A Magnetic Resonance Spectroscopy Study on Polarity Subphenotypes in Bipolar Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Participants
2.3. Clinical Evaluation
2.4. MRI Data Acquisition
2.5. MRS Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. MRS in Cingulum (ACC, PCC) and Bilateral Hippocampus
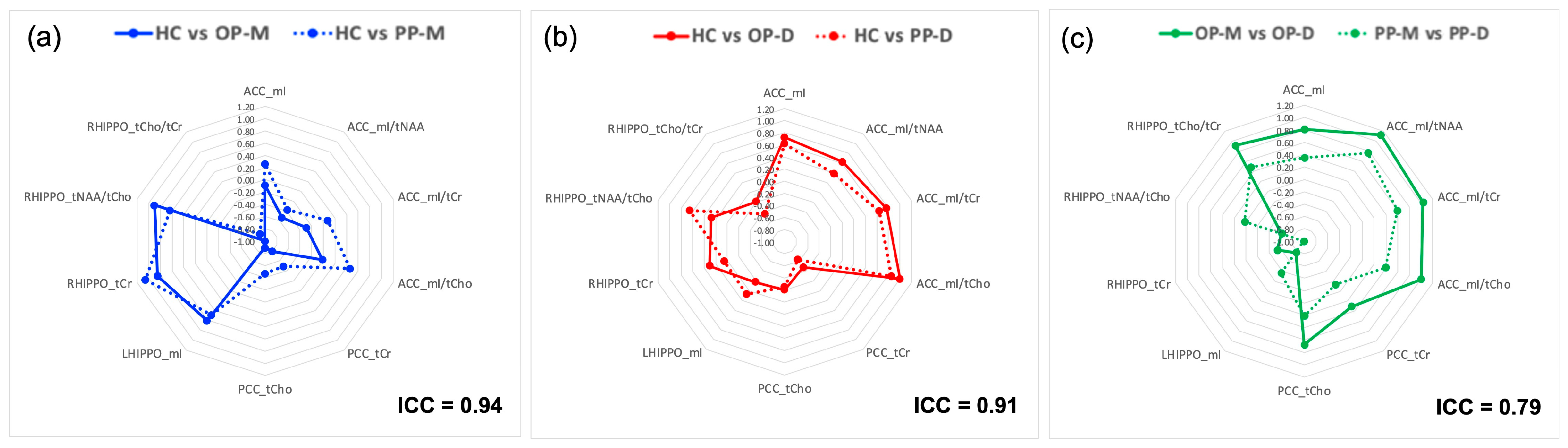
3.2.1. Reproducibility Study of MRS Voxel Placement
3.2.2. Onset Polarity (OP)
3.2.3. Predominant Polarity (PP)
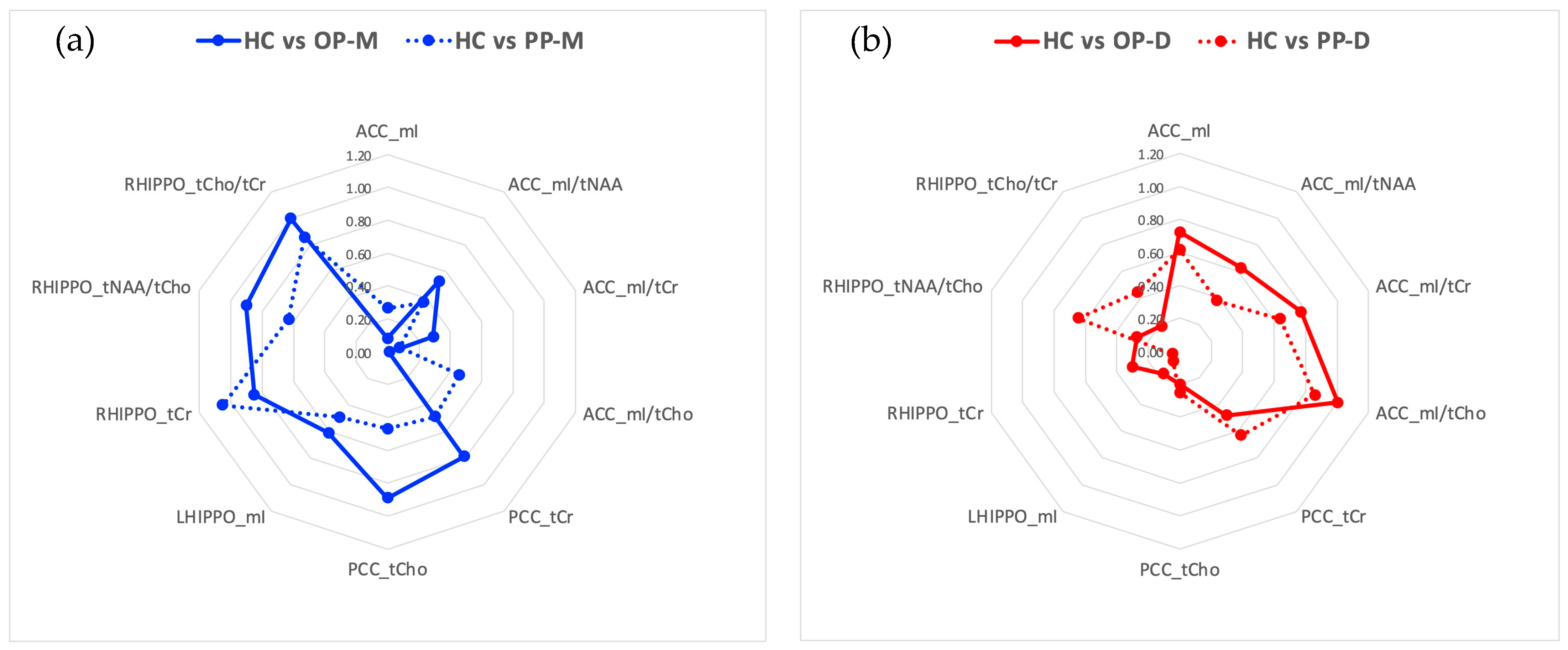
3.2.4. Follow-Up Analysis Based on Effect Sizes
- HC vs. OP-M: PCC tCho (d = −0.89), and RHIPPO tCr (d = 0.85), tNAA/tCho (d = 0.90), and tCho/tCr (d = −1.00).
- HC vs. OP-D: ACC mI/tCr (d = −1.00)
- OP-M vs. OP-D: ACC mI (d = 0.81), mI/tNAA (d = 1.12), mI/tCho (d = 1.04), and mI/tCr (d = 1.00), RHIPPO tCho/tCr (d = 0.91)
- HC vs. PP-M: RHIPPO tCr (d = 1.05) and tCho/tCr (d = −0.86)
- HC vs. PP-D: ACC mI/tCr (d = 0.86)
- HC vs. PP-U: PCC tCr (d = −0.80) and tCho (d = −0.88)
- PP-M vs. PP-D: RHIPPO tCr (d = −0.99)
- HC vs. OP-M: ACC mI/tNAA (d = −0.53), PCC tCr (d = −0.79), and LHIPPO (d = 0.61)
- HC vs. OP-D: ACC mI (d = 0.72), mI/tNAA (d = 0.63), mI/tCho (d = 0.77)
- OP-M vs. OP-D: PCC tCho (d = 0.68), LHIPPO mI (d = −0.77), RHIPPO tCr (d = −0.54) and tNAA/tCho (d = −0.61)
- HC vs. PP-M: RHIPPO tNAA/tCho (d = 0.63)
- HC vs. PP-D: ACC mI (d = 0.62) and mI/tCho (d = 0.64), PCC tCr (d = −0.63), and RHIPPO tNAA/tCho (d = 0.65)
- PP-M vs. PP-D: ACC mI/tNAA (d = 0.76) and mI/tCho (d = 0.59)
- PP-M vs. PP-U: ACC mI/tNAA (d = 0.54), and RHIPPO tCr (d = −0.56)
- PP-D vs. PP-U: ACC mI (d = −0.59), mI/tCho (d = −0.66), and mI/tCr (d = −0.76), and PCC tCho (d = −0.61).
