Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Detection of Trypanosoma cruzi in Low-Resource Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Loop-Mediated Isothermal Amplification (LAMP)
2.2. Limit of Detection (LOD), Selectivity, and Contrived Clinical Sample Experimental Design
2.3. Gold-Standard Comparison Experimental Design
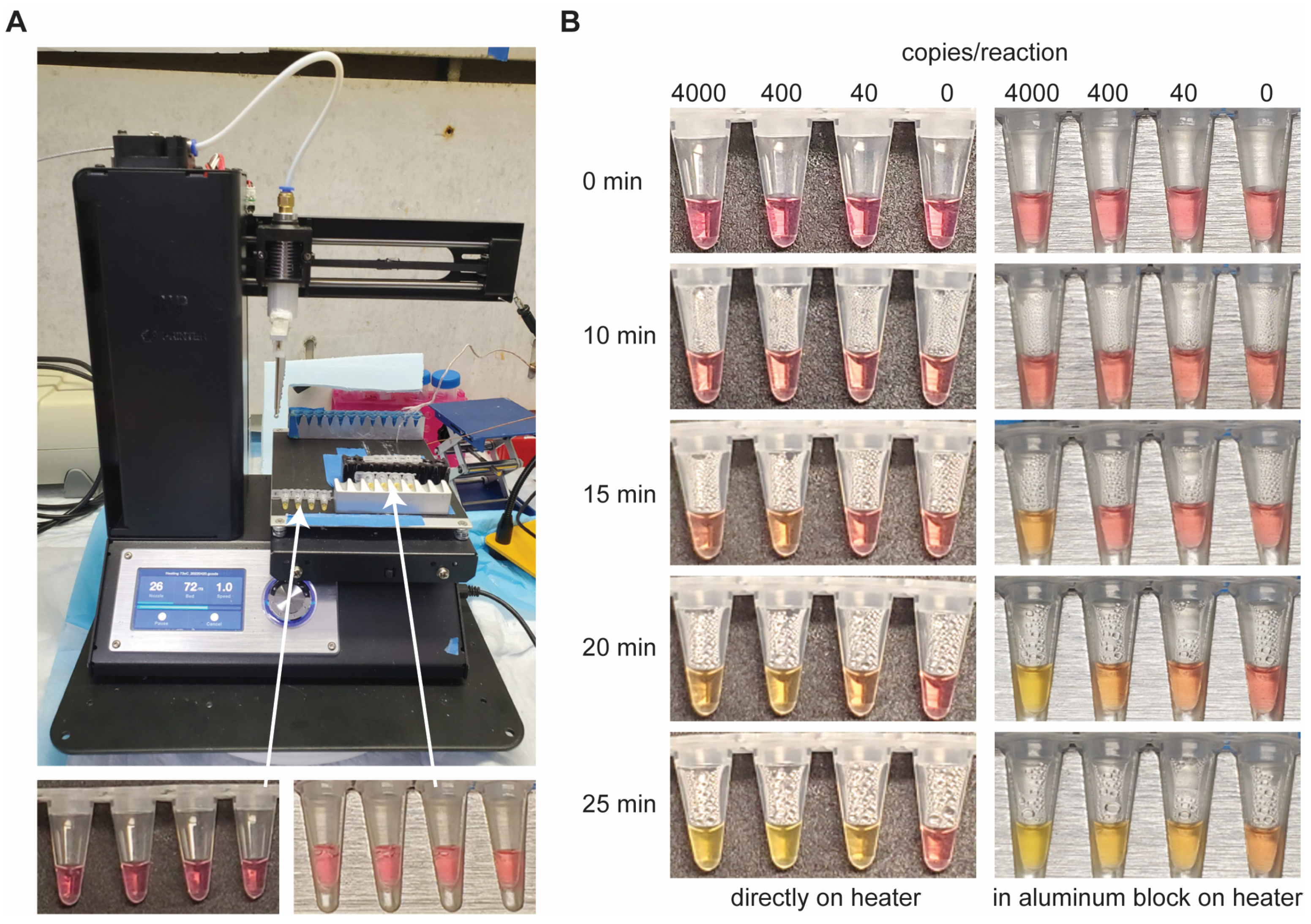
