Neurosurgical Intervention for Nerve and Muscle Biopsies
Abstract
1. Introduction
2. Nerve Biopsy Indications
2.1. Nerve Biopsy: High Importance
2.1.1. Vasculitis
2.1.2. Neurolymphomatosis
2.1.3. Peripheral Nerve Tumors
2.1.4. Pseudoneoplastic Peripheral Nerve Tumors (Pseudotumors)
2.1.5. Neuritic Leprosy
2.2. Nerve Biopsy: Medium Importance
2.2.1. Amyloidosis
2.2.2. Neurosarcoidosis
2.2.3. IgG4-Related Perineural Disease
2.2.4. Paraneoplastic Syndromes
2.3. Nerve Biopsy: Low Importance
2.3.1. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
2.3.2. Paraproteinemic Neuropathy
2.3.3. Adult Polyglucosan Body Disease
2.3.4. Lysosomal and Peroxisomal Storage Disorders
2.3.5. Pure Motor Neuropathy
2.3.6. Diabetic Neuropathy
2.3.7. Cryptogenic Neuropathy
2.3.8. Hereditary Neuropathy
2.3.9. Other Neuropathies
3. Muscle Biopsy Indications
3.1. Muscle Biopsy: High Indication
3.1.1. Polyarteritis Nodosa (PAN)
3.1.2. Dystrophinopathy
3.1.3. Trichinosis
3.2. Muscle Biopsy: Low Indication
3.2.1. Chloroquine Toxicity
3.2.2. Amiodarone Toxicity
3.2.3. Pompe Disease
4. Emerging Biopsy Techniques
4.1. Image-Guided Biopsies
4.2. Optical Biopsy
4.3. Minimally Invasive and Target Fasicular Biopsy
4.4. Shear Wave Elastography (SWE)
4.5. Small Fiber Diagnostic Approach and Needle Biopsies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain Among Adults—United States, 2019–2021. Mmwr. Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Wang, C.-Y. Prevalence of Reduced Muscle Strength in Older U.S. Adults: United States, 2011–2012, NCHS Data Brief. 2015; 179, 1–8.
- AHRQ. Effectiveness of Treatments for Diabetic Peripheral Neuropathy, SubStance (2016). Available online: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/diabetic-neuropathy_research-protocol.pdf (accessed on 16 January 2024).
- Joyce, N.C.; Oskarsson, B.; Jin, L.-W. Muscle Biopsy Evaluation in Neuromuscular Disorders. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 609–631. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, S.I.; Lindal, S. Nerve biopsy—Some comments on procedures and indications. Acta Neurol. Scand. 2011, 124, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nathani, D.; Spies, J.; Barnett, M.H.; Pollard, J.; Wang, M.; Sommer, C.; Kiernan, M.C. Nerve biopsy: Current indications and decision tools. Muscle Nerve 2021, 64, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Grisariu, S.; Avni, B.; Batchelor, T.T.; Bent, M.J.v.D.; Bokstein, F.; Schiff, D.; Kuittinen, O.; Chamberlain, M.C.; Roth, P.; Nemets, A.; et al. Neurolymphomatosis: An International Primary CNS Lymphoma Collaborative Group report. SAE Int. J. Engines 2010, 115, 5005–5011. [Google Scholar] [CrossRef]
- Stern, B.J.; Royal, W.; Gelfand, J.M.; Clifford, D.B.; Tavee, J.; Pawate, S.; Berger, J.R.; Aksamit, A.J.; Krumholz, A.; Pardo, C.A.; et al. Baughman, Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018, 75, 1546–1553. [Google Scholar] [CrossRef]
- Uppin, M.S.; Meena, A.; Hui, M.; Challa, S.; Kaul, S. Pure neuritic leprosy: Resolving diagnostic issues in acid fast bacilli (AFB)-negative nerve biopsies: A Single centre experience from South India. Ann. Indian Acad. Neurol. 2015, 18, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Suhr, O.B.; Hund, E.; Obici, L.; Tournev, I.; Campistol, J.M.; Slama, M.S.; Hazenberg, B.P.; Coelho, T. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr. Opin. Neurol. 2016, 29 (Suppl. S1), S14–S26. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.P.; Dyck, P.J.B.; Gronseth, G.S.; Guillevin, L.; Hadden, R.D.M.; Heuss, D.; Léger, J.; Notermans, N.; Pollard, J.D.; Said, G.; et al. Peripheral Nerve Society Guideline* on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: Executive summary. J. Peripher. Nerv. Syst. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- China, L.; Fernandez, A.; Lacroix, C.; Adams, D.; Plante, V.; Said, G. Contribution of never biopsy findings to the diagnosis of disabling neuropathy in the elderly: A retrospective review of 100 consecutive patients. Brain 1996, 119, 1091–1098. [Google Scholar] [CrossRef]
- Lubec, D.; Müllbacher, W.; Finsterer, J.; Mamoli, B. Diagnostic work-up in peripheral neuropathy: An analysis of 171 cases. Postgrad Med. J. 1999, 75, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Hanewinckel, R.; van Oijen, M.; Ikram, M.A.; van Doorn, P.A. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 2016, 31, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Pasnoor, M.; Dimachkie, M.M.; Barohn, R.J. Cryptogenic Sensory Polyneuropathy. Neurol. Clin. 2013, 31, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, K.G.; Tracy, J.A.; Dyck, P.J.B. Peripheral Nerve Vasculitis: Classification and Disease Associations. Neurol. Clin. 2019, 37, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, A.F.J.E.; Gathier, C.S.; Cats, E.A.; Notermans, N.C.; Collins, M.P. The additional yield of combined nerve/muscle biopsy in vasculitic neuropathy. Eur. J. Neurol. 2011, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Rohatgi, S.; Khan, F. Nonsystemic vasculitic neuropathy. Med. J. Dr. D. Y. Patil Vidyapeeth 2020, 13, 562. [Google Scholar] [CrossRef]