4. Discussion
4.1. Metabolite Changes in Cingulate Cortex (ACC, PCC) and Hippocampus (HIPPO R and L)
4.2. Polarity-Related Regional Specificity of Metabolite Changes within the Emotion Regulation Network
4.3. Metabolite Changes as Consistent Markers of Onset and Predominant Polarity Subphenotypes
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bora, E.; Fornito, A.; Yücel, M.; Pantelis, C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol. Psychiatry 2010, 67, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Kupfer, D.J. Bipolar disorder diagnosis: Challenges and future directions. Lancet 2013, 381, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Alger, J.R. Quantitative Proton Magnetic Resonance Spectroscopy and Spectroscopic Imaging of the Brain: A Didactic Review. Top. Magn. Reson. Imaging 2010, 21, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dager, S.R.; Corrigan, N.M.; Richards, T.L.; Posse, S. Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Top. Magn. Reson. Imaging 2008, 19, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.P.; Law, M. Magnetic resonance spectroscopy of the brain: Review of metabolites and clinical applications. Clin. Radiol. 2009, 64, 12–21. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013. [Google Scholar]
- Prisco, M.D.; Oliva, V.; Fico, G.; Radua, J.; Grande, I.; Roberto, N.; Anmella, G.; Hidalgo-Mazzei, D.; Fornaro, M.; de Bartolomeis, A.; et al. Emotion dysregulation in bipolar disorder compared to other mental illnesses: A systematic review and meta-analysis. Psychol. Med. 2023, 53, 7484–7503. [Google Scholar] [CrossRef] [PubMed]
- Förster, K.; Maliske, L.Z.; Schurz, M.; Henneberg, P.M.; Dannlowski, U.; Kanske, P. How do bipolar disease states affect positive and negative emotion processing? Insights from a meta-analysis on the neural fingerprints of emotional processing. Bipolar Disord. 2023, 25, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Biazus, T.B.; Beraldi, G.H.; Tokeshi, L.; Rotenberg, L.d.S.; Dragioti, E.; Carvalho, A.F.; Solmi, M.; Lafer, B. All-cause and cause-specific mortality among people with bipolar disorder: A large-scale systematic review and meta-analysis. Mol. Psychiatry 2023, 28, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of role due to common physical and mental conditions: Results from the WHO World Mental Health surveys. Mol. Psychiatry 2011, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Primers 2018, 4, 18008. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Dodd, S.; Callaly, P.; Berk, L.; Fitzgerald, P.; de Castella, A.R.; Filia, S.; Filia, K.; Tahtalian, S.; Biffin, F.; et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J. Affect. Disord. 2007, 103, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.A.; Calabrese, J.R.; Weissman, M.M.; Reed, M.; Davies, M.A.; Frye, M.A.; Keck, P.E.; Lewis, L.; McElroy, S.L.; McNulty, J.P.; et al. Screening for bipolar disorder in the community. J. Clin. Psychiatry 2003, 64, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Stensland, M.D.; Schultz, J.F.; Frytak, J.R. Diagnosis of unipolar depression following initial identification of bipolar disorder: A common and costly misdiagnosis. J. Clin. Psychiatry 2008, 69, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M.; Denicoff, K.D.; Leverich, G.S.; Altshuler, L.L.; Frye, M.A.; Suppes, T.M.; Rush, A.J.; Keck, P.E.; McElroy, S.L.; Luckenbaugh, D.A.; et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J. Clin. Psychiatry 2003, 64, 680–690, quiz 738–739. [Google Scholar] [CrossRef] [PubMed]
- Ketter, T.A.; Houston, J.P.; Adams, D.H.; Risser, R.C.; Meyers, A.L.; Williamson, D.J.; Tohen, M. Differential efficacy of olanzapine and lithium in preventing manic or mixed recurrence in patients with bipolar I disorder based on number of previous manic or mixed episodes. J. Clin. Psychiatry 2006, 67, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Menzin, J.; Sussman, M.; Tafesse, E.; Duczakowski, C.; Neumann, P.; Friedman, M. A model of the economic impact of a bipolar disorder screening program in primary care. J. Clin. Psychiatry 2009, 70, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Colom, F.; Vieta, E.; Daban, C.; Pacchiarotti, I.; Sánchez-Moreno, J. Clinical and therapeutic implications of predominant polarity in bipolar disorder. J. Affect. Disord. 2006, 93, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Reinares, M.; Goikolea, J.M.; Bonnin, C.M.; Gonzalez-Pinto, A.; Vieta, E. Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur. Neuropsychopharmacol. 2012, 22, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Reinares, M.; Scott, J.; Nivoli, A.; Murru, A.; Pacchiarotti, I.; Vieta, E.; Colom, F. Polarity index of psychological interventions in maintenance treatment of bipolar disorder. Psychother. Psychosom. 2013, 82, 292–298. [Google Scholar] [CrossRef]