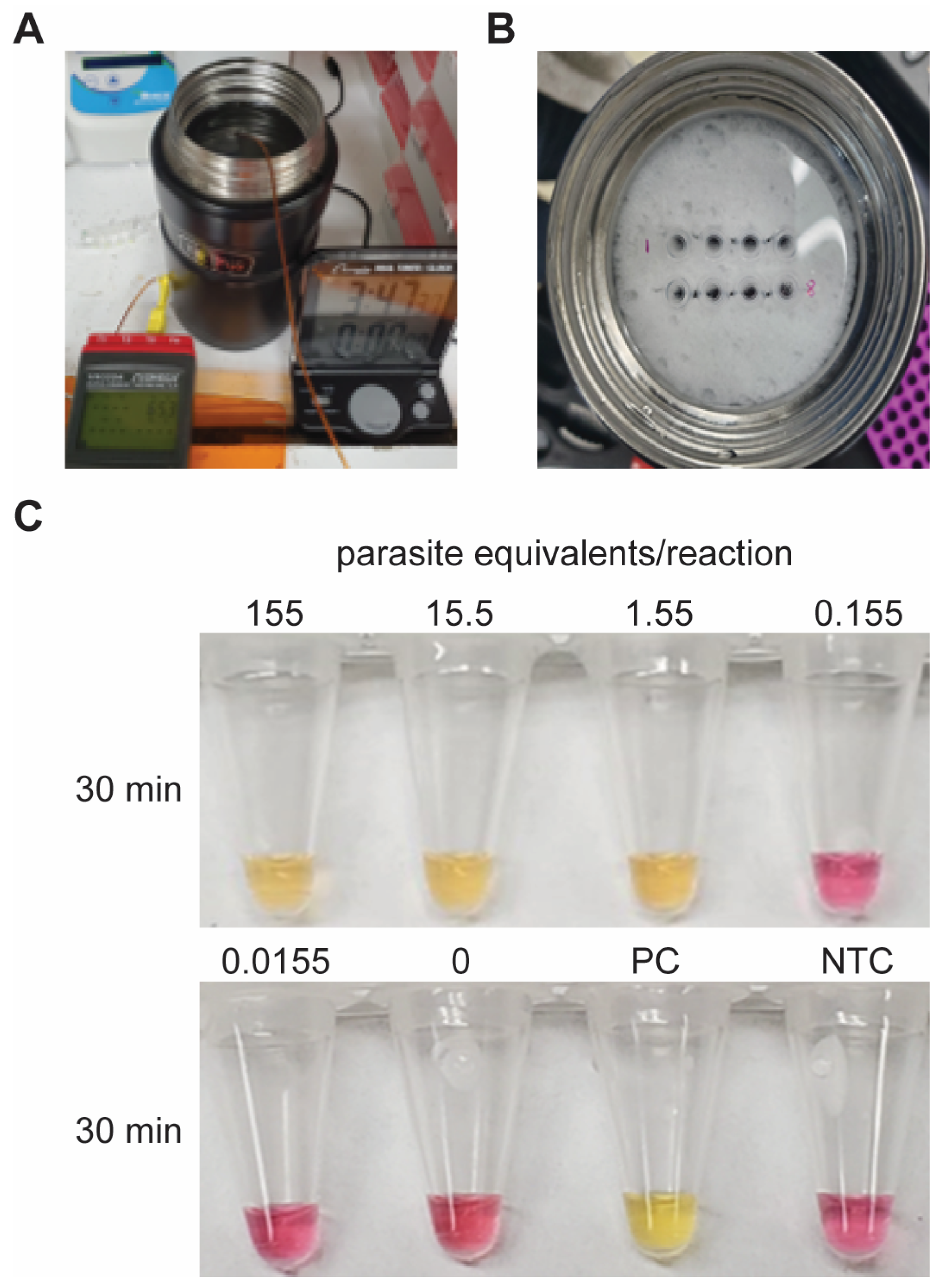
2.4. Low-Cost Heating Experimental Design
2.5. Statistical Analysis
3. Results
3.1. LAMP Assay Optimization
3.2. Assay LOD and Selectivity
3.3. Contrived Clinical Sample Testing
3.4. Direct Comparison of LAMP and PCR Performance with Canine Samples
3.5. LAMP Incubation with Inexpensive Heating Elements
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 2 October 2022).
