Clinical Validation of a Deep Learning-Based Software for Lumbar Bone Mineral Density and T-Score Prediction from Chest X-ray Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging and Data Collection
2.2. BMD Measurement
2.3. Image Acquisition and Pre-Processing
2.4. Image Quality Assessment
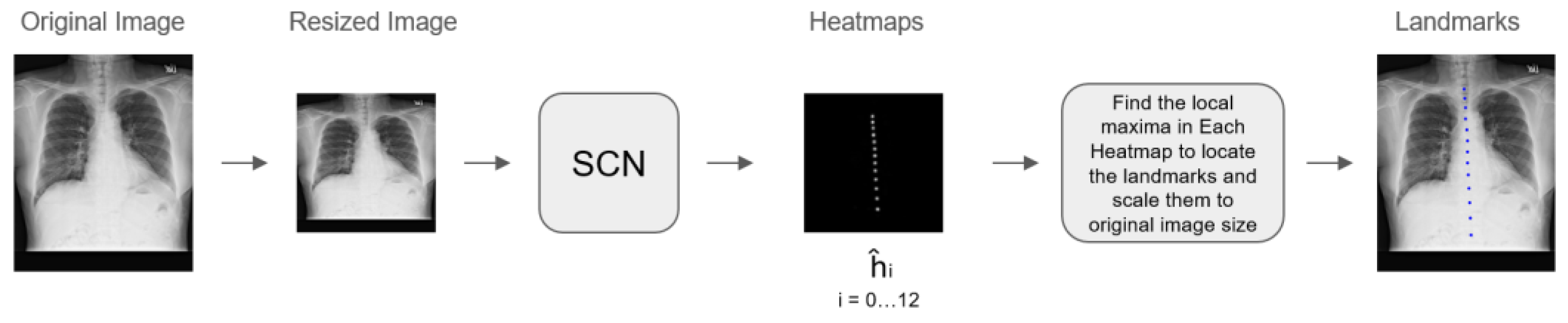
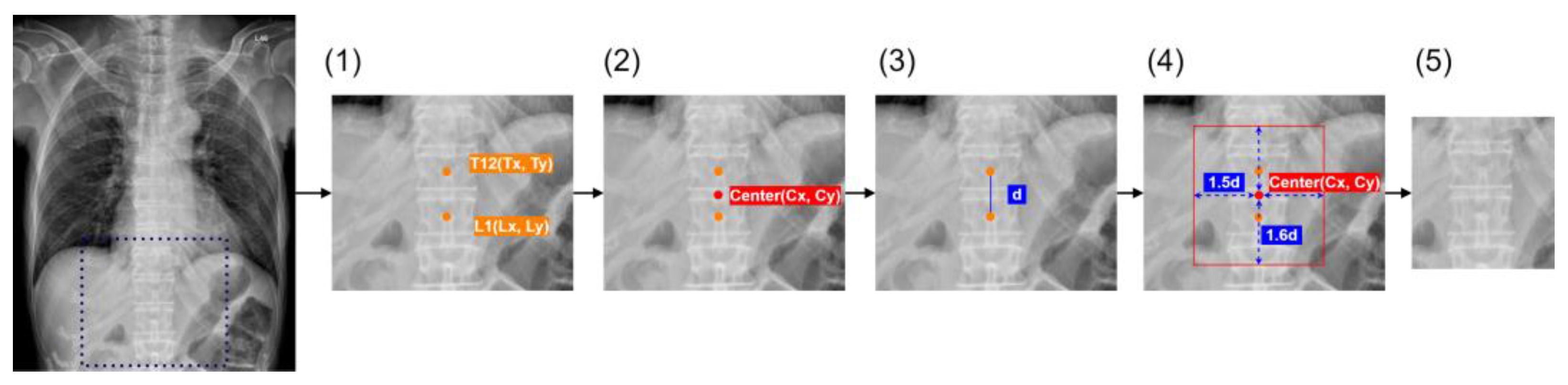
2.5. Algorithm Development
2.6. Clinical Validation
2.7. Evaluation of BMD Prediction Performance and Statistics
3. Results
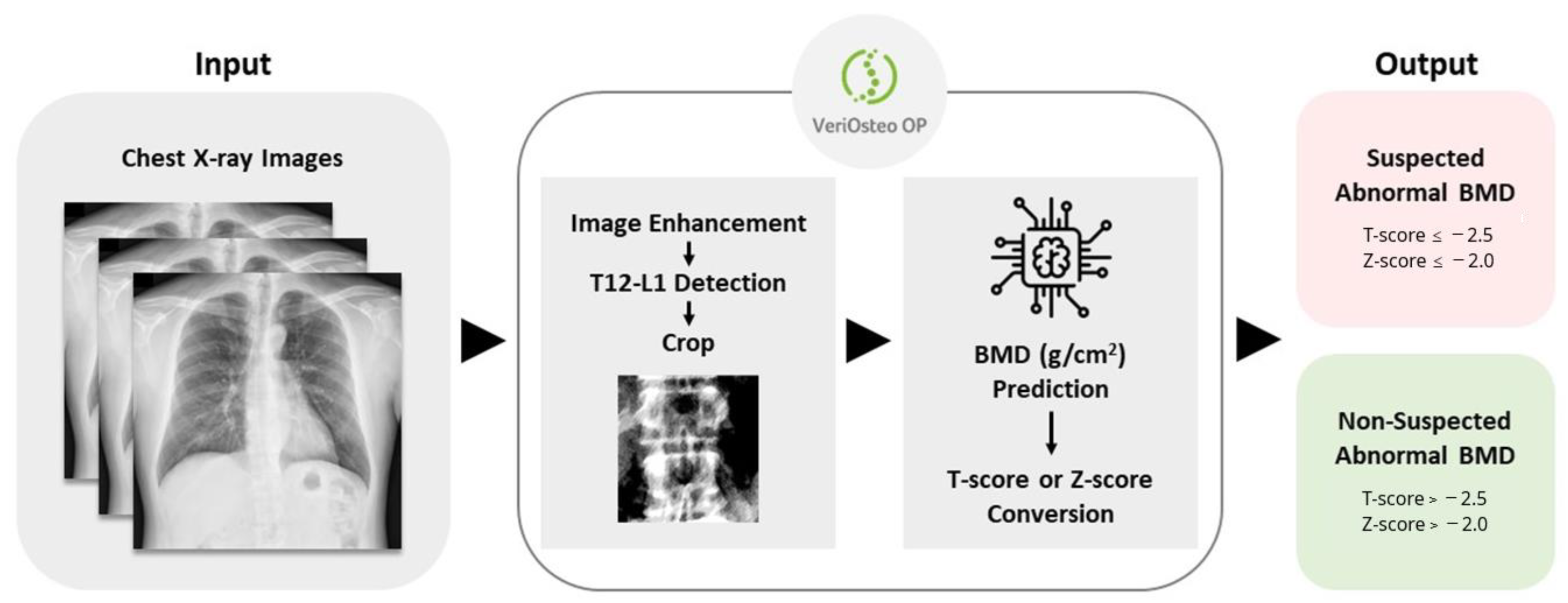
3.1. The Design and Workflow of VeriOsteoTM OP
3.2. Characteristics of the Training/Pre-Clinical Dataset
3.3. Pre-Clinical Performance of VeriOsteoTM OP
3.4. Characteristics of the Clinical Validation Dataset
3.5. Clinical Validation of the Performance of VeriOsteoTM OP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanis, J.A. Diagnosis of Osteoporosis and Assessment of Fracture Risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Consensus Development Conference: Diagnosis, Prophylaxis, and Treatment of Osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef] [PubMed]
- Kanis, J.A. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Synopsis of a WHO Report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Siris, E.S.; Chen, Y.-T.; Abbott, T.A.; Barrett-Connor, E.; Miller, P.D.; Wehren, L.E.; Berger, M.L. Bone Mineral Density Thresholds for Pharmacological Intervention to Prevent Fractures. Arch. Intern. Med. 2004, 164, 1108–1112. [Google Scholar] [CrossRef]
- Li, G.; Thabane, L.; Papaioannou, A.; Ioannidis, G.; Levine, M.A.H.; Adachi, J.D. An Overview of Osteoporosis and Frailty in the Elderly. BMC Musculoskelet. Disord. 2017, 18, 46. [Google Scholar] [CrossRef]
- Melton, L.J.; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. Perspective. How Many Women Have Osteoporosis? J. Bone Miner. Res. 1992, 7, 1005–1010. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare; National Health Research Institutes; The Taiwanese Osteoporosis Association. Osteoporosis Clinical Treatment. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=1151 (accessed on 16 April 2024).
