Changes of Dissociative Properties of Hemoglobin in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
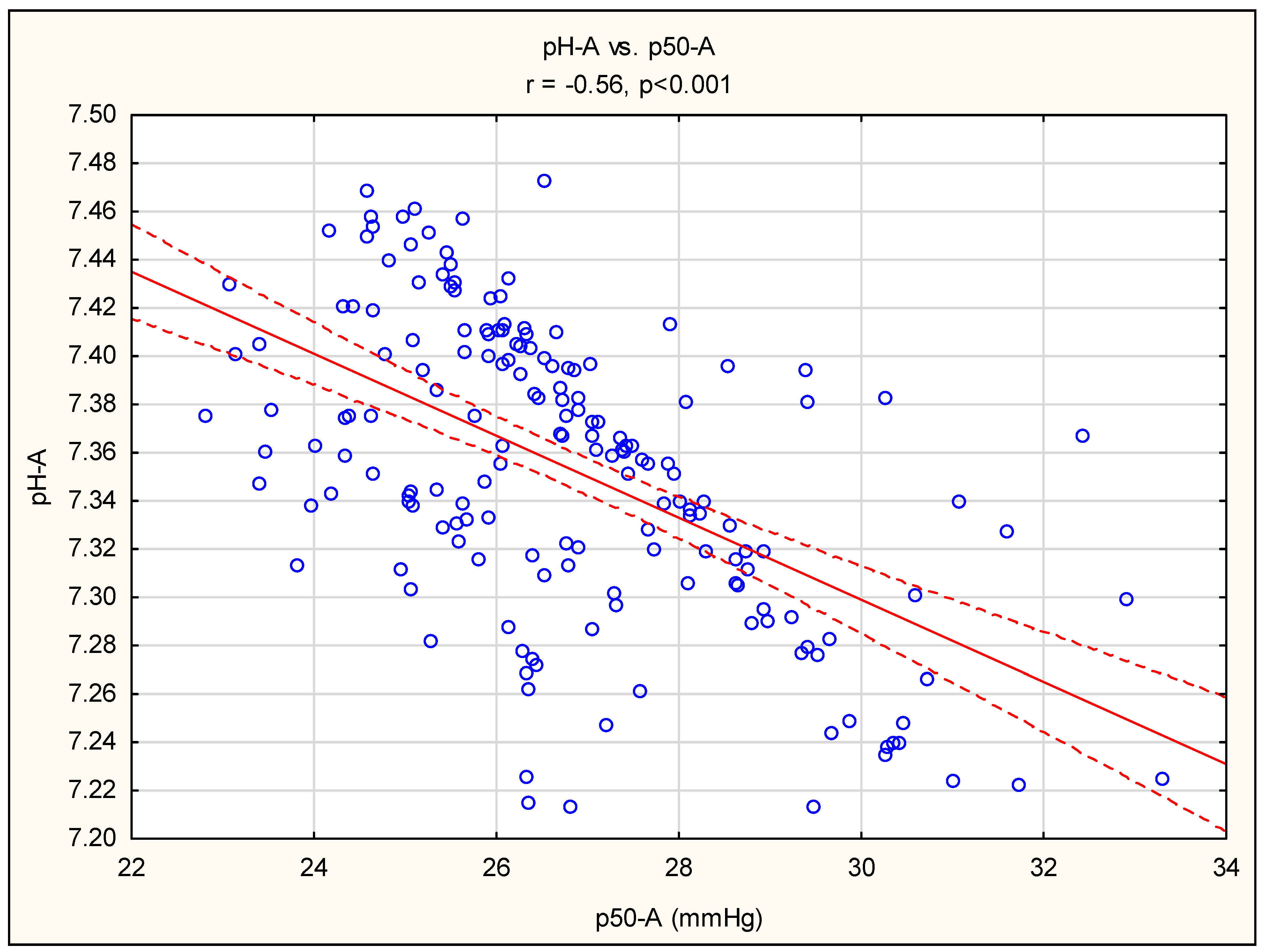
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poyart, C.; Bursaux, E. Current conception of the Bohr effect. Poumon Coeur 1975, 31, 173–177. [Google Scholar] [PubMed]
- Malte, H.; Lykkeboe, G. The Bohr/Haldane effect: A model-based uncovering of the full extent of its impact on O2 delivery to and CO2 removal from tissues. J. Appl. Physiol. 2018, 125, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in chronic kidney disease: From pathophysiology and current treatments, to future agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef] [PubMed]
- Balcerek, B.; Steinach, M.; Lichti, J.; Maggioni, M.A.; Becker, P.N.; Labes, R.; Gunga, H.-C.; Persson, P.B.; Fähling, M. A broad diversity in oxygen affinity to haemoglobin. Sci. Rep. 2020, 10, 16920. [Google Scholar] [CrossRef] [PubMed]
- Ekkernkamp, E.; Welte, L.; Schmoor, C.; Huttmann, S.E.; Dreher, M.; Windisch, W.; Storre, J.H. Spot check analysis of gas exchange: Invasive versus noninvasive methods. Respiration 2015, 89, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Gewin, L.S. Erythrocytes a new/old target for hypoxia in chronic kidney disease? Circ. Res. 2020, 127, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, A. Shifts in the haemoglobin-oxygen dissociation curve: Can we manipulate P50 to good effect? Vet. J. 2007, 175, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.B. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 2004, 182, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.; Murphy, M.; Whitbeck, A.; Kearney, E. Oxygen binding to haemoglobin in subjects with hypoproliferative anaemia, with and without chronic renal disease: Role of pH. Br. J. Haematol. 1974, 27, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Stancu, S.; Mircescu, G.; Mocanu, A.; Capusa, C.; Stefan, G. Metabolic acidosis of chronic kidney disease and cardiovascular disorders. Maedica 2018, 13, 267. [Google Scholar] [PubMed]
- Kim, Y.; Jung, J.H.; Kim, G.E.; Park, M.; Lee, M.; Kim, S.Y.; Kim, M.J.; Kim, Y.H.; Kim, K.W.; Sohn, M.H. P50 implies adverse clinical outcomes in pediatric acute respiratory distress syndrome by reflecting extrapulmonary organ dysfunction. Sci. Rep. 2022, 12, 13666. [Google Scholar] [CrossRef] [PubMed]
- Swenson, E.R. The many acid–base manifestations and consequences of hypoxia. Curr. Opin. Physiol. 2019, 7, 72–81. [Google Scholar] [CrossRef]