- Hui, M.; Meena, A.K.; Rajasekhar, L.; Sireesha, Y.; Afshan, J.; Mridula, R.; Borgohain, R.; Uppin, M.S. Vasculitic Neuropathy: A Retrospective Analysis of Nerve Biopsies and Clinical Features from a Single Tertiary Care Center. Ann. Indian Acad. Neurol. 2019, 22, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Misawa, S.; Arai, K.; Oide, T.; Shibuya, K.; Isose, S.; Sekiguchi, Y.; Nasu, S.; Mitsuma, S.; Kuwabara, S. Combined nerve/muscle/skin biopsy could increase diagnostic sensitivity for vasculitic neuropathy. Clin. Exp. Neuroimmunol. 2015, 6, 312–317. [Google Scholar] [CrossRef]
- Hadden, R.D.; Collins, M.P.; Živković, S.A.; Hsieh, S.-T.; Bonetto, C.; Felicetti, P.; Marchione, P.; Santuccio, C.; Bonhoeffer, J. Vasculitic peripheral neuropathy: Case definition and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine 2017, 35, 1567–1578. [Google Scholar] [CrossRef]
- Üçeyler, N.; Braunsdorf, S.; Kunze, E.; Riediger, N.; Scheytt, S.; Divisova, Š; Bekircan-Kurt, C.E.; Toyka, K.V.; Sommer, C. Cellular infiltrates in skin and sural nerve of patients with polyneuropathies. Muscle Nerve 2017, 55, 884–893. [Google Scholar] [CrossRef]
- Üçeyler, N.; Devigili, G.; Toyka, K.V.; Sommer, C. Skin biopsy as an additional diagnostic tool in non-systemic vasculitic neuropathy. Acta Neuropathol. 2010, 120, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Brandner, S.; Lammens, M.; Sommer, C.; Vallat, J.-M. Processing of nerve biopsies: A practical guide for neuropathologists. Clin. Neuropathol. 2012, 31, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, M.; Mathis, S.; Corcia, P.; Richard, L.; Ghorab, K.; Jaccard, A.; Magy, L.; Vallat, J.-M. Value of nerve biopsy in patients with latent malignant hemopathy and peripheral neuropathy: A case series. Medicine 2015, 94, e394. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zamora, A.; Morales-Vidal, S.; Chawla, J.; Biller, J. Autopsy Proven Peripheral Nervous System Neurolymphomatosis Despite Negative Bilateral Sural Nerve Biopsy. Front. Neurol. 2013, 4, 197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duchesne, M.; Roussellet, O.; Maisonobe, T.; Gachard, N.; Rizzo, D.; Armand, M.; Viala, K.; Richard, L.; Delage-Corre, M.; Jaccard, A.; et al. Pathology of Nerve Biopsy and Diagnostic Yield of PCR-Based Clonality Testing in Neurolymphomatosis. J. Neuropathol. Exp. Neurol. 2018, 77, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Shree, R.; Goyal, M.K.; Modi, M.; Gaspar, B.L.; Radotra, B.D.; Ahuja, C.K.; Mittal, B.R.; Prakash, G. The Diagnostic Dilemma of Neurolymphomatosis. J. Clin. Neurol. 2016, 12, 274–281. [Google Scholar] [CrossRef]
- Bishop, A.J.; Zagars, G.K.; Torres, K.E.; Bird, J.E.; Feig, B.W.; Guadagnolo, B.A. Malignant Peripheral Nerve Sheath Tumors A Single Institution’s Experience Using Combined Surgery and Radiation Therapy. Am. J. Clin. Oncol. 2018, 41, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kourea, H.P.; Bilsky, M.H.; Leung, D.H.; Lewis, J.J.; Woodruff, J.M. Woodruff, Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumors: A clinicopathologic study of 25 patients and 26 tumors. Cancer 1998, 82, 2191–2203. [Google Scholar] [CrossRef]
- Ducatman, B.S.; Scheithauer, B.W.; Piepgras, D.G.; Reiman, H.M.; Ilstrup, D.M. Ilstrup, Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986, 57, 2006–2021. [Google Scholar] [CrossRef]
- Zou, C.; Smith, K.D.; Liu, J.; Lahat, G.; Myers, S.B.; Wang, W.-L.; Zhang, W.; McCutcheon, I.E.; Slopis, J.M.; Lazar, A.J.; et al. Clinical, Pathological, and Molecular Variables Predictive of Malignant Peripheral Nerve Sheath Tumor Outcome. Ann. Surg. 2009, 249, 1014–1022. [Google Scholar] [CrossRef]
- Mauermann, M.L.; Amrami, K.K.; Kuntz, N.L.; Spinner, R.J.; Bosch, E.P.; Engelstad, J.; Felmlee, J.P.; Dyck, P.J.B. Longitudinal study of intraneural perineurioma—A benign, focal hypertrophic neuropathy of youth. Brain 2009, 132, 2265–2276. [Google Scholar] [CrossRef]
- Niederhauser, B.D.; Spinner, R.J.; Jentoft, M.E.; Everist, B.M.; Matsumoto, J.M.; Amrami, K.K. Neuromuscular choristoma: Characteristic magnetic resonance imaging findings and association with post-biopsy fibromatosis. Skelet. Radiol. 2013, 42, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Mauermann, M.L.; Scheithauer, B.W.; Spinner, R.J.; Amrami, K.K.; Nance, C.S.; Kline, D.G.; O’Connor, M.I.; Engelstad, J.; Dyck, P.J.B. Inflammatory pseudotumor of nerve: Clinicopathological characteristics and a potential therapy. J. Peripher. Nerv. Syst. 2010, 15, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Garbino, J.A.; Ura, S.; Belone, A.d.F.F.; Marciano, L.H.S.C.; Fleury, R.N. Clinical and diagnostic aspects of the primarily neural leprosy. Hansenol. Int. 2004, 29, 130–136. [Google Scholar] [CrossRef]