- Brenière, S.F.; Waleckx, E.; Barnabé, C. Over Six Thousand Trypanosoma cruzi Strains Classified into Discrete Typing Units (DTUs): Attempt at an Inventory. PLoS Negl. Trop. Dis. 2016, 10, e0004792. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M. Chagas disease in the 21st century: A public health success or an emerging threat? Parasite 2014, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- CDC. Parasites—American Trypanosomiasis (Also Known as Chagas Disease). Available online: https://www.cdc.gov/parasites/chagas/ (accessed on 1 October 2022).
- Getahun, M.N.; Ngiela, J.; Makwatta, J.O.; Ahuya, P.; Simon, T.K.; Kamau, S.K.; Torto, B.; Masiga, D. Metabolites From Trypanosome-Infected Cattle as Sensitive Biomarkers for Animal Trypanosomosis. Front. Microbiol. 2022, 13, 922760. [Google Scholar] [CrossRef] [PubMed]
- Altcheh, J.; Moscatelli, G.; Mastrantonio, G.; Moroni, S.; Giglio, N.; Marson, M.E.; Ballering, G.; Bisio, M.; Koren, G.; García-Bournissen, F. Population Pharmacokinetic Study of Benznidazole in Pediatric Chagas Disease Suggests Efficacy despite Lower Plasma Concentrations than in Adults. PLoS Neglected Trop. Dis. 2014, 8, e2907. [Google Scholar] [CrossRef] [PubMed]
- Qvarnstrom, Y.; Schijman, A.G.; Veron, V.; Aznar, C.; Steurer, F.; da Silva, A.J. Sensitive and specific detection of Trypanosoma cruzi DNA in clinical specimens using a multi-target real-time PCR approach. PLoS Neglected Trop. Dis. 2012, 6, e1689. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.M.; Ebell, M.H.; Tarleton, R.L. A Systematic Review of High Quality Diagnostic Tests for Chagas Disease. PLoS Neglected Trop. Dis. 2012, 6, e1881. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Flores-Chavez, M.D.; Abras, A.; Ballart, C.; Perez, I.I.; Perez-Gordillo, P.; Gállego, M.; Muñoz, C.; Moure, Z.; Igual, E.S.; Nieto, J.; et al. Evaluation of the Performance of the Loopamp Trypanosoma cruzi Detection Kit for the Diagnosis of Chagas Disease in an Area Where It Is Not Endemic, Spain. J. Clin. Microbiol. 2021, 59, e01860-20. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, F.; Stolowicz, F.; Vojnov, A.; Coltro, W.K.T.; Larocca, L.; Carrillo, C.; Cortón, E. Towards a versatile and economic Chagas Disease point-of-care testing system, by integrating loop-mediated isothermal amplification and contactless/label-free conductivity detection. PLoS Neglected Trop. Dis. 2021, 15, e0009406. [Google Scholar] [CrossRef]
- Ordóñez, D.; Fernández-Soto, P.; Fernández-Martín, A.M.; Crego-Vicente, B.; Febrer-Sendra, B.; Diego, J.G.; Vicente, B.; López-Abán, J.; Belhassen-García, M.; Muro, A.; et al. A Trypanosoma cruzi Genome Tandem Repetitive Satellite DNA Sequence as a Molecular Marker for a LAMP Assay for Diagnosing Chagas’ Disease. Dis. Markers 2020, 2020, 8074314. [Google Scholar] [CrossRef]