- Miguel, V.; Rojo, A. Hypoxia-Driven Responses in Chronic Kidney Disease. Oxygen 2023, 3, 300–321. [Google Scholar] [CrossRef]
- Fu, Q.; Colgan, S.P.; Shelley, C.S. Hypoxia: The force that drives chronic kidney disease. Clin. Med. Res. 2016, 14, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Pastore, Y.D.; Divoky, V.; Liu, E.; Mlodnicka, A.E.; Rainey, K.; Ponka, P.; Semenza, G.L.; Schumacher, A.; Prchal, J.T. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J. Biol. Chem. 2006, 281, 25703–25711. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Wish, J.B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: A potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.W. Minimizing Error in the Determination of P 50. Clin. Chem. 2002, 48, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Gołębiowski, T.; Zmonarski, S.; Rożek, W.; Powązka, M.; Jerzak, P.; Gołębiowski, M.; Kusztal, M.; Olczyk, P.; Stojanowski, J.; Letachowicz, K. Point-of-Care Testing to Differentiate Various Acid–Base Disorders in Chronic Kidney Disease. Diagnostics 2023, 13, 3367. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, A.; Marti, H.R. Adaptation to anemia by decreased oxygen affinity of hemoglobin in patients on dialysis. Kidney Int. 1972, 1, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Rörth, M. Dependency on Acid-base Status of Blood of Oxyhemoglobin Dissociation and 2, 3-Diphosphoglycerate Level in Human Erythrocytes: I. In Vitro Studies on Reduced and Oxygenated Blood. Scand. J. Clin. Lab. Investig. 1970, 26, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Hubchak, S.C.; Liang, X.; Rozen-Zvi, B.; Schumacker, P.T.; Hayashida, T.; Schnaper, H.W. Hypoxia-inducible factor-2α and TGF-β signaling interact to promote normoxic glomerular fibrogenesis. Am. J. Physiol.-Ren. Physiol. 2013, 305, F1323–F1331. [Google Scholar] [CrossRef] [PubMed]
- Nagami, G.T.; Kraut, J.A. The Role of the Endocrine System in the Regulation of Acid–Base Balance by the Kidney and the Progression of Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 2420. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Lubin, B.H. Unstable hemoglobins, hemoglobins with altered oxygen affinity, and m-hemoglobins. Pediatr. Clin. N. Am. 1980, 27, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Hertig, A.; Ferrer-Marin, F. Correction of anaemia on dialysis: Did we forget physiology? Nephrol. Dial. Transplant. 2011, 26, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Wang, Y.; You, G.; Wang, Q.; Ma, N.; Li, B.; Zhao, L.; Zhou, H. The P50 value detected by the oxygenation-dissociation analyser and blood gas analyser. Artif. Cells Nanomed. Biotechnol. 