- Suneetha, S.; Arunthathi, S.; Kurian, N.; Chacko, C.J. Histological changes in the nerve, skin and nasal mucosa of patients with primary neuritic leprosy. Acta Leprol. 2001, 12, 11–18. [Google Scholar]
- DE Freitas, M.R.G.; Nascimento, O.J.; Drago, M.J.; DE Freitas, A.R.; Hahn, M.D. Ulnar nerve palsy in leprosy without skin changes: Biopsy of the superficial branch of the ulnar nerve in the hand. Arq. Neuro Psiquiatria 1998, 56, 585–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loavenbruck, A.J.; Singer, W.; Mauermann, M.L.; Sandroni, P.; Dyck, P.J.B.; Gertz, M.; Klein, C.J.; Low, P.A. Transthyretin amyloid neuropathy has earlier neural involvement but better prognosis than primary amyloid counterpart: An answer to the paradox? Ann. Neurol. 2016, 80, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Hashimoto, R.; Tomita, M.; Kawagashira, Y.; Iijima, M.; Tanaka, F.; Sobue, G. Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: A practical analysis. Amyloid 2011, 18, 53–62. [Google Scholar] [CrossRef]
- Vaxman, I.; Gertz, M. Recent Advances in the Diagnosis, Risk Stratification, and Management of Systemic Light-Chain Amyloidosis. Acta Haematol. 2019, 141, 93–106. [Google Scholar] [CrossRef]
- D’Sa, S.; Kersten, M.J.; Castillo, J.J.; Dimopoulos, M.; Kastritis, E.; Laane, E.; Leblond, V.; Merlini, G.; Treon, S.P.; Vos, J.M.; et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: Recommendations from the IWWM-8 consensus panel. Br. J. Haematol. 2017, 176, 728–742. [Google Scholar] [CrossRef]
- de Larrea, C.F.; Verga, L.; Morbini, P.; Klersy, C.; Lavatelli, F.; Foli, A.; Obici, L.; Milani, P.; Capello, G.L.; Paulli, M.; et al. A practical approach to the diagnosis of systemic amyloidoses. Blood 2015, 125, 2239–2244. [Google Scholar] [CrossRef]
- Said, G. Indications and usefulness of nerve biopsy. Arch. Neurol. 2002, 59, 1532–1535. [Google Scholar] [CrossRef]
- Mazzeo, A.; Russo, M.; Di Bella, G.; Minutoli, F.; Stancanelli, C.; Gentile, L.; Baldari, S.; Carerj, S.; Toscano, A.; Vita, G. Transthyretin-Related Familial Amyloid Polyneuropathy (TTR-FAP): A Single-Center Experience in Sicily, an Italian Endemic Area. J. Neuromuscul. Dis. 2015, 2, S39–S48. [Google Scholar] [CrossRef] [PubMed]
- Vital, C.; Vital, A.; Bouillot-Eimer, S.; Brechenmacher, C.; Ferrer, X.; Lagueny, A. Amyloid neuropathy: A retrospective study of 35 peripheral nerve biopsies. J. Peripher. Nerv. Syst. 2004, 9, 232–241. [Google Scholar] [CrossRef]
- Burns, T.; Dyck, P.; Aksamit, A. The natural history and long-term outcome of 57 limb sarcoidosis neuropathy cases. J. Neurol. Sci. 2006, 244, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, R.T.; Wilkins, A.; Scolding, N.J. Neurosarcoidosis: A clinical approach to diagnosis and management. J. Neurol. 2017, 264, 1023–1028. [Google Scholar] [CrossRef]
- Said, G.; Lacroix, C.; Planté-Bordeneuve, V.; Le Page, L.; Pico, F.; Presles, O.; Senant, J.; Remy, P.; Rondepierre, P.; Mallecourt, J. Nerve granulomas and vasculitis in sarcoid peripheral neuropathy. A clinicopathological study of 11 patients. Brain 2002, 125, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Lacomis, D. Neurosarcoidosis. Curr. Neuropharmacol. 2011, 9, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Zen, Y.; Sato, Y.; Abo, H.; Demachi, H.; Uchiyama, A.; Gabata, T.; Matsui, O. IgG4-Related Perineural Disease. Int. J. Rheumatol. 2012, 2012, 401890. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shiraishi, M.; Yamada, K.; Doi, M.; Kato, M.; Hasegawa, Y. A case of refractory IgG4-related peripheral neuropathy with severe axonal damage. Rinsho Shinkeigaku 2016, 56, 323–327. [Google Scholar] [CrossRef]
- Waheed, W.; Nickerson, J.; Ambaye, A.B.; Babi, M.-A.; Tandan, R. IgG4-Related Neuromyopathy Associated With Recurrent Pleural Effusion. J. Clin. Neuromuscul. Dis. 2015, 16, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, S.; Kawagashira, Y.; Ohyama, K.; Iijima, M.; Koike, H.; Watanabe, H.; Tatematsu, A.; Nakamura, S.; Sobue, G. Mononeuritis multiplex with tumefactive cellular infiltration in a patient with reactive lymphoid hyperplasia with increased immunoglobulin G4–positive cells. Hum. Pathol. 2014, 45, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Fan, M.; Cai, N.; Cai, B. Combination of autoimmune pancreatitis and peripheral neuropathy on an IgG4-related disease patient with 4 years following-up. J. Neuroimmunol. 2020, 348, 577378. [Google Scholar] [CrossRef] [PubMed]
