inflammatory cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 2011, 432595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, S.; Chen, Q.; Xu, D.; Xie, N.; Dai, Y. Clinical application of protein biomarkers in lupus erythematosus and lupus nephritis. Lupus 2018, 27, 1582–1590. [Google Scholar] [CrossRef]
- Ouyang, X.; Dai, Y.; Wen, J.L.; Wang, L.X. 1H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus. Lupus 2011, 20, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Trzupek, D.; Lee, M.; Hamey, F.; Wicker, L.S.; Todd, J.A.; Ferreira, R.C. Single-cell multi-omics analysis reveals IFN-driven alterations in T lymphocytes and natural killer cells in systemic lupus erythematosus. Wellcome Open Res. 2022, 6, 149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, B.; Yi, K.; Zhang, Y.; Pang, T.; Zhou, J.; He, J.; Lan, H.; Xian, H.; Li, R. Multi-omics analysis of multiple myeloma patients with differential response to first-line treatment. Clin. Exp. Med. 2023, 23, 3833–3846. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Gridley, G.; Check, D.; Landgren, O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood 2008, 111, 3388–3394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renier, G.; Renier, J.C.; Gardembas-Pain, M.; Chevailler, A.; Boasson, M.; Hurez, D. Ankylosing spondylitis and monoclonal gammopathies. Ann. Rheum. Dis. 1992, 51, 951–954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardalan, M.R.; Shoja, M.M. Multiple myeloma presented as acute interstitial nephritis and rheumatoid arthritis-like polyarthritis. Am. J. Hematol. 2007, 82, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Baldursdóttir, T.R.; Löve, Þ.J.; Gíslason, G.K.; Björkholm, M.; Mellqvist, U.H.; Lund, S.H.; Blimark, C.H.; Turesson, I.; Hultcrantz, M.; Landgren, O.; et al. Autoimmune disease is associated with a lower risk of progression in monoclonal gammopathy of undetermined significance. Eur. J. Haematol. 2021, 106, 380–388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eriksson, M. Rheumatoid arthritis as a risk factor for multiple myeloma: A case-control study. Eur. J. Cancer. 1993, 29, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Liu, X.; Försti, A.; Ji, J.; Sundquist, J.; Sundquist, K. Effect of autoimmune diseases on incidence and survival in subsequent multiple myeloma. J. Hematol. Oncol. 2012, 5, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ugur, M.C.; Gediz, F. Evaluation of patients diagnosed with psoriasis and multiple myeloma after autologous stem cell transplantation. Transfus. Apher. Sci. 2021, 60, 103137. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Purkot, J.; Karczmarczyk, A.; Chojnacki, M.; Zaleska, J.; Własiuk, P.; Grząśko, N.; Morawska, M.; Walter-Croneck, A.; Usnarska-Zubkiewicz, L.; et al. Differential Function of a Novel Population of the CD19+CD24hiCD38hi Bregs in Psoriasis and Multiple Myeloma. Cells 2021, 10, 411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Candando, K.M.; Lykken, J.M.; Tedder, T.F. B10 cell regulation of health and disease. Immunol. Rev. 2014, 259, 259–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bommarito, D.; Hall, C.; Taams, L.S.; Corrigall, V.M. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin. Exp. Immunol. 2017, 188, 455–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, X.; Zhang, L.; Wei, W. Regulatory B cells in inflammatory diseases and tumor. Int. Immunopharmacol. 2019, 67, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musolino, C.; Allegra, A.; Pioggia, G.; Gangemi, S. Immature myeloid-derived suppressor cells: A bridge between inflammation and cancer (Review). Oncol. Rep. 2017, 37, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tai, Y.T.; Ho, M.; Xing, L.; Chauhan, D.; Gang, A.; Qiu, L.; Anderson, K.C. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017, 7, e547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakasheva, T.A.; Waldron, T.J.; Eruslanov, E.; Kim, S.B.; Lee, J.S.; O’Brien, S.; Hicks, P.D.; Basu, D.; Singhal, S.; Malavasi, F.; et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015, 75, 4074–4085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Görgün, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Maguire, C.; Laubach, J.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013, 121, 2975–2987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouaziz, J.D.; Calbo, S.; Maho-Vaillant, M.; Saussine, A.; Bagot, M.; Bensussan, A.; Musette, P. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur. J. Immunol. 2010, 40, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013, 5, 173ra23. [Google Scholar] [CrossRef] [PubMed]
- Daien, C.I.; Gailhac, S.; Mura, T.; Audo, R.; Combe, B.; Hahne, M.; Morel, J. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol. 2014, 66, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Soekojo, C.Y.; Chng, W.J. Treatment horizon in multiple myeloma. Eur. J. Haematol. 2022, 109, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanchez, L.; Wang, Y.; Siegel, D.S.; Wang, M.L. Daratumumab: A first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J. Hematol. Oncol. 2016, 9, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- de Weers, M.; Tai, Y.T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Walsh, A.; Yin, X.; Wechalekar, M.D.; Smith, M.D.; Proudman, S.M.; Veale, D.J.; Fearon, U.; Pitzalis, C.; Humby, F.; et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nocturne, G.; Marmontel, O.; di Filippo, M.; Chretien, P.; Krzysiek, R.; Lifermann, F.; Rahal, N.; Belkhir, R.; Moulin, P.; Mariette, X. Efficacy of daratumumab in refractory primary Sjögren disease. RMD Open 2023, 9, e003464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roccatello, D.; Fenoglio, R.; Caniggia, I.; Kamgaing, J.; Naretto, C.; Cecchi, I.; Rubini, E.; Rossi, D.; De Simone, E.; Del Vecchio, G.; et al. Daratumumabmonotherapy for refractory lupus nephritis. Nat. Med. 2023, 29, 2041–2047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bengtsson, A.A.; Rönnblom, L. Role of interferons in SLE. Best Pract. Res. Clin. Rheumatol. 