- Nayak, S.; Edwards, D.L.; Saleh, A.A.; Greenspan, S.L. Systematic Review and Meta-Analysis of the Performance of Clinical Risk Assessment Instruments for Screening for Osteoporosis or Low Bone Density. Osteoporos. Int. 2015, 26, 1543. [Google Scholar] [CrossRef]
- Dimai, H.P. Use of Dual-Energy X-ray Absorptiometry (DXA) for Diagnosis and Fracture Risk Assessment; WHO-Criteria, T- and Z-Score, and Reference Databases. Bone 2017, 104, 39–43. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.A.; Moraes, R.; Castanha, E.B.; Prevedello, A.S.; Vieira Filho, J.; Bussolaro, F.A.; García Cava, D. Osteoporosis Screening: Applied Methods and Technological Trends. Med. Eng. Phys. 2022, 108, 103887. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Baim, S. Quantitative Ultrasound (QUS) in the Management of Osteoporosis and Assessment of Fracture Risk. J. Clin. Densitom. 2017, 20, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Njeh, C.F.; Hans, D.; Li, J.; Fan, B.; Fuerst, T.; He, Y.Q.; Tsuda-Futami, E.; Lu, Y.; Wu, C.Y.; Genant, H.K. Comparison of Six Calcaneal Quantitative Ultrasound Devices: Precision and Hip Fracture Discrimination. Osteoporos. Int. 2000, 11, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.C.; Sundram, F.X. Bone Mineral Density–Correlation between Quantitative Ultrasound Characteristics and Dual Energy X-ray Absorptiometry. Ann. Acad. Med. Singap. 1998, 27, 524–526. [Google Scholar] [PubMed]
- Villa, P.; Lassandro, A.P.; Moruzzi, M.C.; Amar, I.D.; Vacca, L.; Di Nardo, F.; De Waure, C.; Pontecorvi, A.; Scambia, G. A Non-Invasive Prevention Program Model for the Assessment of Osteoporosis in the Early Postmenopausal Period: A Pilot Study on FRAX® and QUS Tools Advantages. J. Endocrinol. Investig. 2016, 39, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.J.; Deyo, R.A. Patient Self-Referral for Radiologic Screening Tests: Clinical and Ethical Concerns. J. Am. Board. Fam. Pract. 2003, 16, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-C.; Lin, W.-C.; Wang, T.-H.; Chen, G.-F.; Chou, D.-Y.; Lin, D.-M.; Lin, S.-Y.; Chan, M.-H.; Wu, J.-M.; Tseng, C.-D.; et al. Pre-Screening for Osteoporosis with Calcaneus Quantitative Ultrasound and Dual-Energy X-ray Absorptiometry Bone Density. Sci. Rep. 2021, 11, 15709. [Google Scholar] [CrossRef] [PubMed]