- Baptista, B.; Casian, A.; Gunawardena, H.; D’Cruz, D.; Rice, C.M. Neurological Manifestations of IgG4-Related Disease. Curr. Treat. Options Neurol. 2017, 19, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Mahendraker, N.; Ramphul, K. Paraneoplastic Syndromes; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pelosof, L.C.; Gerber, D.E. Paraneoplastic Syndromes: An Approach to Diagnosis and Treatment. Mayo Clin. Proc. 2010, 85, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Gultekin, H.S.; Posner, J.B. Paraneoplastic Neurologic Syndromes: Pathogenesis and Physiopathology. Brain Pathol. 1999, 9, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Warriach, S.A. Rare case: Paraneoplastic syndrome affecting peripheral nerves, associated with anti-collapsin-response mediator protein-5 (anti-CRMP5) antibodies, as early manifestation of small cell lung cancer confined to a solitary lymph node without evidence of lung mass on routine CT thorax. BMJ Case Rep. 2020, 13, e232656. [Google Scholar] [CrossRef] [PubMed]
- Vallat, J.-M.; Sommer, C.; Magy, L. Chronic inflammatory demyelinating polyradiculoneuropathy: Diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010, 9, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, S.; Misawa, S. Chronic Inflammatory Demyelinating Polyneuropathy. Adv. Exp. Med. Biol. 2019, 1190, 333–343. [Google Scholar] [CrossRef]
- Viala, K.; Maisonobe, T.; Stojkovic, T.; Koutlidis, R.; Ayrignac, X.; Musset, L.; Fournier, E.; Léger, J.; Bouche, P. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J. Peripher. Nerv. Syst. 2010, 15, 50–56. [Google Scholar] [CrossRef]
- Rajabally, Y.A.; Stettner, M.; Kieseier, B.C.; Hartung, H.-P.; Malik, R.A. CIDP and other inflammatory neuropathies in diabetes—Diagnosis and management. Nat. Rev. Neurol. 2017, 13, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline* on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First revision. J. Peripher. Nerv. Syst. 2010, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Nemni, R.; Corbo, M.; Fazio, R.; Quattrini, A.; Comi, G.; Canal, N. Cryoglobulinaemic neuropathy. A clinical, morphological and immunocytochemical study of 8 cases. Brain 1988, 111 Pt 3, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ciompi, M.; Marini, D.; Siciliano, G.; Melchiorre, D.; Bazzichi, L.; Sartucci, F.; Murri, L. Cryoglobulinemic peripheral neuropathy: Neurophysiologic evaluation in twenty-two patients. Biomed. Pharmacother. 1996, 50, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Cerri, F.; Falzone, Y.M.; Riva, N.; Quattrini, A. An update on the diagnosis and management of the polyneuropathy of POEMS syndrome. J. Neurol. 2019, 266, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Vallat, J.; Magy, L.; Richard, L.; Sturtz, F.; Couratier, P. Contribution of electron microscopy to the study of neuropathies associated with an IgG monoclonal paraproteinemia. Micron 2008, 39, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Vital, C.; Vital, A.; Ferrer, X.; Viallard, J.; Pellegrin, J.; Bouillot, S.; Lequen, L.; Larrieu, J.; Brechenmacher, C.; Petry, K.G.; et al. Crow–Fukase (POEMS) syndrome: A study of peripheral nerve biopsy in five new cases. J. Peripher. Nerv. Syst. 2003, 8, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Vallat, J.-M.; Magy, L.; Sindou, P.; Magdelaine, C.; Cros, D. IgG Neuropathy: An Immunoelectron Microscopic Study. J. Neuropathol. Exp. Neurol. 2005, 64, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Vallat, J.M.; Tabaraud, F.; Sindou, P.; Preux, P.M.; Vandenberghe, A.; Steck, A. Myelin widenings and MGUS-IgA: An immunoe-lectron microscopic study. Ann. Neurol. 2000, 47, 808–811. [Google Scholar] [CrossRef]
- Ellie, E.; Vital, A.; Steck, A.; Boiron, J.-M.; Vital, C.; Julien, J. Neuropathy associated with “benign” anti-myelin-associated glycoprotein IgM gammopathy: Clinical, immunological, neurophysiological pathological findings and response to treatment in 33 cases. J. Neurol. 1996, 243, 34–43. [Google Scholar] [CrossRef]
- van de Mortel, J.P.M.; D’sa, S.; Vrancken, A.F.J.E.; Notermans, N.C.; Vos, J.M.I.; Minnema, M.C. Polyneuropathy Associated with IgM Monoclonal Gammopathy; Advances in Genetics and Treatment, Focusing on Anti-MAG Antibodies. Hemato 2022, 3, 663–688. [Google Scholar] [CrossRef]
- Magy, L.; Kaboré, R.; Mathis, S.; Lebeau, P.; Ghorab, K.; Caudie, C.; Vallat, J.-M. Heterogeneity of Polyneuropathy Associated with Anti-MAG Antibodies. J. Immunol. Res. 2015, 2015, 450391. [Google Scholar] [CrossRef] [PubMed]
- Bleasel, A.F.; Hawke, S.H.; Pollard, J.D.; McLeod, J.G. IgG monoclonal paraproteinaemia and peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry 1993, 56, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kawagashira, Y.; Koike, H.; Tomita, M.; Morozumi, S.; Iijima, M.; Nakamura, T.; Katsuno, M.; Tanaka, F.; Sobue, G. Morphological Progression of Myelin Abnormalities in IgM-Monoclonal Gammopathy of Undetermined Significance Anti-Myelin-Associated Glycoprotein Neuropathy. J. Neuropathol. Exp. Neurol. 2010, 69, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Mochel, F.; Schiffmann, R.; Steenweg, M.E.; Akman, H.O.; Wallace, M.; Sedel, F.; Laforêt, P.; Levy, R.; Powers, J.M.; Demeret, S.; et al. Adult polyglucosan body disease: Natural History and Key Magnetic Resonance Imaging Findings. Ann. Neurol. 2012, 72, 433–441. [Google Scholar] [CrossRef]
