Frozen Section of Placental Membranes and Umbilical Cord: A Valid Diagnostic Tool for Early-Onset Neonatal Sepsis Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Population Inclusion Criteria
2.2. Data Sources
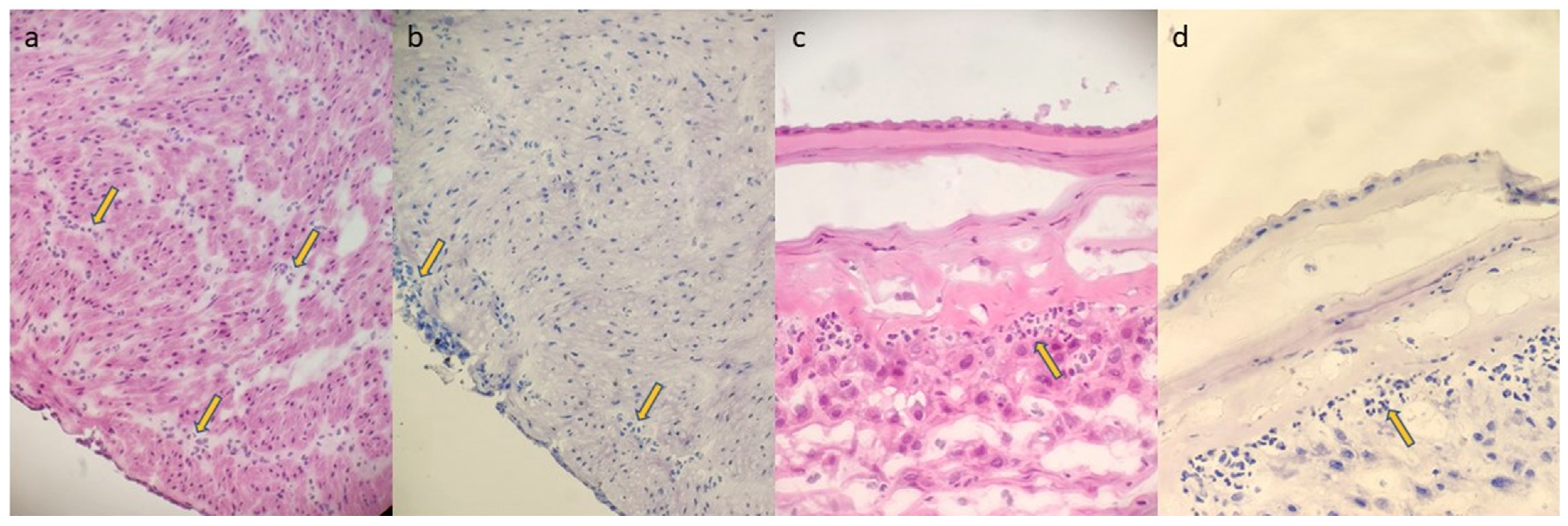
2.3. FSMU Technique
2.4. Data Analysis
3. Results
3.1. Patient Demographics
3.2. Indications for FSMU
3.3. FSMU and FPR Concordance
3.4. Clinical Outcomes and Antibiotic Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EONS | Early-onset neonatal sepsis |
| FPR | Final pathology report |
| FSMU | Frozen section examination of placental membranes and umbilical cord |
| PPROMs | Premature rupture of the amniotic membranes |
| PROM | Prolonged rupture of the membranes |
References
- Van Herk, W.; Stocker, M.; van Rossum, A.M. Recognising early onset neonatal sepsis: An essential step in appropriate anti-microbial use. J. Infect. 2016, 72, S77–S82. [Google Scholar] [CrossRef]
- MSD Manuals. Neonatal Sepsis. Available online: https://www.merckmanuals.com/en-pr/professional/pediatrics/infections-in-neonates/neonatal-sepsis (accessed on 15 February 2024).
- National Institutes of Health. Neonatal Sepsis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531478 (accessed on 15 February 2024).