- Popovic, D.; Torrent, C.; Goikolea, J.M.; Cruz, N.; Sánchez-Moreno, J.; González-Pinto, A.; Vieta, E. Clinical implications of predominant polarity and the polarity index in bipolar disorder: A naturalistic study. Acta Psychiatr. Scand. 2014, 129, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Berk, M.; Wang, W.; Colom, F.; Tohen, M.; Baldessarini, R.J. Predominant previous polarity as an outcome predictor in a controlled treatment trial for depression in bipolar I disorder patients. J. Affect. Disord. 2009, 119, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Tundo, A.; Musetti, L.; Benedetti, A.; Berti, B.; Massimetti, G.; Dell’Osso, L. Onset polarity and illness course in bipolar I and II disorders: The predictive role of broadly defined mixed states. Compr. Psychiatry 2015, 63, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Tondo, L.; Visioli, C. First-episode types in bipolar disorder: Predictive associations with later illness. Acta Psychiatr. Scand. 2014, 129, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Delbello, M.P.; Miyahara, S.; Wisniewski, S.R.; Sachs, G.S.; Nierenberg, A.A.; STEP-BD investigators. Revisiting depressive-prone bipolar disorder: Polarity of initial mood episode and disease course among bipolar I systematic treatment enhancement program for bipolar disorder participants. Biol. Psychiatry 2005, 58, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Perugi, G.; Micheli, C.; Akiskal, H.S.; Madaro, D.; Socci, C.; Quilici, C.; Musetti, L. Polarity of the first episode, clinical characteristics, and course of manic depressive illness: A systematic retrospective investigation of 320 bipolar I patients. Compr. Psychiatry 2000, 41, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kraguljac, N.V.; Reid, M.; White, D.; Jones, R.; den Hollander, J.; Lowman, D.; Lahti, A.C. Neurometabolites in schizophrenia and bipolar disorder—A systematic review and meta-analysis. Psychiatry Res. 2012, 203, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Yildiz-Yesiloglu, A.; Ankerst, D.P. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: A systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 969–995. [Google Scholar] [CrossRef]
- Winsberg, M.E.; Sachs, N.; Tate, D.L.; Adalsteinsson, E.; Spielman, D.; Ketter, T.A. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol. Psychiatry 2000, 47, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, A.; Frye, M.; Callicott, J.H.; Mattay, V.S.; Rakow, R.; Shelton-Repella, J.; Post, R.; Weinberger, D.R. Neuronal pathology in the hippocampal area of patients with bipolar disorder: A study with proton magnetic resonance spectroscopic imaging. Biol. Psychiatry 2003, 53, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Yildirim, H.; Ozdemir, H.; Ogur, E.; Tezcan, E. Hippocampal 1H MRS in patients with bipolar disorder taking valproate versus valproate plus quetiapine. Psychol. Med. 2007, 37, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bhagwagar, Z.; Wylezinska, M.; Jezzard, P.; Evans, J.; Ashworth, F.; Sule, A.; Matthews, P.M.; Cowen, P.J. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol. Psychiatry 2007, 61, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Sager, T.N.; Topp, S.; Torup, L.; Hanson, L.G.; Egestad, B.; Møller, A. Evaluation of CA1 damage using single-voxel 1H-MRS and un-biased stereology: Can non-invasive measures of N-acetyl-asparate following global ischemia be used as a reliable measure of neuronal damage? Brain Res. 2001, 892, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.; Stanley, J.A.; Nicoletti, M.A.; Sassi, R.B.; Mallinger, A.G.; Frank, E.; Kupfer, D.; Keshavan, M.S.; Soares, J.C. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J. Affect. Disord. 2005, 86, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Iosifescu, D.V.; Moore, C.M.; Deckersbach, T.; Tilley, C.A.; Ostacher, M.J.; Sachs, G.S.; Nierenberg, A.A. Galantamine-ER for cognitive dysfunction in bipolar disorder and correlation with hippocampal neuronal viability: A proof-of-concept study. CNS Neurosci. Ther. 2009, 15, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Colla, M.; Schubert, F.; Bubner, M.; Heidenreich, J.O.; Bajbouj, M.; Seifert, F.; Luborzewski, A.; Heuser, I.; Kronenberg, G. Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol. Psychiatry 2009, 14, 647, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hamakawa, H.; Shioiri, T.; Murashita, J.; Takahashi, Y.; Takahashi, S.; Inubushi, T. Choline-containing compounds detected by proton magnetic resonance spectroscopy in the basal ganglia in bipolar disorder. J. Psychiatry Neurosci. 1996, 21, 248–254. [Google Scholar] [PubMed]
- Scotti-Muzzi, E.; Umla-Runge, K.; Soeiro-de-Souza, M.G. Anterior cingulate cortex neurometabolites in bipolar disorder are influenced by mood state and medication: A meta-analysis of 1H-MRS studies. Eur. Neuropsychopharmacol. 2021, 47, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, P.H.; McGrath, B.M.; Kim, H. Bipolar disorder and myo-inositol: A review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Öngür, D.; Jensen, J.E.; Prescot, A.P.; Stork, C.; Lundy, M.; Cohen, B.M.; Renshaw, P.F. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol. Psychiatry 2008, 64, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Watzl, J.; Banakar, S.; O’Neill, J.; Mintz, J.; Davanzo, P.; Fischer, J.; Chirichigno, J.W.; Ventura, J.; Elman, S.; et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology 2007, 32, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, R.; Milne, A.M.; MacQueen, G.M.; Hall, G.B.C. Increased choline-containing compounds in the orbitofrontal cortex and hippocampus in euthymic patients with bipolar disorder: A proton magnetic resonance spectroscopy study. Psychiatry Res. 2009, 172, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Janiri, D.; Simonetti, A.; Piras, F.; Ciullo, V.; Spalletta, G.; Sani, G. Predominant polarity and hippocampal subfield volumes in Bipolar disorders. Bipolar Disord. 