- Dubois, E.F.; van den Bergh, J.P.; Smals, A.G.; van de Meerendonk, C.W.; Zwinderman, A.H.; Schweitzer, D.H. Comparison of Quantitative Ultrasound Parameters with Dual Energy X-ray Absorptiometry in Pre- and Postmenopausal Women. Neth. J. Med. 2001, 58, 62–70. [Google Scholar] [CrossRef]
- Yen, T.-Y.; Ho, C.-S.; Chen, Y.-P.; Pei, Y.-C. Diagnostic Accuracy of Deep Learning for the Prediction of Osteoporosis Using Plain X-rays: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 207. [Google Scholar] [CrossRef]
- Areeckal, A.S.; Jayasheelan, N.; Kamath, J.; Zawadynski, S.; Kocher, M.; David, S.S. Early Diagnosis of Osteoporosis Using Radiogrammetry and Texture Analysis from Hand and Wrist Radiographs in Indian Population. Osteoporos. Int. 2018, 29, 665–673. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, K.; Wang, Y.; Zhou, X.; Lu, L.; Xiao, J.; Wu, M.; Kuo, C.-F.; Miao, S. Opportunistic Screening of Osteoporosis Using Plain Film Chest X-ray; Rekik, I., Adeli, E., Park, S.H., Schnabel, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 12928, pp. 138–146. [Google Scholar]
- Jang, M.; Kim, M.; Bae, S.J.; Lee, S.H.; Koh, J.-M.; Kim, N. Opportunistic Osteoporosis Screening Using Chest Radiographs with Deep Learning: Development and External Validation with a Cohort Dataset. J. Bone Miner. Res. 2022, 37, 369–377. [Google Scholar] [CrossRef]
- Asamoto, T.; Takegami, Y.; Sato, Y.; Takahara, S.; Yamamoto, N.; Inagaki, N.; Maki, S.; Saito, M.; Imagama, S. External Validation of a Deep Learning Model for Predicting Bone Mineral Density on Chest Radiographs. Arch. Osteoporos. 2024, 19, 15. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, N.; Inagaki, N.; Iesaki, Y.; Asamoto, T.; Suzuki, T.; Takahara, S. Deep Learning for Bone Mineral Density and T-Score Prediction from Chest X-rays: A Multicenter Study. Biomedicines 2022, 10, 2323. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.-J.; Lin, C.; Lin, C.-S.; Lee, C.-C.; Wang, C.-H.; Fang, W.-H. Artificial Intelligence-Enabled Chest X-ray Classifies Osteoporosis and Identifies Mortality Risk. J. Med. Syst. 2024, 48, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, K.; Lu, L.; Xiao, J.; Wu, M.; Kuo, C.-F.; Miao, S. Lumbar Bone Mineral Density Estimation from Chest X-ray Images: Anatomy-Aware Attentive Multi-ROI Modeling. IEEE Trans. Med. Imaging 2023, 42, 257–267. [Google Scholar] [CrossRef]
