Abstract
Introduction: The Enterococcus genus is a common cause of nosocomial infections, with vancomycin-resistant enterococci (VRE) posing a significant treatment challenge. Method: This retrospective study, spanning ten years (2012 to 2021), analyzes antimicrobial susceptibility patterns of Enterococcus species from clinical samples in a Saudi Arabian tertiary care hospital. Result: A total of 1034 Enterococcus isolates were collected, 729 from general wards and 305 from intensive care unit (ICU) patients. VRE accounted for 15.9% of isolates. E. faecalis was the most common species (54.3% of isolates and 2.7% of VRE), followed by E. faecium (33.6% of isolates and 41.2% of VRE). E. faecium exhibited the highest resistance to ciprofloxacin (84.1%), ampicillin (81.6%), and rifampicin (80%), with daptomycin (0.6%) and linezolid (3.1%) showing the lowest resistance. In E. faecalis, ciprofloxacin resistance was highest (59.7%), followed by rifampicin (20.1%) and ampicillin (11.8%). Daptomycin (0%), linezolid (1.5%), and vancomycin (2.7%) had the lowest resistance. VRE cases had higher mortality rates compared to vancomycin-sensitive enterococci (VSE). Conclusion: Eight different strains of Enterocci were identified. E. faecalis was the most commonly identified strain, while E. faecium had the highest percentage of VRE. VRE cases had a significantly higher mortality rate than VSE cases.
1. Introduction
Enterococci are gram-positive bacteria typically found in the human gut in short chains and pairs. They are of great concern worldwide due to their nosocomial nature and emerging drug resistance patterns [1,2]. Several Enterococcus species have been identified; among them, Enterococcus faecalis (E. faecalis) is the most commonly isolated species, accounting for 80–90% of nosocomial enterococcal infections. E. faecium accounts for 10–15% of these enterococcal infections [1,2,3]. The most common infections caused by Enterococcus species are lower urinary tract infections, such as prostatitis, cystitis, and epididymitis. Other infections caused by enterococci include bacteremia; catheter-related infections; intra-abdominal, pelvic, and soft tissue infections; wound infections; endocarditis; and respiratory tract infections.
Patients in the ICU are at a high risk of nosocomial infections due to lowered immunity, indwelling catheterization, and increased antimicrobial use. This group is at high risk from penicillin-resistant VRE, with limited treatment options [4].
Many antimicrobial agents, including cephalosporins, aminoglycosides, clindamycin, and semisynthetic penicillinase-stable penicillins, are intrinsically resistant to enterococci. Enterococci can also develop antimicrobial resistance through genetic mutations, further limiting treatment options [5]. Ampicillin is a convenient option for managing infections in penicillin-susceptible VRE. Reserved antimicrobials such as linezolid and daptomycin are used in life-threatening penicillin-resistant VRE cases, but tigecycline and quinupristin/dalfopristin should be individually evaluated before administration [5,6]. Antimicrobial resistance has increased significantly in the last decade, particularly since the COVID-19 pandemic [7]. The rising trend in antimicrobial resistance during the pandemic could be attributed to poor infection control practices and antimicrobial overuse [8,9].
This retrospective study was carried out in a tertiary care hospital in Saudi Arabia on clinical isolates of nosocomial infections from 2012 to 2021 to characterize and discover the antimicrobial susceptibility patterns of Enterococcus species; we think this will address a common problem in the world of hospital-acquired infection, especially among our national health care professionals.