2020, 22, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, G.D.; Christidi, F.; Karavasilis, E.; Velonakis, G.; Antoniou, A.; Bede, P.; Seimenis, I.; Kelekis, N.; Douzenis, A.; Papakonstantinou, O.; et al. Cerebro-cerebellar white matter connectivity in bipolar disorder and associated polarity subphenotypes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110034. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, G.D.; Christidi, F.; Karavasilis, E.; Bede, P.; Antoniou, A.; Velonakis, G.; Seimenis, I.; Kelekis, N.; Smyrnis, N.; Papakonstantinou, O.; et al. Predominant polarity as a neurobiological specifier in bipolar disorder: Evidence from a multimodal neuroimaging study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110718. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Colbourne, L.; Harrison, C.H. The neuropathology of bipolar disorder: Systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 1787–1808. [Google Scholar] [CrossRef] [PubMed]
- Nurnberger, J.I.; Blehar, M.C.; Kaufmann, C.A.; York-Cooler, C.; Simpson, S.G.; Harkavy-Friedman, J.; Severe, J.B.; Malaspina, D.; Reich, T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch. Gen. Psychiatry 1994, 51, 849–859; discussion 863–864. [Google Scholar] [CrossRef] [PubMed]
- Ferentinos, P.; Fountoulakis, K.N.; Lewis, C.M.; Porichi, E.; Dikeos, D.; Papageorgiou, C.; Douzenis, A. Validating a two-dimensional bipolar spectrum model integrating DSM-5’s mixed features specifier for Major Depressive Disorder. Compr. Psychiatry 2017, 77, 89–99. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. User’s Guide for the SCID-5-CV Structured Clinical Interview for DSM-5® Disorders: Clinical Version; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2016. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. Available online: https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/abs/rating-scale-for-mania-reliability-validity-and-sensitivity/34DE3C8ED1EB65C54E87970C87BC0528 (accessed on 4 January 2024). [CrossRef] [PubMed]
- Wilson, M.; Reynolds, G.; Kauppinen, R.A.; Arvanitis, T.N.; Peet, A.C. A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn. Reson. Med. 2011, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Wilson, M.; Davies, N.P.; MacPherson, L.; English, M.; Arvanitis, T.N.; Peet, A.C. Diagnosing relapse in children’s brain tumors using metabolite profiles. Neuro Oncol. 2014, 16, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, P.; Henriksen, O.; Stubgaard, M.; Gideon, P.; Larsson, H.B. In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn. Reson. Imaging 1993, 11, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Dhamala, E.; Abdelkefi, I.; Nguyen, M.; Hennessy, T.J.; Nadeau, H.; Near, J. Validation of in vivo MRS measures of metabolite concentrations in the human brain. NMR Biomed. 2019, 32, e4058. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, C.; Song, T.; Devier, D.; Bockholt, H.J.; Caprihan, A.; Mullins, P.G.; Posse, S.; Jung, R.E.; Morrison, L.A. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006, 55, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.; Reiter, D.; Kapogiannis, D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer’s brain. Ann. Clin. Transl. Neurol. 2018, 5, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Miola, A.; Cattarinussi, G.; Antiga, G.; Caiolo, S.; Solmi, M.; Sambataro, F. Difficulties in emotion regulation in bipolar disorder: A systematic review and meta-analysis. J. Affect. Disord. 2022, 302, 352–360. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H.; Hurley, R.A.; Hayman, L.A.; Taber, K.H. Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.; Garrison, K.; Whitfield-Gabrieli, S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Front. Hum. Neurosci. 2013, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Mechanisms of memory. Science 1986, 232, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Zola-Morgan, S.; Squire, L.R.; Amaral, D.G. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986, 6, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Milner, B. Disorders of learning and memory after temporal lobe lesions in man. Clin. Neurosurg. 1972, 19, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol. Psychiatry 2003, 54, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry 2003, 54, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Bubb, E.J.; Kinnavane, L.; Aggleton, J.P. Hippocampal–diencephalic–cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci. Adv. 2017, 1, 2398212817723443. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.D. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR Biomed. 1991, 4, 59–63. [Google Scholar] [CrossRef]