- Klein, C.J.; Boes, C.J.; Chapin, J.E.; Lynch, C.D.; Campeau, N.G.; Dyck, P.J.B. Adult polyglucosan body disease: Case description of an expanding genetic and clinical syndrome. Muscle Nerve 2004, 29, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pinto, M.; Sun, C.; Engelstad, J.K.; Dyck, P.J.; Klein, C.J. Expanded teased nerve fibre pathological conditions in disease association. J. Neurol. Neurosurg. Psychiatry 2019, 90, 138–140. [Google Scholar] [CrossRef]
- Mrak, R.E. The Big Eye in the 21st Century: The Role of Electron Microscopy in Modern Diagnostic Neuropathology. J. Neuropathol. Exp. Neurol. 2002, 61, 1027–1039. [Google Scholar] [CrossRef]
- Riva, N.; Iannaccone, S.; Corbo, M.; Casellato, C.; Sferrazza, B.; Lazzerini, A.; Scarlato, M.; Cerri, F.; Previtali, S.C.; Nobile-Orazio, E.; et al. Motor nerve biopsy: Clinical usefulness and histopathological criteria. Ann. Neurol. 2011, 69, 197–201. [Google Scholar] [CrossRef]
- Lozeron, P.; Nahum, L.; Lacroix, C.; Ropert, A.; Guglielmi, J.-M.; Said, G. Symptomatic diabetic and non-diabetic neuropathies in a series of 100 diabetic patients. J. Neurol. 2002, 249, 569–575. [Google Scholar] [CrossRef]
- Rappaport, W.D.; Valente, J.; Hunter, G.C.; Rance, N.E.; Lick, S.; Lewis, T.; Neal, D. Clinical utilization and complications of sural nerve biopsy. Am. J. Surg. 1993, 166, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Deprez, M.; Groote, C.C.-D.; Gollogly, L.; Reznik, M.; Martin, J. Clinical and neuropathological parameters affecting the diagnostic yield of nerve biopsy. Neuromuscul. Disord. 2000, 10, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, M.; Mathis, S.; Richard, L.; Magdelaine, C.; Corcia, P.; Nouioua, S.; Tazir, M.; Magy, L.; Vallat, J.-M. Nerve Biopsy Is Still Useful in Some Inherited Neuropathies. J. Neuropathol. Exp. Neurol. 2018, 77, 88–99. [Google Scholar] [CrossRef]
- England, J.; Gronseth, G.; Franklin, G.; Carter, G.; Kinsella, L.; Cohen, J.; Asbury, A.; Szigeti, K.; Lupski, J.; Latov, N.; et al. Practice Parameter: The Evaluation of Distal Symmetric Polyneuropathy: The Role of Laboratory and Genetic Testing (An Evidence-Based Review). PM&R 2009, 1, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Chahin, N.; Temesgen, Z.; Kurtin, P.J.; Spinner, R.J.; Dyck, P.J.B. HIV lumbosacral radiculoplexus neuropathy mimicking lymphoma: Diffuse infiltrative lymphocytosis syndrome (DILS) restricted to nerve? Muscle Nerve 2010, 41, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; Ewing-Wilson, D.; Chou, S.M.; Mitsumoto, H.; Hanson, M.; Shirey, E.; Ratliff, N.B. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am. J. Med. 1987, 82, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, J.; Pouget, J.; Cros, D.; De Victor, B.; Serratrice, G.; Toga, M. Peripheral neuropathy induced by amiodarone chlorhydrate: A clinicopathological study. J. Neurol. Sci. 1984, 63, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Little, A.A.; Albers, J.W. Clinical description of toxic neuropathies. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Weis, S.; Büttner, A. Neurotoxicology and drug-related disorders. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Katona, I.; Weis, J. Diseases of the peripheral nerves. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Ubogu, E.E. Inflammatory neuropathies: Pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol. 2015, 130, 445–468. [Google Scholar] [CrossRef]
- Querol, L.; Devaux, J.; Rojas-Garcia, R.; Illa, I. Autoantibodies in chronic inflammatory neuropathies: Diagnostic and therapeutic implications. Nat. Rev. Neurol. 2017, 13, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Rutchik, J.S. Occupational Medicine Physician’s Guide to Neuropathy in the Workplace, Part 2: Electromyography and Cryptogenic and Toxic Neuropathy. J. Occup. Environ. Med. 2009, 51, 622–625. [Google Scholar] [CrossRef]
- Walters, J.; Baborie, A. Muscle biopsy: What and why and when? Pract. Neurol. 2020, 20, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Meola, G.; Bugiardini, E.; Cardani, R. Muscle biopsy. J. Neurol. 2012, 259, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; Alba, M.A.; Prieto-González, S.; Cid, M.C. Diagnosis and classification of polyarteritis nodosa. J. Autoimmun. 2014, 48–49, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; Hoffman, G.S. Updating single-organ vasculitis. Curr. Opin. Rheumatol. 2012, 24, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C.; Kiriaev, L.; Kueh, S.; Morley, J.W.; North, K.N.; Houweling, P.J.; Head, S.I.; Barker, R.G.; et al. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2015, 96, 253–305. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V.; Pavlakis, S. Duchenne Muscular Dystrophy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Thada, P.K.; Bhandari, J.; Umapathi, K.K. Becker Muscular Dystrophy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Carlson, C.R.; Moore, S.A.; Mathews, K.D. Dystrophinopathy muscle biopsies in the genetic testing ERA: One center’s data. Muscle Nerve 2018, 58, 149–153. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology, 10th ed.; Elsevier—Health Sciences Division: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Bruschi, F.; Brunetti, E.; Pozio, E. Neurotrichinellosis. Handb. Clin. Neurol. 2013, 114, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, S.; Vujosević, M.; Sasić, M.; Poluga, J.; Misić, S.; Najdanović, L.; Dulović, O.; Dragojlović, J.; Milosević, B. Neurologic man-ifestations in trichinosis. Srp. Arh. Celok. Lek. 1998, 126, 209–213. [Google Scholar]