2017, 31, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, L.; Burns, M.; Durek, P.; Heinz, G.A.; Heinrich, F.; Garantziotis, P.; Enghard, P.; Richter, U.; Biesen, R.; Schneider, U.; et al. Targeting CD38 with Daratumumab in Refractory Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 383, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Tung, D.; Cheung, P.H.; Kaur, P.; Foreman, O.; Kavirayani, A.; Hain, H.S.; Saha, S. Anti-inflammatory and immunomodulatory effects of bortezomib in various in vivo models. Pharmacology 2011, 88, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Škrott, Z.; Cvek, B. Linking the activity of bortezomib in multiple myeloma and autoimmune diseases. Crit. Rev. Oncol. Hematol. 2014, 92, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Citrin, R.; Foster, J.B.; Teachey, D.T. The role of proteasome inhibition in the treatment of malignant and non-malignant hematologic disorders. Expert Rev. Hematol. 2016, 9, 873–889. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montefusco, V.; Galli, M.; Spina, F.; Stefanoni, P.; Mussetti, A.; Perrone, G.; De Philippis, C.; Dalto, S.; Maura, F.; Bonini, C.; et al. Autoimmune diseases during treatment with immunomodulatory drugs in multiple myeloma: Selective occurrence after lenalidomide. Leuk. Lymphoma 2014, 55, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Darabi, K.; Kantamnei, S.; Wiernik, P.H. Lenalidomide-induced warm autoimmune hemolytic anemia. J. Clin. Oncol. 2006, 24, e59. [Google Scholar] [CrossRef] [PubMed]
- Mioso, G.; Gnesotto, L.; Russo, I.; Piaserico, S.; Alaibac, M. Exacerbation of psoriasis induced by lenalidomide in a patient with multiple myeloma. J. Dermatol. Treat. 2023, 34, 2182619. [Google Scholar] [CrossRef] [PubMed]
- Braiteh, F.; Hymes, S.R.; Giralt, S.A.; Jones, R. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J. Clin. Oncol. 2008, 26, 4511–4513. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, I.; Okamoto, S.; Kakimoto, T.; Chen, C.K.; Mori, T.; Yokoyama, K.; Hattori, Y.; Ikeda, Y. Recurrence of autoimmune disease after autologous peripheral blood stem cell transplantation for multiple myeloma. Int. J. Hematol. 2006, 84, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.; Bakalov, V.; Shaikh, S.; Khattab, A.; Sadashiv, S. Coincident remission of ankylosing spondylitis after autologous stem cell transplantation for multiple myeloma. J. Oncol. Pharm. Pract. 2021, 27, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Stannard, K.; Chen, J.; Krumeich, S.; Miles, K.; Nakamura, K.; Smith, J.; Yu, Y.; Ng, S.; Harjunpää, H.; et al. Systemic administration of IL-33 induces a population of circulating KLRG1hi type 2 innate lymphoid cells and inhibits type 1 innate immunity against multiple myeloma. Immunol. Cell Biol. 2021, 99, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Profita, M.; Alonci, A.; Saitta, S.; Russo, S.; Bonanno, A.; Innao, V.; Gangemi, S. Reduced IL-33 plasma levels in multiple myeloma patients are associated with more advanced stage of disease. Br. J. Haematol. 2013, 160, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Schirrmacher, V. Bispecific antibodies and trispecificimmunocytokines for targeting the immune system against cancer: Preparing for the future. BioDrugs 2013, 27, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Husain, B.; Ellerman, D. Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies. BioDrugs 2018, 32, 441–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Q. Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date. BioDrugs 2020, 34, 111–119. [Google Scholar] [CrossRef] [PubMed]
| Cytokines and Other Factors | Role in ADs | Role in MM |
|---|---|---|
| Interleukin 6 | Activation of B cells → abnormal growth → autoantibodies production | Plasmablasts growth, maturations of plasmablasts in pathological PCs |
| Interleukins 13 and 4 | Anti-apoptotic effect, IgE production | B cells proliferation |
| APRIL and BAFF | Chronic inflammation (activation of keratinocytes in Ps and joint erosion in RA) | Survival of MM cells Inhibition of apoptosis Ig class-switch recombination |
| Interleukin 10 | Immunosuppression | Progression of MM B cells differentiation in MM PCs |
| Interleukin 35 | B- and Tregs expansion | B cells differentiation in MM PCs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, L.; Cacciola, R.; Barone, P.; Vecchio, V.; Nasso, M.E.; Alvaro, M.E.; Gangemi, S.; Cacciola, E.; Allegra, A. Autoimmune Diseases and Plasma Cells Dyscrasias: Pathogenetic, Molecular and Prognostic Correlations. Diagnostics 2024, 14, 1135. https://doi.org/10.3390/diagnostics14111135
Giordano L, Cacciola R, Barone P, Vecchio V, Nasso ME, Alvaro ME, Gangemi S, Cacciola E, Allegra A. Autoimmune Diseases and Plasma Cells Dyscrasias: Pathogenetic, Molecular and Prognostic Correlations. Diagnostics. 2024; 14(11):1135. https://doi.org/10.3390/diagnostics14111135
Chicago/Turabian StyleGiordano, Laura, Rossella Cacciola, Paola Barone, Veronica Vecchio, Maria Elisa Nasso, Maria Eugenia Alvaro, Sebastiano Gangemi, Emma Cacciola, and Alessandro Allegra. 2024. "Autoimmune Diseases and Plasma Cells Dyscrasias: Pathogenetic, Molecular and Prognostic Correlations" Diagnostics 14, no. 11: 1135. https://doi.org/10.3390/diagnostics14111135