2020, 48, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Cambier, C.; Di Passio, N.; Clerbaux, T.; Amory, H.; Marville, V.; Detry, B.; Frans, A.; Gustin, P. Blood–oxygen binding in healthy Standardbred horses. Vet. J. 2005, 169, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, T.; Kusztal, M.; Konieczny, A.; Kuriata-Kordek, M.; Gawryś, A.; Augustyniak-Bartosik, H.; Letachowicz, K.; Zielińska, D.; Wiśniewska, M.; Krajewska, M. Exhausted capacity of bicarbonate buffer in renal failure diagnosed using point of care analyzer. Diagnostics 2021, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Andreucci, M.; Liberti, M.E.; Bellizzi, V.; Conte, G.; De Nicola, L. New-onset anemia and associated risk of ESKD and death in non-dialysis CKD patients: A multicohort observational study. Clin. Kidney J. 2022, 15, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Aнемії, K. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012, 2, 279. [Google Scholar]
- Pisoni, R.L.; Bragg-Gresham, J.L.; Young, E.W.; Akizawa, T.; Asano, Y.; Locatelli, F.; Bommer, J.; Cruz, J.M.; Kerr, P.G.; Mendelssohn, D.C. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2004, 44, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Nassar, G.M.; Fishbane, S.; Ayus, J.C. Occult infection of old nonfunctioning arteriovenous grafts: A novel cause of erythropoietin resistance and chronic inflammation in hemodialysis patients. Kidney Int. 2002, 61, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.-U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef] [PubMed]

| Causes of CKD | ||||
|---|---|---|---|---|
| non-HD, N = 115 | HD, N = 65 | p * | No (%) | |
| DM and HA (%) | 55 (48) | 18 (28) | >0.05 | 86 (48) |
| Chronic GN (%) | 31 (27) | 18 (28) | >0.05 | 49 (27) |
| ADPKD (%) | 10 (9) | 2 (0) | >0.05 | 12 (6) |
| IN (%) | 5 (4) | 4 (3) | >0.05 | 9 (5) |
| Others (%) | 12 (10) | 12 (18) | >0.05 | 24 (13) |
| Comorbidities ** | ||||
| non-HD, N = 115 | HD, N = 65 | p | All study patients N = 180 | |
| CCI (points) | 6.46 (3.05) | 6.52 (3.21) | >0.05 | 6.48 (3.09) |
| non-HD N = 115 | HD, N = 65 | p * | No (%) | |
| Heart diseases (%) | 74 (64) | 54 (83) | >0.05 | 128 (71) |
| Peripheral vascular disease (%) | 27 (23) | 16 (25) | >0.05 | 43 (23) |
| Cerebrovascular accident (%) | 34 (39) | 21 (32) | >0.05 | 55 (31) |
| Chronic obstructive pulmonary disease (%) | 10 (9) | 6 (9) | >0.05 | 16 (8) |
| Connective tissue disease (%) | 12 (10) | 2 (3) | >0.05 | 14 (8) |