- Hsieh, C.-I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.-P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated Bone Mineral Density Prediction and Fracture Risk Assessment Using Plain Radiographs via Deep Learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, K.; Ning, Z.; Wang, K.; Dong, Y.; Liu, X.; Liu, S.; Wang, J.; Zhu, C.; Yu, Q.; et al. Deep Learning of Lumbar Spine X-ray for Osteopenia and Osteoporosis Screening: A Multicenter Retrospective Cohort Study. Bone 2020, 140, 115561. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-S.; Chen, Y.-P.; Fan, T.-Y.; Kuo, C.-F.; Yen, T.-Y.; Liu, Y.-C.; Pei, Y.-C. Application of Deep Learning Neural Network in Predicting Bone Mineral Density from Plain X-ray Radiography. Arch. Osteoporos. 2021, 16, 153. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.R.; Chae, H.-D.; Lee, J.; Ye, S.-J.; Kim, D.H.; Hong, S.H.; Choi, J.-Y.; Yoo, H.J. Deep Radiomics-Based Approach to the Diagnosis of Osteoporosis Using Hip Radiographs. Radiol. Artif. Intell. 2022, 4, e210212. [Google Scholar] [CrossRef]
- Tai, T.-W.; Huang, C.-F.; Huang, H.-K.; Yang, R.-S.; Chen, J.-F.; Cheng, T.-T.; Chen, F.-P.; Chen, C.-H.; Chang, Y.-F.; Hung, W.-C.; et al. Clinical Practice Guidelines for the Prevention and Treatment of Osteoporosis in Taiwan: 2022 Update. J. Formos. Med. Assoc. 2023, 122 (Suppl. S1), S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Choksi, P.; Gay, B.L.; Haymart, M.R.; Papaleontiou, M. Physician-Reported Barriers to Osteoporosis Screening: A Nationwide Survey. Endocr. Pract. 2023, 29, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Muratore, M.; Quarta, E.; Paola, M.D.; Casciaro, S. Screening and Early Diagnosis of Osteoporosis through X-ray and Ultrasound Based Techniques. World J. Radiol. 2013, 5, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Marín, F.; López-Bastida, J.; Díez-Pérez, A.; Sacristán, J.A.; ECOSAP DXA Substudy Group Investigators. Bone Mineral Density Referral for Dual-Energy X-ray Absorptiometry Using Quantitative Ultrasound as a Prescreening Tool in Postmenopausal Women from the General Population: A Cost-Effectiveness Analysis. Calcif. Tissue Int. 2004, 74, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Constanze, P.; Eren, Y.; Carsten, M.; Hyungseok, J.; Olav, J.; Cristian, L.; Christian, B.; Claus, C.G.; Sam, S. AI-based automated detection and stability analysis of traumatic vertebral body fractures on computed tomography. Eur. J. Radiol. 2024, 173, 111364. [Google Scholar] [CrossRef]