2. Materials and Methods
This retrospective study was conducted in a tertiary care hospital over a 10-year period (2012–2021) to characterize Enterococcus species and evaluate resistance patterns. The hospital information system was used to collect data on 1034 isolates, with 729 from general wards and 305 from the ICU. We included in this data set all adult patients aged 18 years and above. Along with the requirements that the samples were from nosocomial infections and that the infections had been identified to be hospital-acquired (nosocomial), based on the fact that these infections are usually acquired after hospitalization and manifest 48 h after admission to the hospital, we also excluded any samples collected within 48 h of hospital admission. Thus, we excluded any samples from the pediatric population and any infections from community-acquired sources. The isolates included samples from abscesses, body fluids, blood, CSF, respiratory samples, soft tissue, urine, and wounds. All samples were collected in a sterile manner and sent to the microbiology laboratory. Samples were processed on defined culture media plates, including blood, chocolate, MacConkey, and cysteine lactose electrolyte-deficient (CLED) media, according to the standard operating procedure (SOP) of the microbiology laboratory, which was the Clinical and Laboratory Standards Institute (CLSI). Culture plates were incubated aerobically at 35–37 °C for 24–48 h. Gram staining was performed on samples from abscesses, body fluids, blood, CSF, respiratory samples, soft tissue, and wounds. Culture media plates were observed after 24 h for the presence of pathogenic isolates, their colony morphology, and Gram staining results. In the case of non-significant growth, the culture media plates were re-incubated for an additional 24 h. Enterococcus species were identified by Gram stain results as gram-positive cocci in pairs or short chains, morphology on culture media plates, and biochemical tests such as bile esculin and 6.5% salt broth.
Antimicrobial sensitivity was assessed for all positive isolates using the automated VITEK system, and antibiotic selection was performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. The results were interpreted according to CLSI guidelines.
Statistical Analysis
All data were entered and evaluated using Microsoft Excel 360 and Stata version 17 using Fisher’s exact test and a binomial generalized linear model for trends.
3. Results
3.1. Characteristics of Study Population
This study included 1034 Enterococcus species isolates: 647 (62.6%) from men, with 88 (53.7%) VRE and 559 (64.3%) VSE, and 387 (37.4%) from women, with 76 (46.3%) VRE and 311 (35.7%) VSE (p = 0.001). Of the isolates, 729 (70.5%) were from hospital wards, with 88 (53.6%) VRE and 641 (73.7%) VSE, while 305 (29.5%) were from the ICU, with 76 (46.4%) VRE and 229 (26.3%) VSE (p = 0.001). Overall, there were 164 (15.9%) VRE and 870 (84.1%) VSE (p = 0.01). Of the isolates, 561 (54.3%) were E. faecalis, with 15 (2.7%) VRE and 546 (97.3%) VSE; 347 (33.5%) were E. faecium, with 143 (41.2%) VRE and 204 (58.8%) VSE; and 126 (12.2%) were from other species, with 6 (4.8%) VRE and 120 (95.2%) VSE (p = 0.001) (Figure 1).
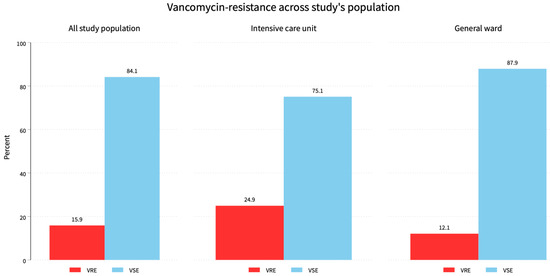
Figure 1.
Vancomycin resistance across the all the study’s population. The overall vancomycin resistance was 15.9% while ICU patients had a 46.4% resistance burden and 53.6% for general ward patients.
We used the following cultural sources: Abscess 60 (5.8%): 8 VRE (4.9%) and 52 VSE (6%); ascitic fluid 21 (2%): 7 VRE (4.3%) and 14 VSE (1.6%); blood 203 (19.6%): 54 VRE (32.9%) and 149 VSE (17.1%); CSF 11 (1.1%): 2 VRE (1.2%) and 9 VSE (1%); respiratory samples 49 (4.7%): 3 VRE (1.8%) and 46 VSE (5.3%); Soft tissue 10 (1%): 1 VRE (0.6%) and 9 VSE (1%); urine 521 (50.4%): 69 VRE (42.1%) and 452 VSE (52%); wounds 120 (11.6%): 17 VRE (10.4%) and 103 VSE (11.8%); other sources 39 (3.8%): 3 VRE (1.8%) and 36 VSE (4.1%) (p = 0.001). See Table 1, Figure 2.

Table 1.
Characteristics of the study population.
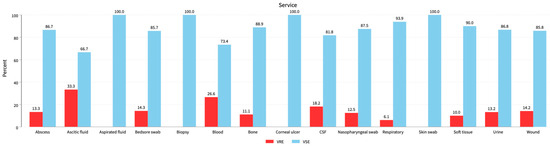
Figure 2.