- Ross, B.; Bluml, S. Magnetic resonance spectroscopy of the human brain. Anat. Rec. 2001, 265, 54–84. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Inubushi, T.; Kato, N. Magnetic resonance spectroscopy in affective disorders. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Downes, C.P.; Hanley, M.R. Neural and developmental actions of lithium: A unifying hypothesis. Cell 1989, 59, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.B.; Cheng, L.; Mudge, A.W.; Harwood, A.J. A common mechanism of action for three mood-stabilizing drugs. Nature 2002, 417, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Valenzuela, M.; Wen, W.; Sachdev, P. Magnetic resonance spectroscopy and its applications in psychiatry. Aust. N. Z. J. Psychiatry 2002, 36, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takahashi, S.; Shioiri, T.; Inubushi, T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J. Affect. Disord. 1992, 26, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takahashi, S.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Inubushi, T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J. Affect. Disord. 1994, 31, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Takahashi, Y.; Inubushi, T.; Takahashi, S. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol. Med. 1995, 25, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.; DelBello, M.P.; Weber, W.A.; Adler, C.M.; Strakowski, S.M.; Lee, J.-H. Tissue-dependent cerebral energy metabolism in adolescents with bipolar disorder. J. Affect. Disord. 2016, 191, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.B.; Stanley, J.A.; Axelson, D.; Brambilla, P.; Nicoletti, M.A.; Keshavan, M.S.; Ramos, R.T.; Ryan, N.; Birmaher, B.; Soares, J.C. Reduced NAA levels in the dorsolateral prefrontal cortex of young bipolar patients. Am. J. Psychiatry 2005, 162, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; DelBello, M.P.; Sellars, M.C.; Strakowski, S.M. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J. Child. Adolesc. Psychopharmacol. 2003, 13, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Caetano, S.C.; Olvera, R.L.; Hatch, J.P.; Sanches, M.; Chen, H.H.; Nicoletti, M.; Stanley, J.A.; Fonseca, M.; Hunter, K.; Lafer, B.; et al. Lower N-acetyl-aspartate levels in prefrontal cortices in pediatric bipolar disorder: A 1H magnetic resonance spectroscopy study. J. Am. Acad. Child. Adolesc. Psychiatry 2011, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; Cecil, K.M.; Strakowski, S.M.; Adler, C.M.; DelBello, M.P. Neurochemical alterations in adolescent bipolar depression: A proton magnetic resonance spectroscopy pilot study of the prefrontal cortex. J. Child. Adolesc. Psychopharmacol. 2008, 18, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Ongür, D.; Prescot, A.P.; Jensen, J.E.; Cohen, B.M.; Renshaw, P.F. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009, 172, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Bio, D.S.; Moreno, R.A.; Garcia-Otaduy, M.C.; Nery, F.; Lafer, B.; Soeiro-de-Souza, M.G. Altered brain creatine cycle metabolites in bipolar I disorder with childhood abuse: A 1H magnetic resonance spectroscopy study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110233. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, H.; Lin, F.; Zheng, W.; Zhang, H.; Wu, R. Neurochemical and microstructural alterations in bipolar and depressive disorders: A multimodal magnetic resonance imaging study. Front. Neurol. 2023, 14, 1089067. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Zhang, Y.; Guan, J.; Zhang, J.; Xu, C.; Shen, Z.; Xiao, B.; Liang, C.; Chen, K.; et al. Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: A two-dimensional proton magnetic resonance spectroscopy study. Bipolar Disord. 2016, 18, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Z.; Li, H.; Liu, C.-F.; Guan, J.-T.; Guo, X.-B.; Wen, C.-H.; Ou, S.-M.; Zhang, Y.-N.; Zhang, J.; Xu, C.-T.; et al. Main Effects of Diagnoses, Brain Regions, and their Interaction Effects for Cerebral Metabolites in Bipolar and Unipolar Depressive Disorders. Sci. Rep. 2016, 6, 37343. [Google Scholar] [CrossRef] [PubMed]
- Allin, M.P.G.; Marshall, N.; Schulze, K.; Walshe, M.; Hall, M.-H.; Picchioni, M.; Murray, R.M.; McDonald, C. A functional MRI study of verbal fluency in adults with bipolar disorder and their unaffected relatives. Psychol. Med. 2010, 40, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, S.M.; Adler, C.M.; Cerullo, M.A.; Eliassen, J.C.; Lamy, M.; Fleck, D.E.; Lee, J.-H.; DelBello, M.P. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Interv. Psychiatry 2008, 2, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, S.; Yu, W.; Gao, Y.; Ma, J. Regional Homogeneity of the Left Posterior Cingulate Gyrus May Be a Potential Imaging Biomarker of Manic Episodes in First-Episode, Drug-Naive Bipolar Disorder. Neuropsychiatr. Dis. Treat. 2023, 19, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Haarman, B.; Riemersma-van der Lek, R.; Burger, H.; Renken, R.; Kuiper, A.; Marsman, J.-B.; Groot, J.; Nolen, W. Metabolites and volumes of the hippocampi in bipolar disorder. In Bipolar Disorders; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mak, C.S.W.; Waldvogel, H.J.; Dodd, J.R.; Gilbert, R.T.; Lowe, M.T.J.; Birch, N.P.; Faull, R.L.M.; Christie, D.L. Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience 2009, 163, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Giannakopoulos, P.; Kövari, E.; Bouras, C. Assessing the role of cingulate cortex in bipolar disorder: Neuropathological, structural and functional imaging data. Brain Res. Rev. 2008, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Schlaug, G.; Armstrong, E.; Schleicher, A.; Zilles, K. Layer V pyramidal cells in the adult human cingulate cortex. Anat. Embryol. 