- Azizi, S.; Culp, L.; Freyberg, J.; Mustafa, B.; Baur, S.; Kornblith, S.; Chen, T.; Tomasev, N.; Mitrović, J.; Strachan, P.; et al. Robust and Data-Efficient Generalization of Self-Supervised Machine Learning for Diagnostic Imaging. Nat. Biomed. Eng. 2023, 7, 756–779. [Google Scholar] [CrossRef]
| Training/Pre-Clinical Dataset | Clinical Validation Dataset | ||||||
|---|---|---|---|---|---|---|---|
| Training | Validation | Test | TCVGH | Joy Clinic | Overall | p-Value * | |
| Number, n | 4188 | 400 | 534 | 304 | 136 | 440 | – |
| Demographics | |||||||
| Female, n (%) | 3263 (78) | 326 (82) | 437 (82) | 250 (82) | 101 (74) | 351 (80) | 0.0544 |
| Male, n (%) | 925 (22) | 74 (18) | 97 (18) | 54 (18) | 35 (26) | 89 (20) | |
| Age (years), mean ± SD | 64.2 ± 13.1 | 62.1 ± 13.8 | 60.7 ± 13.1 | 63.5 ± 12.5 | 60.1 ± 11.9 | 62.5 ± 12.4 | 0.0077 |
| Age ≥ 50 years old, n (%) | 3731 (89) | 334 (84) | 435 (81) | 270 (89) | 106 (78) | 376 (85) | 0.0028 |
| Age < 50 years old, n (%) | 457 (11) | 66 (17) | 99 (19) | 34 (11) | 30 (22) | 64 (15) | |
| Bone Mass Density | |||||||
| BMD (g/cm2), mean ± SD | 1.01 ± 0.20 | 0.99 ± 0.18 | 0.99 ± 0.18 | 0.90 ± 0.18 | 0.95 ± 0.18 | 0.92 ± 0.18 | 0.0074 |
| T-score, mean ± SD | −1.44 ± 1.69 | −1.57 ± 1.53 | −1.61± 1.53 | −2.34 ± 1.49 | −1.89 ± 1.48 | −2.20 ± 1.50 | 0.0035 |
| Z-score, mean ± SD | 0.62 ± 1.59 | 0.34 ± 1.42 | 0.26 ± 1.41 | −0.30 ± 1.29 | −0.06 ± 1.13 | −0.23 ± 1.25 | 0.0619 |
| saBMD 1, n (%) | 1129 (27) | 108 (27) | 152 (28) | 184 (61) | 69 (51) | 253 (58) | – |
| Non-saBMD 2, n (%) | 3059 (73) | 292 (73) | 382 (72) | 120 (39) | 67 (49) | 187 (43) | – |
| Collected images, n (%) | |||||||
| Evaluable 3 | 4188 (100) | 400 (100) | 534 (100) | 300 (99) | 136 (100) | 436 (99) | – |
| Not evaluable 4 | – | – | – | 4 (1) | 0 (0) | 4 (1) | – |
| Pre-Clinical Test | Clinical Validation | ||||
|---|---|---|---|---|---|
| Testing | TCVGH | Joy Clinic | Overall | p-Value * | |
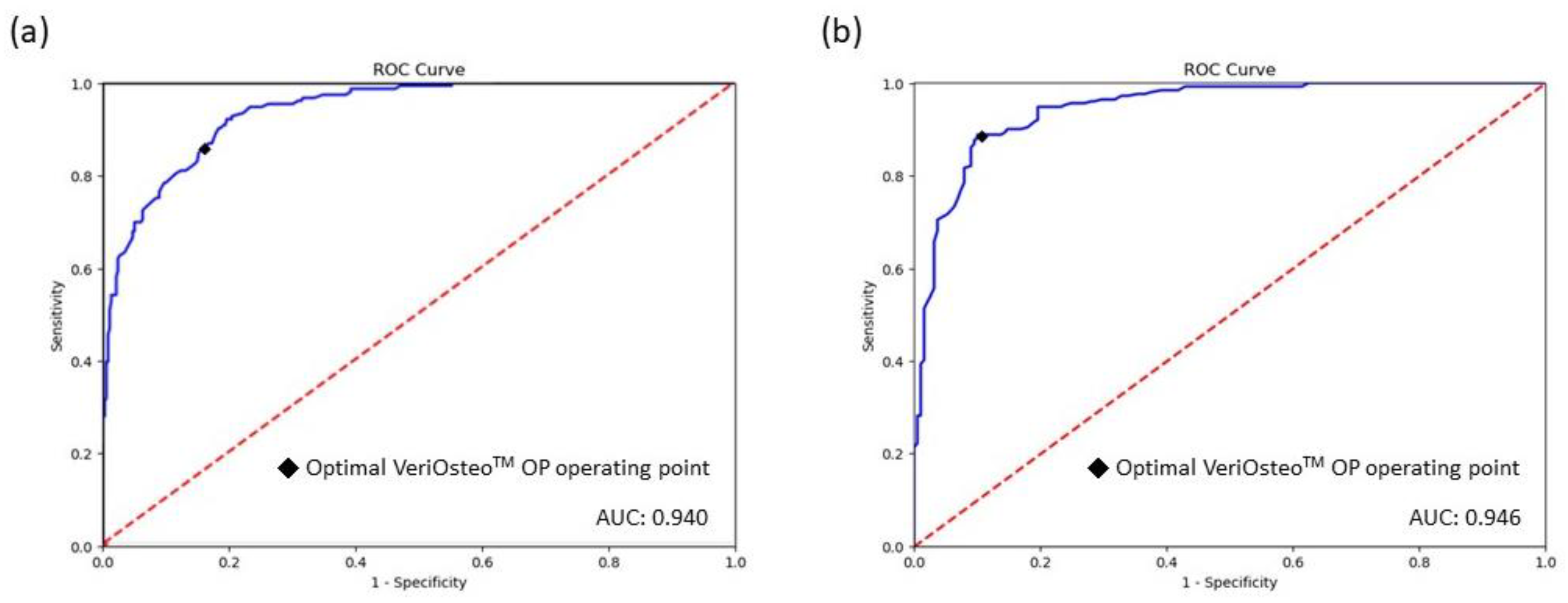