Distribution of VRE isolates among all clinical specimens. Ascitic fluid, blood, and CSF represent the 33.3%, 26.6%, and 18.2% VRE populations followed by bedsore swabs, wounds, and urine samples with 14.3%, 14.2%, and 13.2% VRE populations, respectively.
Patient outcomes showed that 710 (73.3%) survived—85 (56.3%) VRE and 625 (76.5%) VSE—while 258 (26.7%) died—66 (43.7%) VRE and 192 (23.5%) VSE (p < 0.001). See Table 1, Figure 3.
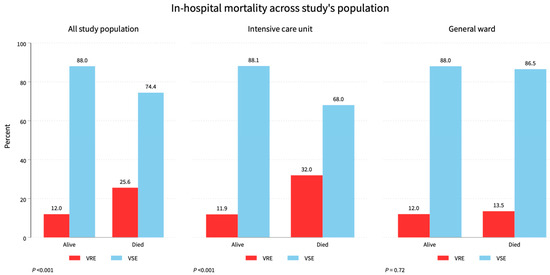
Figure 3.
Mortality status across the study population. Total study mortality was 25.6%, while ICU had 32%, and the general ward had a 13.5% mortality rate of VRE.
The trend of VRE to total study population was 6.1, 7.3, 3.7, 4.9, 5.5, 7.9, 9.1, 17.1, 16.5, and 22 while the trend of VSE to total study population was 4.4, 10.3, 6.2, 6.7, 9.2, 10.2, 10.1, 15.2, 13.1, 14.6 in the years 2012 to 2021, respectively (p = 0.02). Trends of E. faecalis and E. faecium incidence across the years of study are shown in Table 1, Figure 4.
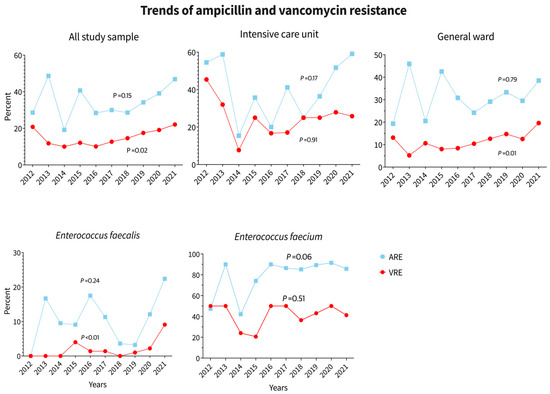
Figure 4.
Trends of E. faecalis and E. faecium incidence across the years of study.
3.2. Resistance Patterns against Selected Antimicrobial Agents
This study identified and reported eight different species of Enterococcus: E. avium, E. casseliflavus, E. durans, E. faecalis, E. faecium, E. gallinarum, E. hirae, and E. raffinosus. E. faecium showed high resistance to ampicillin (81.6%), whereas E. faecalis showed only 11.8% resistance. Linezolid resistance was observed only in E. faecium and E. faecalis cases, with prevalence rates of 3.1% and 1.5% in the total sample population, respectively. The highest resistance in E. faecium was observed against ciprofloxacin (84.1%), followed by ampicillin (81.6%), rifampicin (80%), and vancomycin (41.2%). The least resistant antibiotics for E. faecium were daptomycin (0.6%) and linezolid (3.1%). For E. faecalis, the highest resistance was observed against ciprofloxacin (59.7%), followed by rifampicin (20.1%) and ampicillin (11.8%). The least resistant antibiotics for E. faecalis were daptomycin (0%), linezolid (1.5%), and vancomycin (2.7%). (Table 2). Trends in ampicillin and vancomycin resistance in the whole study population, ICU, and general ward, as well as trends of E. faecalis and E. faecium over the study years, are shown in Figure 5. Ampicillin resistance increased in all groups between 2012 and 2021, particularly among ICU patients. No significant change in antibiotic resistance was observed in the last two years of the COVID-19 pandemic; however, if only ICU data is considered, there was a significant increase in ampicillin resistance in 2020 and 2021. Vancomycin resistance in ICU patients decreased slightly between 2020 and 2021, but it increased in the overall population and general ward patients. See Figure 5.