- Rivero, R.; Bisio, M.; Velázquez, E.B.; Esteva, M.I.; Scollo, K.; González, N.L.; Altcheh, J.; Ruiz, A.M. Rapid detection of Trypanosoma cruzi by colorimetric loop-mediated isothermal amplification (LAMP): A potential novel tool for the detection of congenital Chagas infection. Diagn. Microbiol. Infect. Dis. 2017, 89, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Bisio, M.M.C.; Rivero, R.; Gonzalez, N.; Ballering, G.; D’Amico, I.; Kessler, C.; Moroni, S.; Moscatelli, G.; Ruiz, A.M.; Altcheh, J. Diagnostic Accuracy of Two Molecular Tools for Diagnosis of Congenital Chagas Disease. Mol. Diagn. Ther. 2021, 25, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Besuschio, S.A.; nica Llano Murcia, M.; Benatar, A.F.; Monnerat, S.; Cruz, I.; Picado, A.; de los, M.A.; Curto, N.; Kubota, Y.; Wehrendt, D.P.; et al. Analytical sensitivity and specificity of a loop-mediated isothermal amplification (LAMP) kit prototype for detection of Trypanosoma cruzi DNA in human blood samples. PLoS Neglected Trop. Dis. 2017, 11, e0005779. [Google Scholar] [CrossRef]
- Wehrendt, D.P.; Alonso-Padilla, J.; Liu, B.; Rojas Panozo, L.; Rivera Nina, S.; Pinto, L.; Lozano, D.; Picado, A.; Abril, M.; Pinazo, M.J.; et al. Development and Evaluation of a Three-Dimensional Printer-Based DNA Extraction Method Coupled to Loop Mediated Isothermal Amplification for Point-of-Care Diagnosis of Congenital Chagas Disease in Endemic Regions. J. Mol. Diagn. 2021, 23, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Calderón, A.A.; Besuschio, S.A.; Wong, S.; Fernández, M.; García Cáceres, L.J.; Giorgio, P.; Barcan, L.A.; Markham, C.; Liu, Y.E.; de Noya, B.A.; et al. Loop-Mediated Isothermal Amplification of Trypanosoma cruzi DNA for Point-of-Care Follow-Up of Anti-Parasitic Treatment of Chagas Disease. Microorganisms 2022, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Weaver, S.C.; Wong, P.-Y.; Lie, S.; Wang, E.; Guerbois, M.; Vayugundla, S.P.; Wong, S. Rapid, Affordable and Portable Medium-Throughput Molecular Device for Zika Virus. Sci. Rep. 2016, 6, 38223. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Coen, M.; Hardick, J.; Gaydos, C.A.; Wong, K.-Y.; Smith, C.; Wilson, S.A.; Vayugundla, S.P.; Wong, S. Low-Cost 3D Printers Enable High-Quality and Automated Sample Preparation and Molecular Detection. PLoS ONE 2016, 11, e0158502. [Google Scholar] [CrossRef] [PubMed]
- Busselman, R.E.; Meyers, A.C.; Zecca, I.B.; Auckland, L.D.; Castro, A.H.; Dowd, R.E.; Curtis-Robles, R.; Hodo, C.L.; Saunders, A.B.; Hamer, S.A. High incidence of Trypanosoma cruzi infections in dogs directly detected through longitudinal tracking at 10 multi-dog kennels, Texas, USA. PLoS Neglected Trop. Dis. 2021, 15, e0009935. [Google Scholar] [CrossRef]
- Duffy, T.; Cura, C.I.; Ramirez, J.C.; Abate, T.; Cayo, N.M.; Parrado, R.; Bello, Z.D.; Velazquez, E.; Muñoz-Calderon, A.; Juiz, N.A.; et al. Analytical Performance of a Multiplex Real-Time PCR Assay Using TaqMan Probes for Quantification of Trypanosoma cruzi Satellite DNA in Blood Samples. PLoS Neglected Trop. Dis. 2013, 7, e2000. [Google Scholar] [CrossRef]