1993, 187, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Elston, G.N.; Benavides-Piccione, R.; Defelipe, J. A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb. Cortex 2005, 15, 64–73. [Google Scholar] [CrossRef]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Moore, C.M.; Breeze, J.L.; Gruber, S.A.; Babb, S.M.; Frederick, B.B.; Villafuerte, R.A.; Stoll, A.L.; Hennen, J.; Yurgelun-Todd, D.A.; Cohen, B.M.; et al. Choline, myo-inositol and mood in bipolar disorder: A proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord. 2000, 2, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hamakawa, H.; Kato, T.; Murashita, J.; Kato, N. Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Yildirim, H. Altered Neurochemical Ingredient of Hippocampus in Patients with Bipolar Depression. Depress. Res. Treat. 2012, 2012, 485249. [Google Scholar] [CrossRef] [PubMed]
- Stork, C.; Renshaw, P.F. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 2005, 10, 900–919. [Google Scholar] [CrossRef] [PubMed]
- Chabert, J.; Allauze, E.; Pereira, B.; Chassain, C.; De Chazeron, I.; Rotgé, J.-Y.; Fossati, P.; Llorca, P.-M.; Samalin, L. Glutamatergic and N-Acetylaspartate Metabolites in Bipolar Disorder: A Systematic Review and Meta-Analysis of Proton Magnetic Resonance Spectroscopy Studies. Int. J. Mol. Sci. 2022, 23, 8974. [Google Scholar] [CrossRef] [PubMed]
- Choquette, A.; Dager, A.; Marjańska, M.; Zatony, M.; Pearlson, G.D.; Glahn, D.C.; Knowles, E.E.M. Relating depressive and manic symptomatology to 1H-MRS spectra. J. Affect. Disord. Rep. 2024, 16, 100774. [Google Scholar] [CrossRef]
- Frye, M.A.; Thomas, M.A.; Yue, K.; Binesh, N.; Davanzo, P.; Ventura, J.; O’Neill, J.; Guze, B.; Curran, J.G.; Mintz, J. Reduced concentrations of N-acetylaspartate (NAA) and the NAA-creatine ratio in the basal ganglia in bipolar disorder: A study using 3-Tesla proton magnetic resonance spectroscopy. Psychiatry Res. 2007, 154, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.W.; Dewitt, M.; Hudgens-Haney, M.; Schaeffer, D.J.; Ball, B.H.; Schwarz, N.F.; Hussein, A.A.; Smart, L.M.; Sabatinelli, D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014, 45, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Houenou, J.; Frommberger, J.; Carde, S.; Glasbrenner, M.; Diener, C.; Leboyer, M.; Wessa, M. Neuroimaging-based markers of bipolar disorder: Evidence from two meta-analyses. J. Affect. Disord. 2011, 132, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.; Che, D.; Bai, Y. Neural network of bipolar disorder: Toward integration of neuroimaging and neurocircuit-based treatment strategies. Transl. Psychiatry 2022, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Daban, C.; Colom, F.; Sanchez-Moreno, J.; García-Amador, M.; Vieta, E. Clinical correlates of first-episode polarity in bipolar disorder. Compr. Psychiatry 2006, 47, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Forty, L.; Jones, L.; Jones, I.; Smith, D.J.; Caesar, S.; Fraser, C.; Gordon-Smith, K.; Hyde, S.; Craddock, N. Polarity at illness onset in bipolar I disorder and clinical course of illness. Bipolar Disord. 2009, 11, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Haag, H.; Heidorn, A.; Haag, M.; Greil, W. Sequence of affective polarity and lithium response: Preliminary report on Munich sample. Prog. Neuropsychopharmacol. Biol. Psychiatry 1987, 11, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Pirozzi, R.; Starace, F. Previous pattern of course of the illness as a predictor of response to lithium prophylaxis in bipolar patients. J. Affect. Disord. 1989, 17, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Azorin, J.-M.; Kaladjian, A.; Adida, M.; Fakra, E.; Hantouche, E.; Lancrenon, S. Correlates of first-episode polarity in a French cohort of 1089 bipolar I disorder patients: Role of temperaments and triggering events. J. Affect. Disord. 2011, 129, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tsitsipa, E.; Fountoulakis, K.N. The neurocognitive functioning in bipolar disorder: A systematic review of data. Ann. Gen. Psychiatry 2015, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, H.; Cao, Y.; Song, H.; Jiang, X.; Wei, C.; Yang, Z.; Li, J. Clinical characteristics and cognitive function in bipolar disorder patients with different onset symptom. Front. Psychiatry 2023, 14, 1253088. [Google Scholar] [CrossRef] [PubMed]
- Turvey, C.L.; Coryell, W.H.; Arndt, S.; Solomon, D.A.; Leon, A.C.; Endicott, J.; Mueller, T.; Keller, M.; Akiskal, H. Polarity sequence, depression, and chronicity in bipolar I disorder. J. Nerv. Ment. Dis. 1999, 187, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kassem, L.; Lopez, V.; Hedeker, D.; Steele, J.; Zandi, P.; Bipolar Disorder Consortium NIMH Genetics Initiative; McMahon, F.J. Familiality of polarity at illness onset in bipolar affective disorder. Am. J. Psychiatry 2006, 163, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Haldane, M.; Frangou, S. New insights help define the pathophysiology of bipolar affective disorder: Neuroimaging and neuropathology findings. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Soeiro-de-Souza, M.G.; Otaduy, M.C.G.; Machado-Vieira, R.; Moreno, R.A.; Nery, F.G.; Leite, C.; Lafer, B. Lithium-associated anterior cingulate neurometabolic profile in euthymic Bipolar I disorder: A 1H-MRS study. J. Affect. Disord. 2018, 241, 192–199. [Google Scholar] [CrossRef] [PubMed]