Table 2.
Resistance patterns against selected antimicrobial agents of the whole study population.
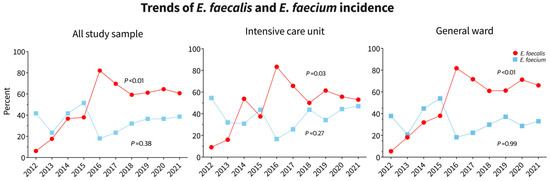
Figure 5.
Trends of ampicillin and vancomycin resistance in the whole study population, ICU, and general ward as well as trends in E. faecalis and E. faecium over the study years.
4. Discussion
In this study, we examined the prevalence and resistance patterns of Enterococcus species in the ICU and general ward of a tertiary care hospital in Saudi Arabia from 2012 to 2021. We identified and reported eight different Enterococcus species: E. avium, E. casseliflavus, E. durans, E. faecalis, E. faecium, E. gallinarum, E. hirae, and E. raffinosus. E. faecalis was the most frequently isolated Enterococcus species (54.3%), followed by E. faecium (33.5%). Our findings support the notion that E. faecalis is the most common cause of enterococcal infections [10,11]. However, similar to other studies, our study showed an increasing proportion of infections caused by E. faecium. This suggests that E. faecium could become the dominant species causing enterococcal infections in the future. Therefore, it is important to monitor the prevalence of Enterococcus species over time.
Our findings revealed that VRE constituted 15.9% of the total sample, while VSE made up 84.1%. This VRE incidence was consistent with recent studies [12,13]. The highest incidence of VRE was observed in E. casseliflavus (75%), followed by E. gallinarum (50%). However, these isolates were few, with only four and six samples respectively, indicating that more positive isolates are needed to represent the true population. These species are known to be intrinsically resistant to vancomycin due to the natural presence of resistant genes [14]. E. faecalis represented 54.3% of the total population, with 2.7% of VRE cases, while E. faecium represented 33.6% of the total population, with 41.2% of VRE cases. This was similar to a previous report [4], in which 41.7% of ICU isolates exhibited resistance to vancomycin, with 60% of these strains being E. faecium and 40% E. faecalis. According to our findings, the resistance pattern (VRE) was more prevalent in the ICU population (24.9% of total ICU samples vs. 12.1% of total general ward samples). A high incidence of VRE in ICU samples has also been variably reported in previous studies [4,7,12], with rates of 41.7%, 12.3%, and 17.3%, respectively. This could be because ICU patients are more susceptible to nosocomial infections due to weakened immunity, frequent exposure to antimicrobial agents, and severe illnesses.
In our study, VRE samples in males accounted for 53.7% of total VRE samples. However, the percentage of VRE among total female samples was 19.6%, while it was 13.6% of total male samples. Current literature suggests differences between sexes, with a male preference (59%) in the distribution of VRE [15]. Previous research has found gender differences in infections caused by some pathogens such as Staphylococcus aureus (male predominance) [16] and Escherichia coli (female predominance) [17]. Genetic [18] and hormonal factors [18,19] could contribute to this phenomenon.
According to our findings, linezolid resistance was found to be 3.1% and 1.5% in the total sample population in E. faecalis and E. faecium cases, respectively. Although linezolid resistance is rare, with more than 99% of gram-positive bacteria still susceptible [20], antimicrobial surveillance studies have revealed that the number of linezolid-resistant Enterococci has recently increased [21,22,23]. Also, the highest resistance for E. faecalis was observed against ciprofloxacin (59.7%), followed by rifampicin (20.1%) and ampicillin (11.8%), and the least resistant antibiotics were daptomycin (0%), linezolid (1.5%), and vancomycin (2.7%). On the other hand, the highest resistance for E. faecium was observed against ciprofloxacin (84.1%), followed by levofloxacin (83.6%), penicillin (82.3%), and ampicillin (81.6%), while the least resistant antibiotics were daptomycin (0.6%), tigecycline (2.7%), and linezolid (3.1%). A huge difference in resistance was observed against ampicillin: E. faecalis was only 11.8% resistant to ampicillin, whereas E. faecium was 81.6% resistant. Ampicillin resistance in E. faecium is triggered mainly by increased PBP5 production and/or polymorphisms in the protein’s beta subunit [24].