- Goel, P.; Gerriets, V. Chloroquine; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pasnoor, M.; Barohn, R.J.; Dimachkie, M.M. Toxic Myopathies. Neurol. Clin. 2014, 32, 647–670. [Google Scholar] [CrossRef]
- Casado, E.; Gratacós, J.; Tolosa, C.; Martínez, J.M.; Ojanguren, I.; Ariza, A.; Real, J.; Sanjuán, A.; Larrosa, M. Antimalarial myopathy: An underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann. Rheum. Dis. 2006, 65, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mastaglia, F.; Papadimitriou, J.; Dawkins, R.; Beveridge, B. Vacuolar myopathy associated with chloroquine, lupus erythematosus and thymoma: Report of a case with unusual mitochondrial changes and lipid accumulation in muscle. J. Neurol. Sci. 1977, 34, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Florek, J.B.; Lucas, A.; Girzadas, D. Amiodarone; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Feeney, E.J.; Austin, S.; Chien, Y.-H.; Mandel, H.; Schoser, B.; Prater, S.; Hwu, W.-L.; Ralston, E.; Kishnani, P.S.; Raben, N. The value of muscle biopsies in Pompe disease: Identifying lipofuscin inclusions in juvenile- and adult-onset patients. Acta Neuropathol. Commun. 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Anilkumar, A.C. Glycogen Storage Disease Type II; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Leslie, N.; Bailey, L. Pompe Disease; StatPearls Publishing: Treasure Island, FL, USA, 1993. [Google Scholar]
- Chhabra, A.; Madhuranthakam, A.J.; Andreisek, G. Magnetic resonance neurography: Current perspectives and literature review. Eur. Radiol. 2018, 28, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Ku, V.; Cox, C.; Mikeska, A.; MacKay, B. Magnetic Resonance Neurography for Evaluation of Peripheral Nerves. J. Brachial Plex. Peripher. Nerve Inj. 2021, 16, E17–E23. [Google Scholar] [CrossRef] [PubMed]
- Loizides, A.; Gruber, L.; Peer, S.; Plaikner, M.; Gruber, H. Ultraschallgesteuerte Interventionen am peripheren Nervensystem. Der Radiol. 2017, 57, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, J.A. Ultrasound-Guided Peripheral Nerve Procedures. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 687–715. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Goindi, A.S.; Gupta, K. Reference values for the cross-sectional area of normal radial nerve at two levels using high-resolution ultrasonography. J. Ultrason. 2021, 21, e112–e126. [Google Scholar] [CrossRef] [PubMed]
- Pianta, M.; Chock, E.; Schlicht, S.; McCombe, D. Accuracy and complications of CT-guided core needle biopsy of peripheral nerve sheath tumours. Skelet. Radiol. 2015, 44, 1341–1349. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.G.; Du, K.P.; Li, S.; Li, Y.D.; Gao, F.; Gao, M.Y.; Gao, J.B. Analysis of the safety and diagnostic efficiency of CT-guided percutaneous biopsy of pancreatic space-occupying lesions using large needle: Comparison of trans-organ biopsy approach and non-trans-organ biopsy approach. Zhonghua Yi Xue Za Zhi 2023, 103, 364–369. [Google Scholar] [CrossRef]
- Holzgrefe, R.E.; Wagner, E.R.; Singer, A.D.; Daly, C.A. Imaging of the Peripheral Nerve: Concepts and Future Direction of Magnetic Resonance Neurography and Ultrasound. J. Hand Surg. 2019, 44, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Gibbs, S.L. Improving precision surgery: A review of current intraoperative nerve tissue fluorescence imaging. Curr. Opin. Chem. Biol. 2023, 76, 102361. [Google Scholar] [CrossRef] [PubMed]
- Chlebicki, C.A.; Lee, A.D.; Jung, W.; Li, H.; Liaw, L.; Chen, Z.; Wong, B.J. Preliminary investigation on use of high-resolution optical coherence tomography to monitor injury and repair in the rat sciatic nerve. Lasers Surg. Med. 2010, 42, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, J.; Jeon, M.; Kim, J. In Vivo Fascicle Bifurcation Imaging of Rat Sciatic Nerve Using Swept-Source Optical Coherence Tomography. IEEE Access 2018, 6, 7713–7718. [Google Scholar] [CrossRef]
- Vasudevan, S.; Vo, J.; Shafer, B.; Nam, A.S.; Vakoc, B.J.; Hammer, D.X. Toward optical coherence tomography angiography-based biomarkers to assess the safety of peripheral nerve electrostimulation. J. Neural Eng. 2019, 16, 036024. [Google Scholar] [CrossRef] [PubMed]