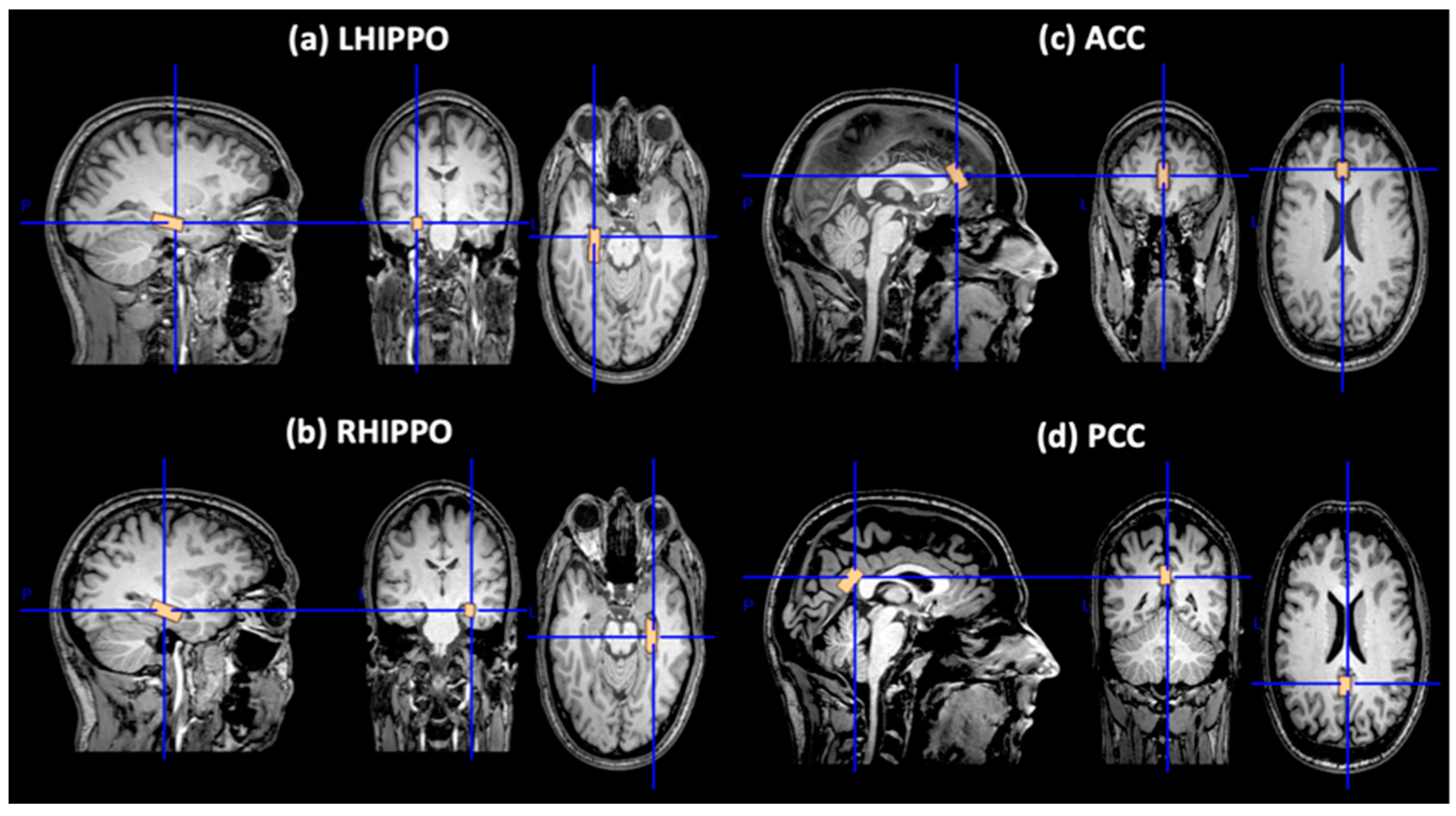

| HC (n = 16) | OP-M (n = 17) | OP-D (n = 24) | PP-M (n = 12) | PP-D (n = 14) | PP-U (n = 15) | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (yrs) | 40.31 ± 10.05 | 42.53 ± 12.73 | 49.21 ± 10.91 | 38.00 ± 10.02 | 51.50 ± 11.13 | 48.47 ± 11.30 |
| Sex (M/F) | 6/10 | 9/8 | 11/13 | 5/7 | 7/7 | 8/7 |
| Education (years) | 14.44 ± 2.07 | 14.35 ± 3.66 | 14.42 ± 3.80 | 15.17 ± 2.86 | 14.64 ± 4.13 | 13.53 ± 3.93 |
| Clinical characteristics | ||||||
| BD-type (I/II) | - | 17/0 | 13/11 | 12/0 | 7/7 | 11/4 |
| Illness duration (years) | - | 12.59 ± 8.93 | 16.25 ± 9.35 | 12.58 ± 8.11 | 15.71 ± 10.40 | 15.53 ± 9.30 |
| Hospitalizations (n) | - | 2.59 ± 2.00 | 2.33 ± 1.79 | 2.58 ± 1.98 | 2.43 ± 1.79 | 2.25 ± 1.92 |
| Depressive episodes (n) | - | 2.94 ± 4.84 | 4.50 ± 4.03 | 0.67 ± 0.78 | 4.21 ± 2.49 | 6.07 ± 5.91 |
| Hyperthymis episodes (n) | - | 4.47 ± 4.60 | 3.04 ± 2.96 | 3.50 ± 2.02 | 1.64 ± 0.84 | 5.60 ± 5.29 |
| Lifetime suicidal attempts (Y/N) | - | 2/15 | 15/9 | 4/8 | 7/7 | 6/9 |
| Lifetime psychosis (Y/N) | - | 12/5 | 10/14 | 9/3 | 4/10 | 9/6 |
| Lifetime Axis I comorbidity (Y/N) | - | 4/13 | 8/16 | 3/9 | 4/10 | 5/10 |
| Current lithium (Y/N) | - | 5/12 | 5/19 | 3/9 | 3/11 | 4/11 |
| Current anticonvulsants (Y/N) | - | 14/3 | 20/4 | 8/4 | 13/1 | 13/2 |
| Current antidepressants (Y/N) | - | 3/14 | 9/15 | 2/10 | 7/7 | 3/12 |
| Current antipsychotics (Y/N) | - | 15/2 | 20/4 | 10/2 | 12/2 | 13/2 |
| FH-FDR of BD (Y/N) | - | 5/12 | 3/21 | 2/10 | 4/10 | 2/13 |
| FH-FDR of schizophrenia (Y/N) | - | 2/15 | 1/23 | 1/11 | 1/13 | 1/14 |
| FH-FDR of MDD (Y/N) | - | 4/13 | 9/15 | 4/8 | 3/11 | 6/9 |
| PP-M | PP-D | PP-U | Total | |
|---|---|---|---|---|
| OP-M | 9 (52.9%) | 2 (11.8%) | 6 (35.3%) | 17 |
| OP-D | 3 (12.5%) | 12 (50.0%) | 9 (37.5%) | 24 |
| Total | 12 | 14 | 15 | 41 |
| Anatomical Region/Metabolite | E.M.M. ± S.E. for Groups | Statistics | ||||
|---|---|---|---|---|---|---|
| HC | OP-M | OP-D | Univariate p-Value | Partial η2 | Post Hoc Comparisons (Corrected p < 0.05) | |
| Dependent variables: absolute values (tNAA, tCr, tCho, mI) | Pillai’s Trace Multivariate F = 1.672, p = 0.039 | |||||
| ACC | ||||||
| tNAA | 8.44 ± 0.58 | 7.26 ± 0.54 | 8.53 ± 0.49 | |||
| tCr | 10.58 ± 0.50 | 10.43 ± 0.46 | 11.23 ± 0.42 | |||
| tCho | 2.89 ± 0.32 | 3.25 ± 0.30 | 3.43 ± 0.27 | |||
| mI | 5.83 ± 0.58 | 6.02 ± 0.54 | 4.19 ± 0.49 | 0.038 | 0.128 | (OP-M > OP-D, p = 0.056) |
| PCC | ||||||
| tNAA | 8.53 ± 0.80 | 7.83 ± 0.75 | 8.91 ± 0.68 | |||
| tCr | 10.42 ± 0.41 | 11.67 ± 0.38 | 11.18 ± 0.34 | (0.081) | 0.099 | (HC < OP-M, p = 0.078) |
| tCho | 1.91 ± 0.39 | 3.26 ± 0.37 | 2.22 ± 0.33 | 0.033 | 0.133 | HC < OP-M, p = 0.043 |
| mI | 5.39 ± 0.75 | 5.39 ± 0.70 | 4.67 ± 0.63 | |||
| LHIPPO | ||||||
| tNAA | 5.78 ± 0.58 | 5.49 ± 0.54 | 6.73 ± 0.49 | |||
| tCr | 7.23 ± 0.47 | 6.85 ± 0.44 | 7.35 ± 0.40 | |||
| tCho | 1.85 ± 0.26 | 2.13 ± 0.24 | 1.81 ± 0.22 | |||
| mI | 8.69 ± 0.62 | 7.23 ± 0.58 | 9.11 ± 0.53 | (0.061) | 0.110 | (OP-M < OP-D, p = 0.072) |
| RHIPPO | ||||||
| tNAA | 6.07 ± 0.52 | 4.92 ± 0.48 | 5.67 ± 0.44 | |||
| tCr | 8.63 ± 0.63 | 6.56 ± 0.59 | 7.88 ± 0.53 | (0.054) | 0.115 | (HC > OP-M, p = 0.056) |
| tCho | 2.03 ± 0.23 | 2.52 ± 0.21 | 2.08 ± 0.20 | |||
| mI | 9.90 ± 1.63 | 9.48 ± 1.52 | 11.00 ± 1.38 | |||
| Dependent variables: ratios (tNAA/tCr, tNAA/tCho, tCho/tCr, mI/tNAA, mI/tCr, mI/tCho) | Pillai’s Trace Multivariate F = 2.148, p = 0.004 | |||||
| ACC | ||||||
| tNAA/tCr | 0.80 ± 0.05 | 0.72 ± 0.05 | 0.77 ± 0.04 | |||
| tNAA/tCho | 3.06 ± 0.24 | 2.59 ± 0.22 | 2.70 ± 0.20 | |||
| tCho/tCr | 0.27 ± 0.03 | 0.30 ± 0.02 | 0.31 ± 0.02 | |||
| mI/tNAA | 0.71 ± 0.08 | 0.88 ± 0.08 | 0.51 ± 0.07 | 0.003 | 0.210 | OP-M > OP-D, p = 0.002 |
| mI/tCr | 0.55 ± 0.06 | 0.62 ± 0.06 | 0.37 ± 0.05 | 0.005 | 0.195 | OP-M > OP-D, p = 0.005; (HC > OP-D, p = 0.076) |
| mI/tCho | 2.21 ± 0.24 | 2.22 ± 0.23 | 1.25 ± 0.21 | 0.004 | 0.203 | HC > OP-D, p = 0.016; OP-M > OP-D, p = 0.010 |
| PCC | ||||||
| tNAA/tCr | 0.80 ± 0.06 | 0.67 ± 0.06 | 0.80 ± 0.05 | |||
| tNAA/tCho | 4.54 ± 0.45 | 3.89 ± 0.42 | 4.12 ± 0.38 | |||
| tCho/tCr | 0.19 ± 0.04 | 0.28 ± 0.03 | 0.20 ± 0.03 | |||
| mI/tNAA | 0.80 ± 0.29 | 1.06 ± 0.27 | 0.71 ± 0.24 | |||
| mI/tCr | 0.53 ± 0.08 | 0.45 ± 0.07 | 0.44 ± 0.07 | |||
| mI/tCho | 2.84 ± 0.35 | 2.27 ± 0.33 | 2.14 ± 0.30 | |||
| LHIPPO | ||||||
| tNAA/tCr | 0.84 ± 0.08 | 0.81 ± 0.08 | 0.94 ± 0.07 | |||
| tNAA/tCho | 3.39 ± 0.33 | 3.18 ± 0.31 | 3.77 ± 0.28 | |||
| tCho/tCr | 0.25 ± 0.05 | 0.35 ± 0.05 | 0.25 ± 0.04 | |||
| mI/tNAA | 1.93 ± 0.31 | 1.49 ± 0.29 | 1.60 ± 0.26 | |||
| mI/tCr | 1.29 ± 0.13 | 1.08 ± 0.12 | 1.31 ± 0.11 | |||
| mI/tCho | 5.69 ± 0.56 | 4.28 ± 0.52 | 5.18 ± 0.47 | |||
| RHIPPO | ||||||
| tNAA/tCr | 0.76 ± 0.09 | 0.85 ± 0.09 | 0.76 ± 0.08 | |||
| tNAA/tCho | 3.18 ± 0.26 | 2.28 ± 0.24 | 2.90 ± 0.22 | 0.035 | 0.130 | HC > OP-M, p = 0.038 |
| tCho/tCr | 0.25 ± 0.03 | 0.37 ± 0.03 | 0.27 ± 0.02 | 0.005 | 0.201 | HC < OP-M, p = 0.008; OP-M > OP-D, p = 0.024 |
| mI/tNAA | 1.73 ± 0.69 | 2.12 ± 0.64 | 2.37 ± 0.58 | |||
| mI/tCr | 1.28 ± 0.24 | 1.66 ± 0.23 | 1.38 ± 0.21 | |||
| mI/tCho | 5.26 ± 0.59 | 4.12 ± 0.55 | 5.21 ± 0.50 | |||
| Anatomical Region/Metabolite | E.M.M. ± S.E. for Groups | Statistics | |||||
|---|---|---|---|---|---|---|---|
| HC | PP-Μ | PP-D | PP-U | Univariate p-Value | Partial η2 | Post hoc Comparisons | |