Another significant difference in resistance was observed against rifampin, with rates of 20.1% and 80% against E. faecalis and E. faecium, respectively. Previous research on isolates from bovine milk supported our findings, with 13.6% of E. faecalis and 42.3% of E. faecium being rifampicin-resistant strains [25]. Rifampicin resistance rates were also found to be 71.2% and 94.3% in E. faecalis and E. faecium isolates from various clinical infections, respectively [26]. Previously, research [27] on urinary tract infection isolates found complete resistance to rifampicin in E. faecium (100%) and a significant proportion in E. faecalis (81.2%). Rifampicin is not commonly used in the treatment of enterococci as acquired resistance to rifampicin has been observed in both E. faecium and E. faecalis due to mutations in the gene encoding the RNA polymerase subunit (rpoB) [28,29]. Recently, it was reported that HelD proteins from high G+C Actinobacteria, called HelR, were able to dissociate rifampicin-stalled RNA polymerase from DNA and provide rifampicin resistance [25].
Despite the fact that the COVID-19 pandemic was the primary cause of increased and inappropriate antimicrobial use [30], our study found no conclusive evidence of a rising trend of antimicrobial resistance as a result of the pandemic. Other studies have produced conflicting results, with some indicating that the pandemic was associated with an increase in VRE infection or colonization [31] while others discovered a decrease in VRE resistance during the COVID-19 pandemic [4,32]. This disparity could be explained by the fact that some hospitals, possibly due to the heavy load they were exposed to during the pandemic, had decreased infection control activities, decreased adherence to blood culture and central line bundles, and antibiotic abuse in COVID-19 patients, as well as high rates of staff turnover and the presence of non-strictly qualified staff members in ICU settings during the first pandemic periods [33].
Our findings revealed that the mortality rate for VRE infections was higher (43.7%) than for VSE cases (23.5%). However, the effect of VRE infection on mortality is still debatable, with the associated comorbidities potentially skewing the estimates [34,35,36].
Due to the study’s retrospective design, one limitation was that no genomic analysis of resistant isolates, particularly VRE, was performed. Future research involving genomic analysis is warranted. Also, our study represents a regional—or, at most, a national—observation, which may limit its generalizability.
5. Conclusions
This study provides an overview of enterococcal infections and antibiotic susceptibility trends, guiding clinicians in the selection of appropriate empirical antibiotic therapy to improve clinical outcomes. From clinical specimens in a tertiary care hospital, we identified eight different Enterococcus species. E. faecalis was the most commonly identified strain, while E. faecium specimens had the highest percentage of VRE. VRE cases had a significantly higher mortality rate than VSE cases. Daptomycin, linezolid, and vancomycin had the least resistance among isolated strains.
Author Contributions
A.A.B.: conceptualization, experiment, writing; A.A. (Abdulrahman Alwahhabi): data collection, writing; Y.S.: writing and reviewing; A.A. (Abdullah Algarn), data collection; M.A.: data collection analysis and software; M.A.: data collection; A.A. (Ahmed Alasmari): management, ethical approvals; A.A. (Adil Alshehry): writing and reviewing; W.F.M.: writing and reviewing; N.N.: writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Research ethical committee of the Aseer central hospital (ACH IRB No. 20191218 and the date of approval was 15 December 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferede, Z.T.; Tullu, K.D.; Derese, S.G.; Yeshanew, A.G. Prevalence and antimicrobial susceptibility pattern of Enterococcus species isolated from different clinical samples at Black Lion Specialized Teaching Hospital, Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Yilema, A.; Moges, F.; Tadele, S.; Endris, M.; Kassu, A.; Abebe, W.; Ayalew, G. Isolation of enterococci, their antimicrobial susceptibility patterns and associated factors among patients attending at the University of Gondar Teaching Hospital. BMC Infect. Dis. 2017, 17, 276. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. Emergence and management of drug-resistant Enterococcal infections. Expert. Rev. Anti Infect. Ther. 2008, 6, 637–655. [Google Scholar] [CrossRef] [PubMed]