- Monroy, G.L.; Erfanzadeh, M.; Tao, M.A.; DePaoli, D.T.; Saytashev, I.; Nam, S.A.; Rafi, H.; Kwong, K.C.; Shea, K.; Vakoc, B.J.; et al. Development of polarization-sensitive optical coherence tomography imaging platform and metrics to quantify electrostimulation-induced peripheral nerve injury in vivo in a small animal model. Neurophotonics 2023, 10, 025004. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.; Goodwin, M.; Vanholsbeeck, F. Optical coherence tomography imaging of evoked neural activity in sciatic nerve of rat. J. Phys. D Appl. Phys. 2021, 54, 334002. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sasoh, M.; Ido, M.; Narushima, C.; Uji, Y. Retinal Nerve Fiber Layer Decrease during Glycemic Control in Type 2 Diabetes. J. Ophthalmol. 2010, 2010, 569215. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, S.; Can, E.; Öztürk, F. Comparison of the corneal biomechanical properties, optic nerve head topographic parameters, and retinal nerve fiber layer thickness measurements in diabetic and non-diabetic primary open-angle glaucoma. Int. Ophthalmol. 2016, 36, 727–736. [Google Scholar] [CrossRef]
- Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Bitirgen, G.; Ferdousi, M.; Kalteniece, A.; Azmi, S.; D’Onofrio, L.; Lim, S.H.; Ponirakis, G.; Khan, A.; Gad, H.; et al. Corneal Confocal Microscopy to Image Small Nerve Fiber Degeneration: Ophthalmology Meets Neurology. Front. Pain Res. 2021, 2, 725363. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, X.; Wang, X.; Pan, Q.; Xian, T.; Yu, X.; Guo, L. In Vivo Corneal Confocal Microscopy Detects Improvement of Corneal Nerve Parameters following Glycemic Control in Patients with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 8516276. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Frank, B.; Marshall, A.; Khalil, R.S.; Ponirakis, G.; Petropoulos, I.N.; Cuthbertson, D.J.; Malik, R.A.; Alam, U. Early Detection of Diabetic Peripheral Neuropathy: A Focus on Small Nerve Fibres. Diagnostics 2021, 11, 165. [Google Scholar] [CrossRef]
- Che, N.-N.; Yang, H.-Q. Potential use of corneal confocal microscopy in the diagnosis of Parkinson’s disease associated neuropathy. Transl. Neurodegener. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Creigh, P.D.; Mountain, J.; Sowden, J.E.; Eichinger, K.; Ravina, B.; Larkindale, J.; Herrmann, D.N. Measuring peripheral nerve involvement in Friedreich’s ataxia. Ann. Clin. Transl. Neurol. 2019, 6, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Fan, D.S.; Zhang, S.; Liu, Z.Y. Corneal confocal microscopy detects small-fiber neuropathy in patients with amyo-trophic lateral sclerosis. Zhonghua Nei Ke Za Zhi 2022, 61, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Franca, P.D.d.S.; Jiang, Y.; Pirovano, G.; Kossatz, S.; Guru, N.; Yarilin, D.; Agwa, A.J.; Schroeder, C.I.; Patel, S.G.; et al. Fluorescence Imaging of Peripheral Nerves by a Nav1.7-Targeted Inhibitor Cystine Knot Peptide. Bioconjugate Chem. 2019, 30, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gil, J.; Chow, C.Y.; Chatras, H.; França, P.D.d.S.; Samuels, Z.V.; Cornejo, M.; King, G.F.; Lewis, J.S.; Reiner, T.; Gonzales, J. Development and Validation of Nerve-Targeted Bacteriochlorin Sensors. J. Am. Chem. Soc. 2023, 145, 14276–14287. [Google Scholar] [CrossRef]
- Wei, B.; Su, H.; Chen, P.; Tan, H.-L.; Li, N.; Qin, Z.-E.; Huang, P.; Chang, S. Recent advancements in peripheral nerve-specific fluorescent compounds. Biomater. Sci. 2021, 9, 7799–7810. [Google Scholar] [CrossRef]
- Agadi, J.; Raghav, G.; Mahadevan, A.; Shankar, S. Usefulness of superficial peroneal nerve/peroneus brevis muscle biopsy in the diagnosis of vasculitic neuropathy. J. Clin. Neurosci. 2012, 19, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Marek, T.; Howe, B.M.; Amrami, K.K.; Spinner, R.J. From targeted fascicular biopsy of major nerve to targeted cutaneous nerve biopsy: Implementing clinical anatomy can catalyze a paradigm shift. Clin. Anat. 2018, 31, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Capek, S.; Amrami, K.K.; Dyck, P.J.B.; Spinner, R.J. Targeted fascicular biopsy of the sciatic nerve and its major branches: Rationale and operative technique. Neurosurg. Focus 2015, 39, E12. [Google Scholar] [CrossRef]
- Collins, M.; Mendell, J.; Periquet, M.; Sahenk, Z.; Amato, A.; Gronseth, G.; Barohn, R.; Jackson, C.; Kissel, J. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology 2000, 55, 636–643. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. RadioGraphics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Bedewi, M.A.; Kotb, M.A.; Aldossary, N.M.; Abodonya, A.M.; Alhariqi, B.A.; Swify, S.M. Shear wave elastography of the ulnar nerve at the forearm. Medicine 2021, 100, E24071. [Google Scholar] [CrossRef] [PubMed]
- A Bedewi, M.; A Kotb, M.; Aldossary, N.M.; Abodonya, A.M.; Saleh, A.K.; Swify, S.M. Shear wave elastography of the radial nerve in healthy subjects. J. Int. Med Res. 2021, 49, 0300060520987938. [Google Scholar] [CrossRef] [PubMed]