- Chan, K.; Wong, P.-Y.; Parikh, C.; Wong, S. Moving toward rapid and low-cost point-of-care molecular diagnostics with a repurposed 3D printer and RPA. Anal. Biochem. 2018, 545, 4–12. [Google Scholar] [CrossRef]
- Chernov, V.; Alander, J.; Bochko, V. Integer-based accurate conversion between RGB and HSV color spaces. Comput. Electr. Eng. 2015, 46, 328–337. [Google Scholar] [CrossRef]
- Parikh, R.; Mathai, A.; Parikh, S.; Chandra Sekhar, G.; Thomas, R. Understanding and using sensitivity, specificity and predictive values. Indian. J. Ophthalmol. 2008, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.T.; Layne, T.R.; O’Connell, K.C.; Tanner, N.A.; Landers, J.P. Comparative Evaluation and Quantitative Analysis of Loop-Mediated Isothermal Amplification Indicators. Anal. Chem. 2020, 92, 13343–13353. [Google Scholar] [CrossRef] [PubMed]
- NCCLS. Assessment of the Clinical Accuracy of Laboratory Tests Using Receiver Operating Characteristic (ROC) Plots; Approved Guideline. NCCLS Document GP10-A (ISBN 1-56238-285-3); NCCLS: Wayne, PA, USA, 1995. [Google Scholar]


| Sample | qPCR Ct | LAMP (Positive Replicates) |
|---|---|---|
| 1 | -- | 0/8 |
| 2 | -- | 0/8 |
| 3 | -- | 0/8 |
| 4 | -- | 2/8 |
| 5 | -- | 0/8 |
| 6 | 33.81 | 8/8 |
| 7 | -- | 0/8 |
| 8 | -- | 1/8 |
| 9 | -- | 1/8 |
| 10 | -- | 0/8 |
| 11 | -- | 1/8 |
| 12 | -- | 0/8 |
| 13 | -- | 0/8 |
| 14 | 35.19 | 8/8 |
| 15 | 34.59 | 7/8 |
| 16 | 34.25 | 7/8 |
| 17 | 31.61 | 8/8 |
| 18 | 33.36 | 8/8 |
| 19 | -- | 0/8 |
| 20 | -- | 0/8 |
| qPCR | |||
|---|---|---|---|
| Positive | Negative | ||
| LAMP | Positive | TP = 6 | FP = 0 |
| Negative | FN = 0 | TN = 14 | |
| P = 6 | N = 14 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moehling, T.J.; Worthington, M.D.; Wong, P.-Y.G.; Wong, S.S.; Meagher, R.J. Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Detection of Trypanosoma cruzi in Low-Resource Settings. Diagnostics 2024, 14, 1193. https://doi.org/10.3390/diagnostics14111193
Moehling TJ, Worthington MD, Wong P-YG, Wong SS, Meagher RJ. Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Detection of Trypanosoma cruzi in Low-Resource Settings. Diagnostics. 2024; 14(11):1193. https://doi.org/10.3390/diagnostics14111193
Chicago/Turabian StyleMoehling, Taylor J., Myla D. Worthington, Pui-Yan G. Wong, Season S. Wong, and Robert J. Meagher. 2024. "Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Detection of Trypanosoma cruzi in Low-Resource Settings" Diagnostics 14, no. 11: 1193. https://doi.org/10.3390/diagnostics14111193
APA StyleMoehling, T. J., Worthington, M. D., Wong, P.-Y. G., Wong, S. S., & Meagher, R. J. (2024). Development of a Colorimetric Loop-Mediated Isothermal Amplification Assay for the Detection of Trypanosoma cruzi in Low-Resource Settings. Diagnostics, 14(11), 1193. https://doi.org/10.3390/diagnostics14111193