- M Alatrouny, A.; Amin, M.A.; Shabana, H.S. Prevalence of vancomycin-resistant enterococci among patients with nosocomial infections in intensive care unit. Al-Azhar Med. J. 2020, 49, 1955–1964. [Google Scholar] [CrossRef]
- Agudelo Higuita, N.I.; Huycke, M.M. Enterococcal Disease, Epidemiology, and Implications for Treatment. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar] [PubMed]
- Contreras, G.A.; Munita, J.M.; Arias, C.A. Novel Strategies for the Management of Vancomycin-Resistant Enterococcal Infections. Curr. Infect. Dis. Rep. 2019, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B. The impact of COVID-19 on bacterial antimicrobial resistance: Findings from a narrative review. J. Health Inform. Dev. Ctries. 2022, 16, 1–16. [Google Scholar]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 pandemic on multidrug-resistant gram-positive and gram-negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Jabbari Shiadeh, S.M.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef]
- Horner, C.; Mushtaq, S.; Allen, M.; Hope, R.; Gerver, S.; Longshaw, C.; Reynolds, R.; Woodford, N.; Livermore, D.M. Replacement of Enterococcus faecalis by Enterococcus faecium as the predominant Enterococcus in UK bacteraemias. JAC Antimicrob. Resist. 2021, 3, dlab185. [Google Scholar] [CrossRef]
- Johargy, A.K.; Jamal, A.; Momenah, A.M.; Ashgar, S.S. Vancomycin-resistant Enterococci in Saudi Arabia: Prevalence, antibiotic resistance, and susceptibility array. Pure Appl. Biol. (PAB) 2021, 5, 830–840. [Google Scholar] [CrossRef]
- Cimen, C.; Berends, M.S.; Bathoorn, E.; Lokate, M.; Voss, A.; Friedrich, A.W.; Glasner, C.; Hamprecht, A. Vancomycin-resistant enterococci (VRE) in hospital settings across European borders: A scoping review comparing the epidemiology in the Netherlands and Germany. Antimicrob. Resist. Infect. Control 2023, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Marques, J.; Coelho, M.; Santana, A.R.; Pinto, D.; Semedo-Lemsaddek, T. Dissemination of Enterococcal Genetic Lineages: A One Health Perspective. Antibiotics 2023, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Correa-Martínez, C.L.; Schuler, F.; Kampmeier, S. Sex differences in vancomycin-resistant enterococci bloodstream infections-a systematic review and meta-analysis. Biol. Sex. Differ. 2021, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Allard, C.; Carignan, A.; Bergevin, M.; Boulais, I.; Tremblay, V.; Robichaud, P.; Duperval, R.; Pepin, J. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin. Microbiol. Infect. 2008, 14, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Gregson, D.B.; Church, D.L.; Ross, T.; Pitout, J.D. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin. Microbiol. Infect. 2008, 14, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, E.R.; García-Gómez, E.; Camacho-Arroyo, I.; González-Pedrajo, B. Sexual dimorphism in bacterial infections. Biol. Sex. Differ. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Deshpande, L.; Streit, J.M.; Sader, H.S.; Castanheira, M.; Hogan, P.A.; Flamm, R.K. ZAAPS programme results for 2016: An activity and spectrum analysis of linezolid using clinical isolates from medical centres in 42 countries. J. Antimicrob. Chemother. 2018, 73, 1880–1887. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Feßler, A.T.; Hanke, D.; Eichhorn, I.; Azcona-Gutiérrez, J.M.; Pérez-Moreno, M.O.; Seral, C.; Aspiroz, C.; Alonso, C.A.; Torres, L.; et al. Mechanisms of Linezolid Resistance Among Enterococci of Clinical Origin in Spain—Detection of optrA- and cfr(D)-Carrying E. faecalis. Microorganisms 2020, 8, 1155. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wysocka, M.; Kotłowski, R.; Bronk, M.; Michalik, M.; Samet, A. Linezolid-resistant Enterococcus faecium strains isolated from one hospital in Poland -commensals or hospital-adapted pathogens? PLoS ONE 2020, 15, e0233504. [Google Scholar] [CrossRef] [PubMed]