- Schrier, V.J.; Lin, J.; Gregory, A.; Thoreson, A.R.; Alizad, A.; Amadio, P.C.; Fatemi, M. Shear wave elastography of the median nerve: A mechanical study. Muscle Nerve 2020, 61, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.; Raffoul, W.; Christen, T.; Pedrazzi, N. Post-Operative Assessment of Ulnar Nerve Tension Using Shear-Wave Elastography. Neurol. Int. 2021, 13, 469–476. [Google Scholar] [CrossRef]
- Lauria, G.; Hsieh, S.T.; Johansson, O.; Kennedy, W.R.; Leger, J.M.; Mellgren, S.I.; Nolano, M.; Merkies, I.S.J.; Polydefkis, M.; Smith, A.G.; et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Fe-deration of Neurological Societies and the Peripheral Nerve Society. Eur. J. Neurol. 2010, 17, 903-e49. [Google Scholar] [CrossRef]
- Hovaguimian, A.; Gibbons, C.H. Diagnosis and Treatment of Pain in Small-fiber Neuropathy. Curr. Pain Headache Rep. 2011, 15, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Bonaparte, S.; Segovia, S.; Llauger, J.; Belmonte, I.; Pedrosa, I.; Alejaldre, A.; Mayos, M.; Suárez-Cuartín, G.; Gallardo, E.; Illa, I.; et al. Muscle MRI Findings in Childhood/Adult Onset Pompe Disease Correlate with Muscle Function. PLoS ONE 2016, 11, e0163493. [Google Scholar] [CrossRef] [PubMed]
- Raithatha, A.; Ashraghi, M.R.; Lord, C.; Limback-Stanic, C.; Viegas, S.; Amiras, D. Ultrasound-guided muscle biopsy: A practical alternative for investigation of myopathy. Skelet. Radiol. 2020, 49, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
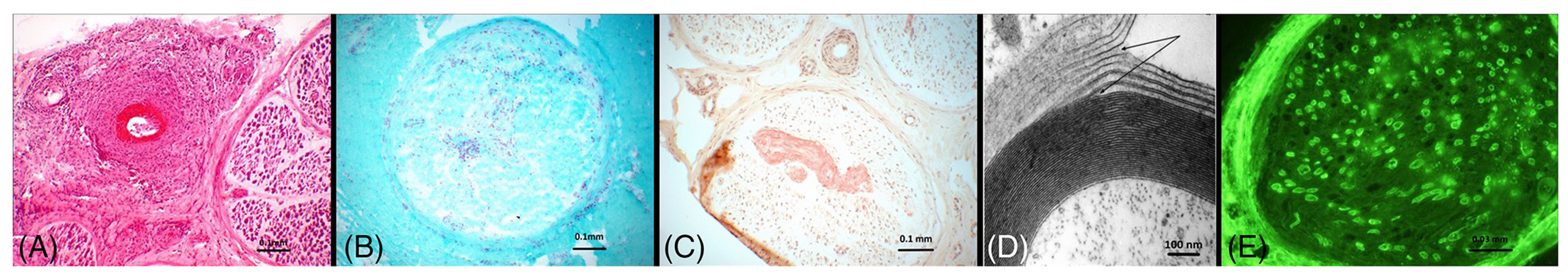
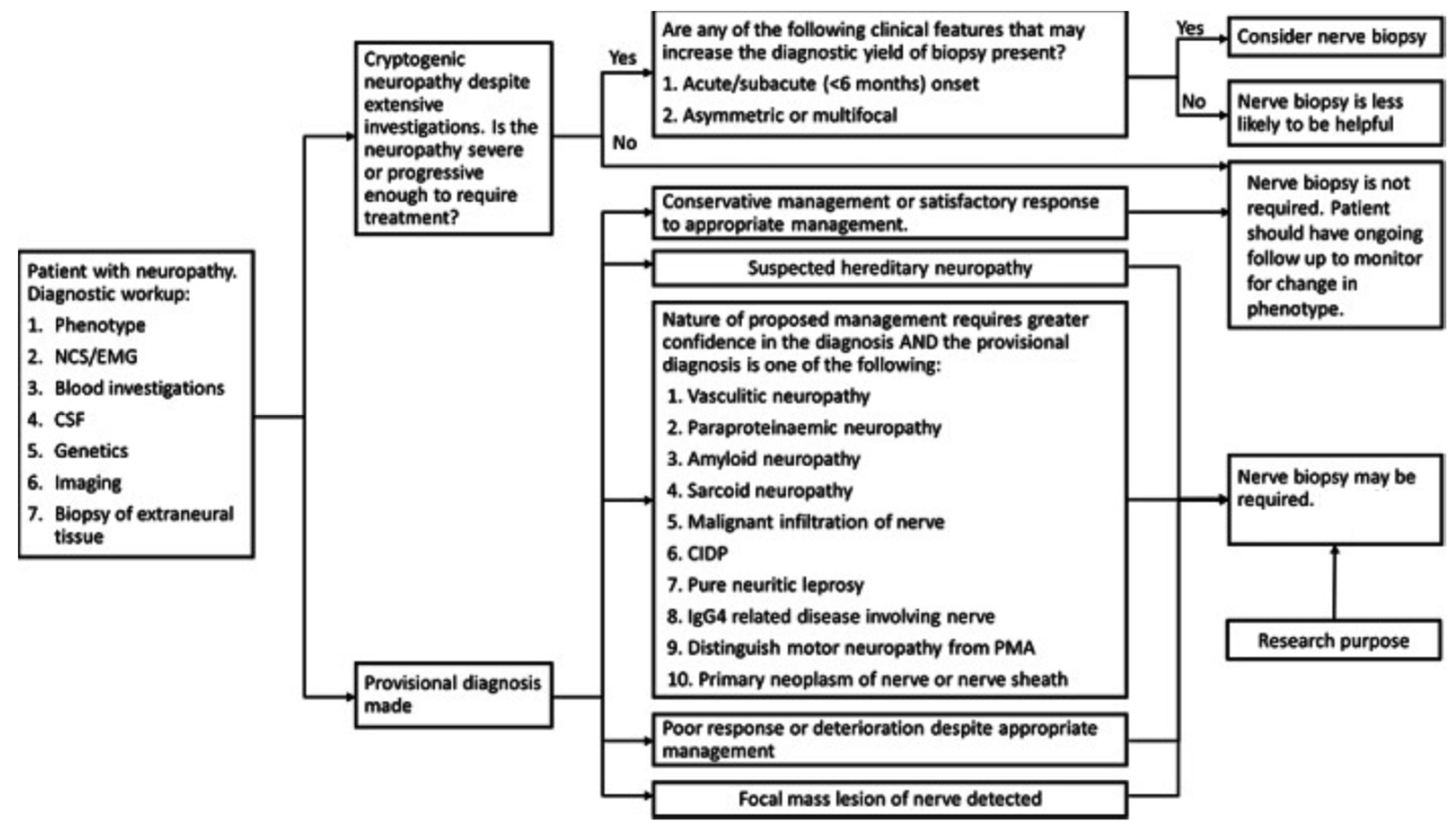
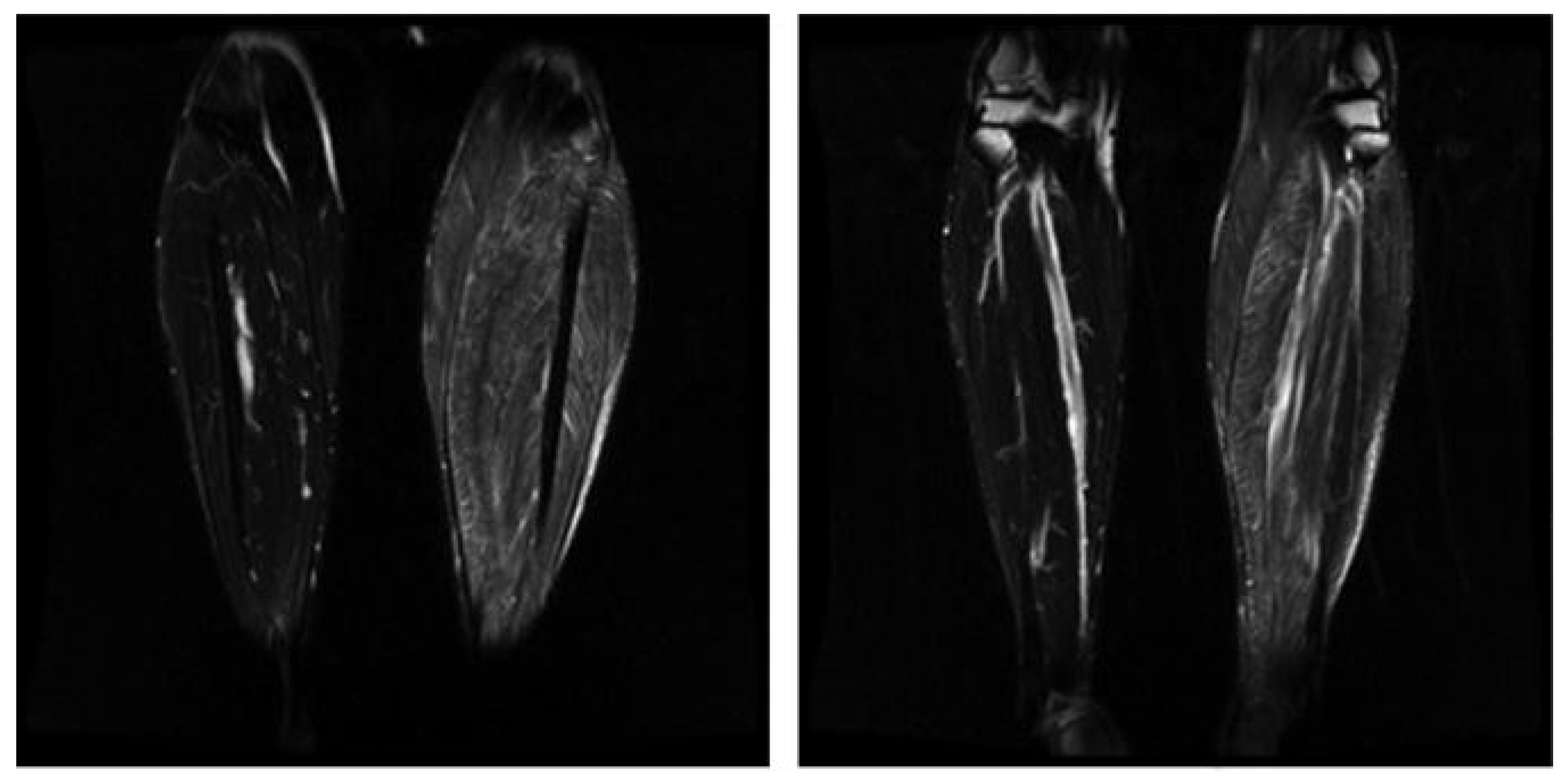
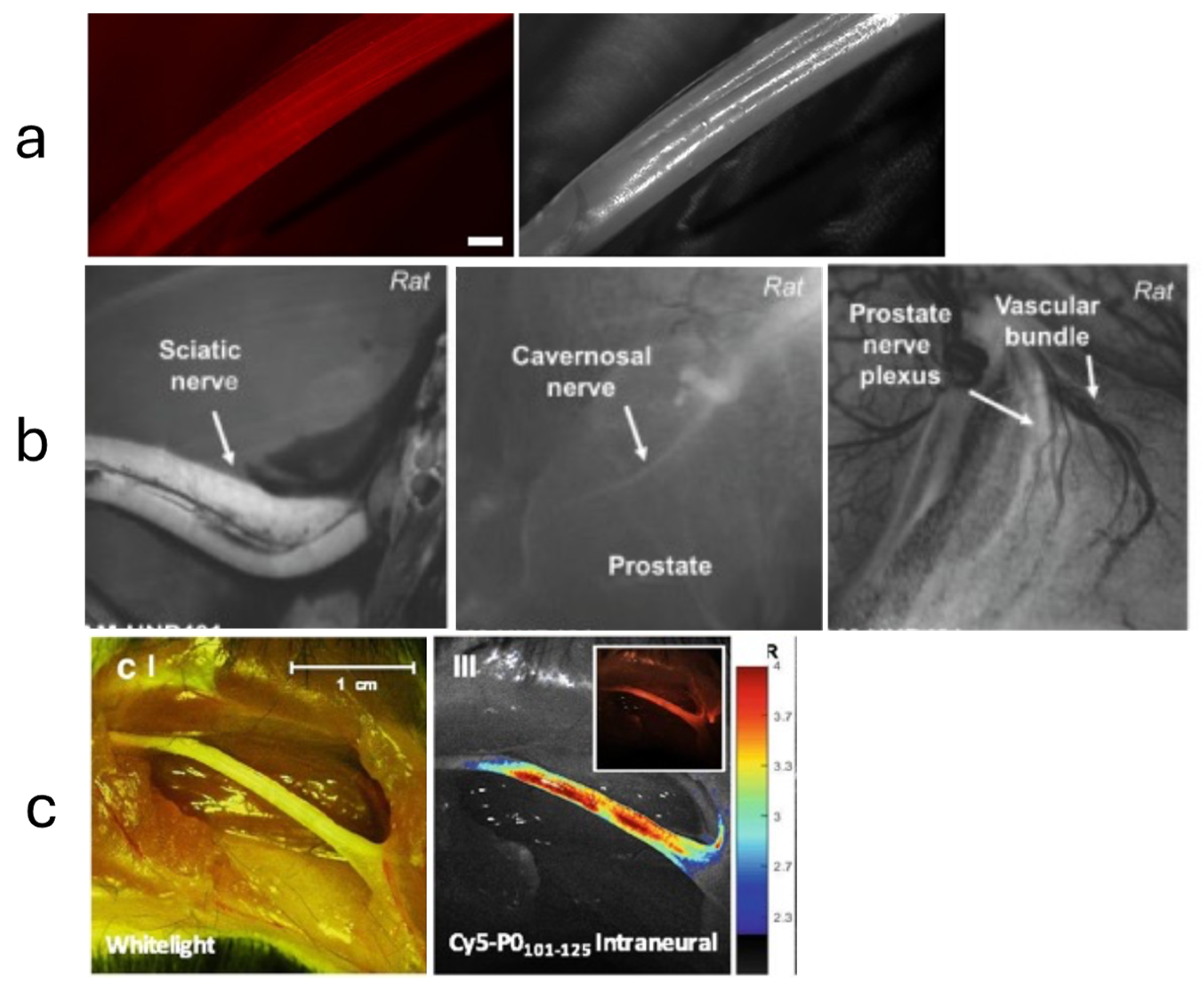
| High | Medium | Low |
|---|---|---|
| -Vasculitic Neuropathy | -Amyloidosis | -Chronic Inflammatory Demyelinating Polyneuropathy |
| -Neurolymphomatosis | -Neurosarcoidosis | -Paraproteinameic Neuropathy |
| -Peripheral Nerve Tumors | -Adult Polyglucosan Body Disease | |
| -Pseudoneoplastic Peripheral Nerve Tumors | -IgG4-related Perineural Disease | -Lysosomal and Peroxisomal Storage Disorders |
| -Pure Motor Neuropathy | ||
| -Neuritic Leprosy | -Paraneoplastic Syndromes | -Diabetic Neuropathy |
| -Cryptogenic Neuropathy | ||
| -Hereditary Neuropathy | ||
| -Other Neuropathies |
| Suspected Diagnosis | Case Where Biopsy Is Indicated |
|---|---|
| High Importance | |
| Vasculitic Neuropathy | Lack of evidence for extraneural vasculitis or progressive symptoms despite treatment |
| Neurolymphomatosis | Primary neurolymphomatosis or secondary neurolymphomatosis in cases of diagnostic ambiguity |
| Peripheral Nerve Tumors | Atypical benign tumors |
| Pseudoneoplastic Peripheral Nerve Tumors | Exclusion of malignancy |
| Neuritic Leprosy | Almost all cases for definitive diagnosis |
| Medium Importance | |
| Amyloidosis | Other tissue biopsy not possible or demonstrates negative results |
| Neurosarcoidosis | Negative results following extraneural biopsy or absence of extraneural symptoms |
| IgG4-Related Perineural Disease | Majority of patients, specifically in cases of atypical presentation or lack of extraneural evidence |
| Paraneoplastic Syndromes | In the setting of unclear etiologies of peripheral neuropathies |
| Low Importance | |
| Chronic Inflammatory Demyelinating Polyneuropathy | Lack of response to treatment or atypical presentation |
| Paraproteinameic Neuropathy | Suspected diagnosis of vasculitic or amyloid neuropathy, or infiltrative malignancy |
| Adult Polyglucosan Body Disease | Suspected diagnosis following inconclusive enzyme and genetic testing |
| Lysosomal and Peroxisomal Storage Disorders | Atypical presentation or suspected diagnosis following inconclusive testing |
| Pure Motor Neuropathy | Inability to determine etiology of neuropathy between motor neuron disease and motor neuropathy |
| Diabetic Neuropathy | Atypical presentation or suspected superimposed etiology |
| Cryptogenic Neuropathy | Suspected diagnosis following inconclusive testing |
| Hereditary Neuropathy | Atypical presentation or suspected diagnosis following inconclusive testing |
| Other Neuropathies | Atypical presentation or suspected diagnosis following inconclusive testing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.A.; Caussat, T.; Mouhawasse, E.; Ali, R.; Johansen, P.M.; Lucke-Wold, B. Neurosurgical Intervention for Nerve and Muscle Biopsies. Diagnostics 2024, 14, 1169. https://doi.org/10.3390/diagnostics14111169
Mohamed AA, Caussat T, Mouhawasse E, Ali R, Johansen PM, Lucke-Wold B. Neurosurgical Intervention for Nerve and Muscle Biopsies. Diagnostics. 2024; 14(11):1169. https://doi.org/10.3390/diagnostics14111169
Chicago/Turabian StyleMohamed, Ali A., Thomas Caussat, Edwin Mouhawasse, Rifa Ali, Phillip M. Johansen, and Brandon Lucke-Wold. 2024. "Neurosurgical Intervention for Nerve and Muscle Biopsies" Diagnostics 14, no. 11: 1169. https://doi.org/10.3390/diagnostics14111169
APA StyleMohamed, A. A., Caussat, T., Mouhawasse, E., Ali, R., Johansen, P. M., & Lucke-Wold, B. (2024). Neurosurgical Intervention for Nerve and Muscle Biopsies. Diagnostics, 14(11), 1169. https://doi.org/10.3390/diagnostics14111169