| Dependent variables: absolute values (tNAA, tCr, tCho, mI) | Pillai’s Trace Multivariate F = 1.052, p = 0.408 | ||||||
| ACC | |||||||
| tNAA | 8.37 ± 0.56 | 6.58 ± 0.65 | 8.22 ± 0.60 | 9.09 ± 0.61 | |||
| tCr | 10.57 ± 0.51 | 10.41 ± 0.58 | 11.18 ± 0.54 | 11.01 ± 0.55 | |||
| tCho | 2.91 ± 0.31 | 3.57 ± 0.36 | 3.60 ± 0.33 | 2.85 ± 0.34 | |||
| mI | 5.76 ± 0.61 | 5.13 ± 0.70 | 4.29 ± 0.64 | 5.69 ± 0.66 | |||
| PCC | |||||||
| tNAA | 8.52 ± 0.81 | 7.88 ± 0.94 | 9.24 ± 0.86 | 8.11 ± 0.88 | |||
| tCr | 10.37 ± 0.41 | 11.17 ± 0.48 | 11.39 ± 0.44 | 11.65 ± 0.45 | |||
| tCho | 1.86 ± 0.40 | 2.60 ± 0.46 | 2.26 ± 0.43 | 3.24 ± 0.44 | |||
| mI | 5.32 ± 0.76 | 4.69 ± 0.88 | 5.03 ± 0.81 | 5.27 ± 0.83 | |||
| LHIPPO | |||||||
| tNAA | 5.85 ± 0.60 | 6.23 ± 0.69 | 6.36 ± 0.64 | 5.90 ± 0.65 | |||
| tCr | 7.22 ± 0.48 | 6.80 ± 0.55 | 7.48 ± 0.51 | 7.09 ± 0.52 | |||
| tCho | 1.86 ± 0.26 | 2.25 ± 0.30 | 1.78 ± 0.28 | 1.84 ± 0.28 | |||
| mI | 8.72 ± 0.45 | 7.62 ± 0.76 | 8.56 ± 0.70 | 8.58 ± 0.71 | |||
| RHIPPO | |||||||
| tNAA | 6.06 ± 0.53 | 4.90 ± 0.61 | 5.57 ± 0.56 | 5.53 ± 0.57 | |||
| tCr | 8.56 ± 0.62 | 6.00 ± 0.71 | 8.44 ± 0.66 | 7.37 ± 0.67 | |||
| tCho | 2.01 ± 0.24 | 2.26 ± 0.27 | 2.46 ± 0.25 | 2.10 ± 0.26 | |||
| mI | 9.75 ± 1.63 | 8.02 ± 1.88 | 11.32 ± 1.73 | 11.60 ± 1.76 | |||
| Dependent variables: ratios (tNAA/tCr, tNAA/tCho, tCho/tCr, mI/tNAA, mI/tCr, mI/tCho) | Pillai’s Trace Multivariate F = 1.218, p = 0.196 | ||||||
| ACC | |||||||
| tNAA/tCr | 0.79 ± 0.05 | 0.66 ± 0.06 | 0.74 ± 0.05 | 0.84 ± 0.05 | |||
| tNAA/tCho | 3.03 ± 0.22 | 2.20 ± 0.26 | 2.51 ± 0.24 | 3.25 ± 0.24 | |||
| tCho/tCr | 0.28 ± 0.02 | 0.33 ± 0.03 | 0.33 ± 0.03 | 0.26 ± 0.03 | |||
| mI/tNAA | 0.70 ± 0.09 | 0.83 ± 0.10 | 0.57 ± 0.09 | 0.64 ± 0.10 | |||
| mI/tCr | 0.55 ± 0.07 | 0.53 ± 0.07 | 0.38 ± 0.07 | 0.55 ± 0.07 | |||
| mI/tCho | 2.18 ± 0.26 | 1.71 ± 0.30 | 1.30 ± 0.28 | 2.08 ± 0.28 | |||
| PCC | |||||||
| tNAA/tCr | 0.81 ± 0.06 | 0.71 ± 0.07 | 0.81 ± 0.07 | 0.69 ± 0.07 | |||
| tNAA/tCho | 4.54 ± 0.45 | 4.04 ± 0.52 | 4.42 ± 0.48 | 3.57 ± 0.49 | |||
| tCho/tCr | 0.18 ± 0.04 | 0.23 ± 0.04 | 0.20 ± 0.04 | 0.28 ± 0.04 | |||
| mI/tNAA | 0.76 ± 0.29 | 0.61 ± 0.33 | 0.90 ± 0.31 | 1.11 ± 0.31 | |||
| mI/tCr | 0.53 ± 0.08 | 0.42 ± 0.09 | 0.48 ± 0.09 | 0.44 ± 0.09 | |||
| mI/tCho | 2.85 ± 0.36 | 2.35 ± 0.41 | 2.28 ± 0.38 | 1.97 ± 0.39 | |||
| LHIPPO | |||||||
| tNAA/tCr | 0.84 ± 0.09 | 0.91 ± 0.10 | 0.87 ± 0.09 | 0.86 ± 0.09 | |||
| tNAA/tCho | 3.43 ± 0.34 | 3.61 ± 0.39 | 3.52 ± 0.36 | 3.38 ± 0.37 | |||
| tCho/tCr | 0.26 ± 0.06 | 0.38 ± 0.06 | 0.24 ± 0.06 | 0.26 ± 0.06 | |||
| mI/tNAA | 1.91 ± 0.31 | 1.23 ± 0.36 | 1.66 ± 0.33 | 1.77 ± 0.33 | |||
| mI/tCr | 1.29 ± 0.13 | 1.18 ± 0.15 | 1.22 ± 0.14 | 1.23 ± 0.15 | |||
| mI/tCho | 5.71 ± 0.57 | 4.56 ± 0.66 | 4.81 ± 0.61 | 4.96 ± 0.62 | |||
| RHIPPO | |||||||
| tNAA/tCr | 0.77 ± 0.09 | 0.93 ± 0.11 | 0.72 ± 0.10 | 0.75 ± 0.10 | |||
| tNAA/tCho | 3.21 ± 0.27 | 2.54 ± 0.31 | 2.52 ± 0.29 | 2.79 ± 0.29 | |||
| tCho/tCr | 0.25 ± 0.03 | 0.36 ± 0.04 | 0.30 ± 0.03 | 0.28 ± 0.03 | |||
| mI/tNAA | 1.68 ± 0.69 | 1.63 ± 0.79 | 2.70 ± 0.73 | 2.43 ± 0.75 | |||
| mI/tCr | 1.28 ± 0.25 | 1.66 ± 0.28 | 1.32 ± 0.26 | 1.55 ± 0.27 | |||
| mI/tCho | 5.27 ± 0.61 | 4.26 ± 0.70 | 4.54 ± 0.64 | 5.36 ± 0.66 | |||
| HC vs. OP-M | HC vs. OP-D | OP-M vs. OP-D | HC vs. PP-M | HC vs. PP-D | HC vs. PP-U | PP-M vs. PP-D | PP-M vs. PP-U | PP-D vs. PP-U | |
|---|---|---|---|---|---|---|---|---|---|
| ACC | |||||||||
| mI | −0.08 | 0.72 | 0.81 | 0.26 | 0.62 | 0.03 | 0.35 | −0.23 | −0.59 |
| mI/tNAA | −0.53 | 0.63 | 1.12 * | −0.37 | 0.38 | 0.17 | 0.76 | 0.54 | −0.20 |
| mI/tCho | −0.29 | 0.77 | 1.04 * | 0.08 | 0.64 | 0.00 | 0.59 | −0.08 | −0.66 |
| mI/tCr | −0.01 | 1.00 * | 1.00 * | 0.46 | 0.86 | 0.10 | 0.39 | −0.36 | −0.76 |
| PCC | |||||||||
| tCr | −0.79 | −0.48 | 0.31 | −0.49 | −0.63 | −0.80 | −0.13 | −0.29 | −0.16 |
| tCho | −0.89 * | −0.20 | 0.68 | −0.47 | −0.25 | −0.88 | 0.21 | −0.40 | −0.61 |
| LHIPPO | |||||||||
| mI | 0.61 | −0.17 | −0.77 | 0.49 | 0.07 | 0.06 | −0.36 | −0.37 | −0.01 |
| RHIPPO | |||||||||
| tCr | 0.85 | 0.30 | −0.54 | 1.05 | 0.05 | 0.49 | −0.99 | −0.56 | 0.44 |
| tNAA/tCho | 0.90 * | 0.27 | −0.61 | 0.63 | 0.65 | 0.40 | 0.02 | −0.24 | −0.25 |
| tCho/tCr | −1.00 * | −0.19 | 0.91 * | −0.86 | −0.44 | −0.27 | 0.48 | 0.64 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argyropoulos, G.D.; Christidi, F.; Karavasilis, E.; Bede, P.; Velonakis, G.; Antoniou, A.; Seimenis, I.; Kelekis, N.; Smyrnis, N.; Papakonstantinou, O.; et al. A Magnetic Resonance Spectroscopy Study on Polarity Subphenotypes in Bipolar Disorder. Diagnostics 2024, 14, 1170. https://doi.org/10.3390/diagnostics14111170
Argyropoulos GD, Christidi F, Karavasilis E, Bede P, Velonakis G, Antoniou A, Seimenis I, Kelekis N, Smyrnis N, Papakonstantinou O, et al. A Magnetic Resonance Spectroscopy Study on Polarity Subphenotypes in Bipolar Disorder. Diagnostics. 2024; 14(11):1170. https://doi.org/10.3390/diagnostics14111170
Chicago/Turabian StyleArgyropoulos, Georgios D., Foteini Christidi, Efstratios Karavasilis, Peter Bede, Georgios Velonakis, Anastasia Antoniou, Ioannis Seimenis, Nikolaos Kelekis, Nikolaos Smyrnis, Olympia Papakonstantinou, and et al. 2024. "A Magnetic Resonance Spectroscopy Study on Polarity Subphenotypes in Bipolar Disorder" Diagnostics 14, no. 11: 1170. https://doi.org/10.3390/diagnostics14111170