- Araújo, B.C.; Guimarães, H. Risk factors for neonatal sepsis: An overview. J. Pediatr. Neonatal Individ. Med. 2020, 9, e090206. [Google Scholar]
- Vatne, A.; Klingenberg, C.; Rettedal, S.; Øymar, K. Early-Onset Sepsis in Neonates—A Population-Based Study in South-West Norway From 1996 to 2018. Front. Pediatr. 2021, 9, 634798. [Google Scholar] [CrossRef]
- Shah, S.S. Early-onset neonatal sepsis: Diagnosis, management, and controversies. Indian J. Pediatr. 2016, 83, 1183–1193. [Google Scholar]
- Ortgies, T.; Rullmann, M.; Ziegelhöfer, D.; Bläser, A.; Thome, U.H. The role of early-onset-sepsis in the neurodevelopment of very low birth weight infants. BMC Pediatr. 2021, 21, 289. [Google Scholar] [CrossRef]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Walsh, W.K.; Randolph, A.G.; McCrindle, B.W. Antimicrobial Resistance in Neonates. Pediatrics 2008, 122, e418–e428. [Google Scholar]
- Boureka, E.; Krasias, D.; Tsakiridis, I.; Karathanasi, A.M.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Prevention of Early-Onset Neonatal Group B Streptococcal Disease: A Comprehensive Review of Major Guidelines. Obstet. Gynecol. Surv. 2023, 78, 766–774. [Google Scholar] [CrossRef]
- Shah, P.S.; Ohlsson, A.; Kimberlin, C.L. Incomplete Blood Count and Blood Culture Findings in Infants with Early-Onset Sepsis. Pediatrics 2015, 135, e371–e377. [Google Scholar]
- Zaidi, A.K.; Thayyil, S. Diagnosis of neonatal sepsis: A continuing challenge. Front. Pediatr. 2017, 5, 291. [Google Scholar]
- Celik, I.H.; Hanna, M.; Canpolat, F.E.; Mohan, P. Diagnosis of neonatal sepsis: The past, present and future. Pediatr. Res. 2022, 91, 337–350. [Google Scholar] [CrossRef]
- Rutay, K.; Mitra, R.; Bhagat, S.; Bhatt, K.; Underwood, M.A. Gut Microbiome and Its Role in Neonatal Sepsis. Front. Pediatr. 2020, 8, 591418. [Google Scholar]
- Fjalstad, J.W.; Esaiassen, E.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: A systematic review. J. Antimicrob. Chemother. 2018, 73, 569–580. [Google Scholar] [CrossRef]
- Ni, J.; Friedman, H.; Boyd, B.C.; McGurn, A.; Babinski, P.; Markossian, T.; Dugas, L.R. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr. 2019, 19, 225. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Lei, S.; Wen, L.; He, Q.; Sun, Z. Neonatal Antibiotic Exposure and Childhood Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151055. [Google Scholar] [CrossRef]
- Rosai, J. Rosai and Ackerman’s Surgical Pathology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bauer, C.R.; Stromberg, B.E.; Agarwal, R. Frozen Section Examination in Neonatal Medicine: A Review of the Literature. Arch. Pathol. Lab. Med. 2010, 134, 102–107. [Google Scholar]
- Kovács, K.; Kovács, Ő.Z.; Bajzát, D.; Imrei, M.; Nagy, R.; Németh, D.; Kói, T.; Szabó, M.; Fintha, A.; Hegyi, P.; et al. The histologic fetal inflammatory response and neonatal outcomes: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2024, 230, 493–511.e3. [Google Scholar] [CrossRef]
- Wong, Y.P.; Khong, T.Y. Changing Laboratory Practice for Early Detection of a Fetal Inflammatory Response: A Contemporary Approach. Diagnostics 2023, 13, 487. [Google Scholar] [CrossRef]
- Lukanović, D.; Batkoska, M.; Kavšek, G.; Druškovič, M. Clinical chorioamnionitis: Where do we stand now? Front. Med. 2023, 10, 1191254. [Google Scholar] [CrossRef]
- Hojsak, I.; Fabiano, V.; Pop, T.L.; Goulet, O.; Zuccotti, G.V.; Çokuğraş, F.C.; Pettoello-Mantovani, M.; Kolaček, S. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr. 2018, 107, 927–937. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Berardi, A.; Spada, C.; Ciccia, M.; Capretti, M.; Brusa, G.; Sandri, F.; Balestri, E.; Rocca, L.; Gambini, L.; Azzalli, M.; et al. Observation on the newborn at risk of early-onset sepsis: The approach of the Emilia-Romagna Region (Italy) [Osservazione nel neonato a rischio di sepsi precoce: L’Approccio della Regione Emilia-Romagna]. Med. Bambino 2019, 38, 370–376. (In Italian) [Google Scholar]
- Mahe, E.; Hamid, J.; Terry, J.; Jansen, J.W.; Bourgeois, J.; Arredondo-Marin, J. Frozen section of placental membranes and umbilical cord: An aid to early postpartum diagnosis of intra-amniotic infection. Am. J. Clin. Pathol. 2014, 142, 202–208. [Google Scholar] [CrossRef]