| AUC (95% CI) | 0.940 (0.923–0.957) | 0.948 (0.924–0.972) | 0.938 (0.895–0.980) | 0.946 (0.925–0.967) | 0.6901 |
| Accuracy (%) (95% CI) | 84.5 (81.1–87.4) | 88.3 (84.2–91.7) | 90.4 (84.2–94.8) | 89.0 (85.7–91.8) | 0.5148 |
| Sensitivity (%) (95% CI) | 86.2 (79.7–91.2) | 86.7 (80.9–91.3) | 94.0 (85.4–98.4) | 88.7 (84.1–92.4) | 0.1072 |
| Specificity (%) (95% CI) | 83.8 (79.7–87.3) | 90.8 (84.1–95.3) | 87.0 (76.7–93.9) | 89.4 (84.1–93.4) | 0.4154 |
| PPV (%) (95% CI) | 67.9 (62.5–72.8) | 93.5 (88.6–96.7) | 87.5 (77.6–94.1) | 91.7 (87.4–94.8) | 0.1263 |
| NPV (%) (95% CI) | 93.8 (91.1–95.8) | 81.8 (74.2–88.0) | 93.8 (84.8–98.3) | 85.7 (80.0–90.3) | 0.0252 |
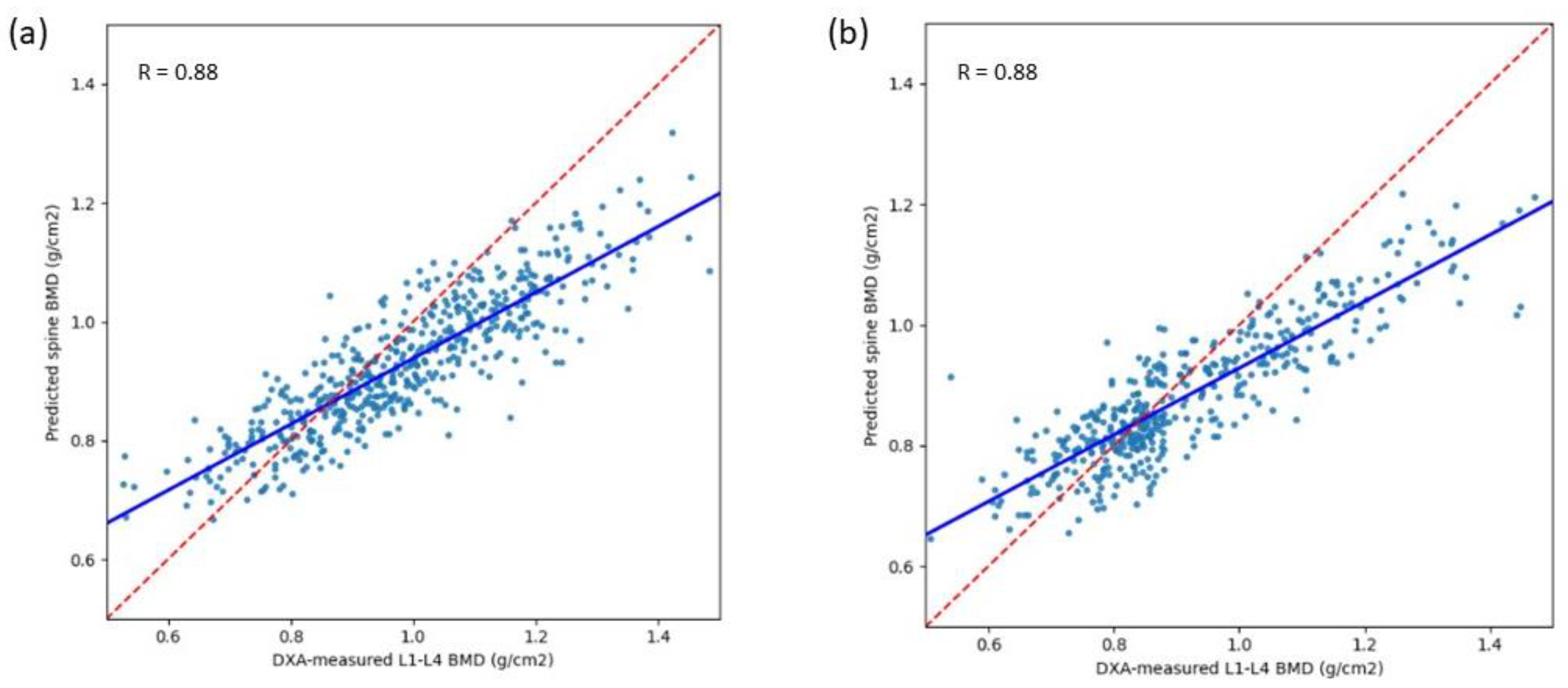
| Pearson’s correlation coefficient (95% CI) | 0.88 (0.86–0.90) | 0.88 (0.85–0.90) | 0.88 (0.84–0.91) | 0.88 (0.86–0.90) | 0.8298 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, S.-C.; Lien, C.-E.; Lee, C.-H.; Tu, K.-C.; Lin, C.-H.; Hsiao, A.Y.; Teng, S.; Chiang, H.-H.; Ke, L.-Y.; Han, C.-L.; et al. Clinical Validation of a Deep Learning-Based Software for Lumbar Bone Mineral Density and T-Score Prediction from Chest X-ray Images. Diagnostics 2024, 14, 1208. https://doi.org/10.3390/diagnostics14121208
Tseng S-C, Lien C-E, Lee C-H, Tu K-C, Lin C-H, Hsiao AY, Teng S, Chiang H-H, Ke L-Y, Han C-L, et al. Clinical Validation of a Deep Learning-Based Software for Lumbar Bone Mineral Density and T-Score Prediction from Chest X-ray Images. Diagnostics. 2024; 14(12):1208. https://doi.org/10.3390/diagnostics14121208
Chicago/Turabian StyleTseng, Sheng-Chieh, Chia-En Lien, Cheng-Hung Lee, Kao-Chang Tu, Chia-Hui Lin, Amy Y. Hsiao, Shin Teng, Hsiao-Hung Chiang, Liang-Yu Ke, Chun-Lin Han, and et al. 2024. "Clinical Validation of a Deep Learning-Based Software for Lumbar Bone Mineral Density and T-Score Prediction from Chest X-ray Images" Diagnostics 14, no. 12: 1208. https://doi.org/10.3390/diagnostics14121208
APA StyleTseng, S.-C., Lien, C.-E., Lee, C.-H., Tu, K.-C., Lin, C.-H., Hsiao, A. Y., Teng, S., Chiang, H.-H., Ke, L.-Y., Han, C.-L., Lee, Y.-C., Huang, A.-C., Yang, D.-J., Tsai, C.-W., & Chen, K.-H. (2024). Clinical Validation of a Deep Learning-Based Software for Lumbar Bone Mineral Density and T-Score Prediction from Chest X-ray Images. Diagnostics, 14(12), 1208. https://doi.org/10.3390/diagnostics14121208