- Misiakou, M.A.; Hertz, F.B.; Schønning, K.; Häussler, S.; Nielsen, K.L. Emergence of linezolid-resistant Enterococcus faecium in a tertiary hospital in Copenhagen. Microb. Genom. 2023, 9, mgen001055. [Google Scholar] [CrossRef] [PubMed]
- Gagetti, P.; Bonofiglio, L.; García Gabarrot, G.; Kaufman, S.; Mollerach, M.; Vigliarolo, L.; von Specht, M.; Toresani, I.; Lopardo, H.A. Resistance to β-lactams in enterococci. Rev. Argent. Microbiol. 2019, 51, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Paschoalini, B.R.; Nuñez, K.V.M.; Maffei, J.T.; Langoni, H.; Guimarães, F.F.; Gebara, C.; Freitas, N.E.; dos Santos, M.V.; Fidelis, C.E.; Kappes, R.; et al. The Emergence of Antimicrobial Resistance and Virulence Characteristics in Enterococcus Species Isolated from Bovine Milk. Antibiotics 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Jahansepas, A.; Aghazadeh, M.; Rezaee, M.A.; Hasani, A.; Sharifi, Y.; Aghazadeh, T.; Mardaneh, J. Occurrence of Enterococcus faecalis and Enterococcus faecium in Various Clinical Infections: Detection of Their Drug Resistance and Virulence Determinants. Microb. Drug Resist. 2018, 24, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, Y.; Hasani, A.; Ghotaslou, R.; Naghili, B.; Aghazadeh, M.; Milani, M.; Bazmany, A. Virulence and Antimicrobial Resistance in Enterococci Isolated from Urinary Tract Infections. Adv. Pharm. Bull. 2013, 3, 197–201. [Google Scholar] [PubMed]
- Urusova, D.V.; Merriman, J.A.; Gupta, A.; Chen, L.; Mathema, B.; Caparon, M.G.; Khader, S.A. Rifampin resistance mutations in the rpoB gene of Enterococcus faecalis impact host macrophage cytokine production. Cytokine 2022, 151, 155788. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Abdel Gawad, A.M.; Ashry, W.M.O.; El-Ghannam, S.; Hussein, M.; Yousef, A. Antibiotic resistance profile of common uropathogens during COVID-19 pandemic: A hospital-based epidemiologic study. BMC Microbiol. 2023, 23, 28. [Google Scholar] [CrossRef]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am. J. Infect. Control 2022, 50, 32–38. [Google Scholar] [CrossRef]
- Cole, J.; Barnard, E. The impact of the COVID-19 pandemic on healthcare-acquired infections with multidrug-resistant organisms. Am. J. Infect. Control 2021, 49, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Parisini, A.; Boni, S.; Vacca, E.B.; Bobbio, N.; Del Puente, F.; Feasi, M.; Prinapori, R.; Lattuada, M.; Sartini, M.; Cristina, M.L.; et al. Impact of the COVID-19 Pandemic on Epidemiology of Antibiotic Resistance in an Intensive Care Unit (ICU): The Experience of a North-West Italian Center. Antibiotics 2023, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Eichel, V.M.; Last, K.; Brühwasser, C.; von Baum, H.; Dettenkofer, M.; Götting, T.; Grundmann, H.; Güldenhöven, H.; Liese, J.; Martin, M.; et al. Epidemiology and outcomes of vancomycin-resistant Enterococcus infections: A systematic review and meta-analysis. J. Hosp. Infect. 2023, 141, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hemapanpairoa, J.; Changpradub, D.; Thunyaharn, S.; Santimaleeworagun, W. Does Vancomycin Resistance Increase Mortality? Clinical Outcomes and Predictive Factors for Mortality in Patients with Enterococcus faecium Infections. Antibiotics 2021, 10, 105. [Google Scholar] [CrossRef]
- Dubler, S.; Lenz, M.; Zimmermann, S.; Richter, D.C.; Weiss, K.H.; Mehrabi, A.; Mieth, M.; Bruckner, T.; Weigand, M.A.; Brenner, T.; et al. Does vancomycin resistance increase mortality in Enterococcus faecium bacteraemia after orthotopic liver transplantation? A retrospective study. Antimicrob. Resist. Infect. Control 2020, 9, 22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).