| Risk Factor | Description |
|---|---|
| Chorioamnionitis or peripartum maternal fever |
|
| Prolonged rupture of membranes (PROMs) | Rupture of membranes for ≥18 h associated with:
|
| Preterm premature rupture of membranes (PPROMs) | Rupture of membranes before 37 weeks of gestation, particularly unexplained cases |
| Positive vagino-rectal swab for fetal pathogens | Positive swab for Group B Streptococcus (GBS) or other pathogens with:
|
| Maternal genitourinary infection during pregnancy | Documented maternal genitourinary infection during pregnancy with:
|
| Co-presence of multiple risk factors | Two or more of the listed risk factors are present |
| Emerging infectious risk factors | Any other potential infectious risk factors identified on an individual basis by the neonatologist |
| Characteristic | Mean | Min–Max |
|---|---|---|
| Maternal age | 32.42 | 22–43 |
| Gestational age | 38.02 | 32–42 |
| Birth weight | 2.915 | 1440–4060 |
| Characteristic | Number | Percentage (%) |
| Gravida | ||
| G1 | 24 | 40.68 |
| G2 | 19 | 32.20 |
| >G3 | 16 | 27.12 |
| Para | ||
| P1 | 34 | 57.63 |
| P2 | 16 | 27.12 |
| >P3 | 9 | 15.25 |
| Sex of the Newborn | ||
| Female | 30 | 50.85 |
| Male | 29 | 49.15 |
| Apgar Score 1′ | ||
| 0–2 | 0 | 0.00 |
| 3–5 | 3 | 5.08 |
| 6–8 | 15 | 25.42 |
| 9–10 | 39 | 66.10 |
| N/A | 2 | 3.39 |
| Apgar Score 5′ | ||
| 0–2 | 0 | 0.00 |
| 3–5 | 0 | 0.00 |
| 6–8 | 9 | 15.25 |
| 9–10 | 48 | 81.36 |
| N/A | 2 | 3.39 |
| Absolute Indication | ||
| Premature rupture of membranes (PROMs) | 35 | 59.32 |
| Vaginal and/or rectal swabs positive for GBS | 12 | 20.34 |
| Vaginal and/or rectal swabs for GBS not performed | 6 | 10.17 |
| Maternal fever | 2 | 3.39 |
| Relative Indication | ||
| Tinted or malodorous amniotic fluid | 10 | 16.95 |
| Pre-eclampsia or gestational diabetes mellitus | 6 | 10.17 |
| FUNISITIS Frozen Sections | FUNISITIS FPR | |||
|---|---|---|---|---|
| NO | Stage 1 | Stage 2 | TOTAL | |
| NO | 54 | 0 | 0 | 54 (91.5%) |
| YES | 1 | 3 | 1 | 5 (8.5%) |
| TOTAL | 55 (93.2%) | 3 (5.1%) | 1 (1.7%) | 59 (100%) |
| SIGNIFICANCE LEVEL | p < 0.0001 | |||
| 95% CI | 0.64801 to 1 | |||
| CHORIONAMIONITIS STAGE Frozen Sections | CHORIONAMNONTIS STAGE FPR | |||
| NO | Stage 1 | Stage 2 | TOTAL | |
| NO | 38 | 5 | 0 | 43 (72.9%) |
| Stage 1 | 4 | 7 | 0 | 11 (18.6%) |
| Stage 2 | 0 | 1 | 4 | 5 (8.5%) |
| TOTAL | 42 (71.2%) | 13 (22%) | 4 (6.8%) | 59 (100%) |
| SIGNIFICANCE LEVEL | p < 0.0001 | |||
| 95% CI | 0.49696 to 0.88002 | |||
| CHORIONAMIONITIS GRADE Frozen Sections | CHORIONAMNONTIS GRADE FPR | |||
| NO | G1 | G2 | TOTAL | |
| NO | 37 | 4 | 0 | 41 |
| G1 | 5 | 9 | 1 | 15 |
| G2 | 0 | 2 | 1 | 3 |
| TOTAL | 42 (71.2%) | 15 (25.4%) | 2 (3.4%) | 59 (100%) |
| SIGNIFICANCE LEVEL | p < 0.0001 | |||
| 95% CI | 0.33121 to 0.77517 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrella, V.; Paudice, M.; Pittaluga, M.; Allodi, A.; Fulcheri, E.; Buffelli, F.; Barra, F.; Ferrero, S.; Arioni, C.; Vellone, V.G. Frozen Section of Placental Membranes and Umbilical Cord: A Valid Diagnostic Tool for Early-Onset Neonatal Sepsis Management. Diagnostics 2024, 14, 1157. https://doi.org/10.3390/diagnostics14111157
Parrella V, Paudice M, Pittaluga M, Allodi A, Fulcheri E, Buffelli F, Barra F, Ferrero S, Arioni C, Vellone VG. Frozen Section of Placental Membranes and Umbilical Cord: A Valid Diagnostic Tool for Early-Onset Neonatal Sepsis Management. Diagnostics. 2024; 14(11):1157. https://doi.org/10.3390/diagnostics14111157
Chicago/Turabian StyleParrella, Veronica, Michele Paudice, Michela Pittaluga, Alessandra Allodi, Ezio Fulcheri, Francesca Buffelli, Fabio Barra, Simone Ferrero, Cesare Arioni, and Valerio Gaetano Vellone. 2024. "Frozen Section of Placental Membranes and Umbilical Cord: A Valid Diagnostic Tool for Early-Onset Neonatal Sepsis Management" Diagnostics 14, no. 11: 1157. https://doi.org/10.3390/diagnostics14111157
APA StyleParrella, V., Paudice, M., Pittaluga, M., Allodi, A., Fulcheri, E., Buffelli, F., Barra, F., Ferrero, S., Arioni, C., & Vellone, V. G. (2024). Frozen Section of Placental Membranes and Umbilical Cord: A Valid Diagnostic Tool for Early-Onset Neonatal Sepsis Management. Diagnostics, 14(11), 1157. https://doi.org/10.3390/diagnostics14111157