| Gastrointerstitial complications (%) | 16 (14) | 6 (9) | >0.05 | 32 (18) |
| Diabetes mellitus (%) | 39 (34) | 24 (37) | >0.05 | 63 (35) |
| Neoplasmatic disease (%) | 15 (13) | 10 (15) | >0.05 | 25 (14) |
| non-HD, N = 115 | HD, N = 65 | p | |
|---|---|---|---|
| pH-A | 7.33 ± 0.06 | 7.38 ± 0.05 | <0.01 |
| pCO2-A (mmHg) | 34.44 ± 4.98 | 37.06 ± 4.66 | <0.01 |
| pO2-A (mmHg) | 85.64 ± 16.33 | 82.71 ± 16.89 | >0.05 |
| HCO3−-A (mmol/L) | 18.49 ± 3.99 | 22.70 ± 2.95 | <0.01 |
| ABE-A (mmol/L) | −6.62 ± 4.34 | −2.07 ± 3.64 | <0.01 |
| sO2-A (%) | 95.81 ± 3.11 | 95.22 ± 2.97 | >0.05 |
| K+-A (mmol/L) | 4.41 ± 0.78 | 4.77 ± 0.75 | <0.01 |
| Na+-A (mmol/L) | 140.56 ± 3.17 | 139.06 ± 3.06 | <0.01 |
| Ca2+-A (mmol/L) | 1.14 ± 0.10 | 1.09 ± 0.09 | <0.05 |
| Cl−-A (mg/dL) | 112.31 ± 5.55 | 105.78 ± 4.17 | <0.01 |
| Anion Gap-A | 9.78 ± 2.33 | 10.49 ± 2.34 | >0.05 |
| pH-V (mmHg) | 7.27 ± 0.07 | 7.32 ± 0.06 | <0.01 |
| pCO2-V (mmHg) | 37.06 ± 5.04 | 41.09 ± 4.65 | <0.01 |
| pO2-V (%) | 60.53 ± 13.30 | 56.20 ± 11.11 | <0.05 |
| HCO3−-V (mmol/L) | 17.62 ± 4.02 | 20.68 ± 2.93 | <0.01 |
| ABE-V (mmol/L) | −8.47 ± 4.67 | −4.41 ± 3.79 | <0.01 |
| sO2-V (%) | 88.56 ± 7.03 | 86.10 ± 8.00 | <0.05 |
| K+-V (mmol/L) | 4.47 ± 0.75 | 4.89 ± 0.68 | <0.01 |
| Na+-V (mmol/L) | 141.06 ± 3.03 | 139.55 ± 2.81 | <0.01 |
| Ca2+-V (mmol/L) | 1.17 ± 0.11 | 1.12 ± 0.09 | <0.05 |
| Cl−-V (mg/dL) | 112.34 ± 5.40 | 105.63 ± 3.93 | <0.01 |
| Anion gap-V (mmol/L) | 11.17 ± 2.49 | 12.39 ± 2.37 | <0.01 |
| ΔpCO2 (mmHg) | −2.69 ± 2.67 | −4.10 ± 3.74 | <0.01 |
| ΔcHCO3− (mmol/L) | 0.87 ± 1.79 | 1.13 ± 2.41 | >0.05 |
| Total protein (g/dL) | 5.95 ± 0.93 | 6.30 ± 0.80 | <0.05 |
| Albumin (g/dL) | 3.37 ± 0.59 | 3.49 ± 0.48 | >0.05 |
| TC (mg/dL) | 192.11 ± 67.62 | 179.10 ± 55.73 | >0.05 |
| TG (mg/dL) | 156.91 ± 81.20 | 141.35 ± 93.61 | >0.05 |
| CRP (mg/L) | 8.78 ± 14.5 | 14.43 ± 19.9 | <0.05 |
| Pi (mg/dL) | 5.48 ± 1.27 | 5.59 ± 1.59 | >0.05 |
| PTH (pg/mL) | 350.01 ± 229.76 | 412.89 ± 348.28 | >0.05 |
| AP (UI/L) | 75.09 ± 28.83 | 91.52 ± 66.71 | <0.05 |
| BNP (pg/mL) | 563.10 ± 771.42 | 2231.29 ± 5930.58 | <0.05 |
| sCr (mg/dL) | 5.38 ± 1.83 | 6.64 ± 2.44 | <0.01 |
| eGFR (mL/min/1.75 m2) | 11.67 ± 4.24 | 10.01 ± 4.54 | <0.05 |
| Urea (mg/dL) | 146.67 ± 38.55 | 103.34 ± 35.42 | <0.01 |
| p50-A (mmHg) | 26.89 ± 2.02 | 26.68 ± 1.99 | >0.05 |
| p50-V (mmHg) | 26.80 ± 1.83 | 27.34 ± 1.84 | >0.05 |
| Δp50 (mmHg) | 0.08 ± 2.05 | −0.66 ± 1.93 | <0.05 |
| Δp50 (%) | −0.01 ± 7.44 | −2.77 ± 7.31 | <0.05 |
| Mean p50 (mmHg) | 26.85 ± 1.63 | 27.01 ± 1.66 | >0.05 |
| Parameters of erythropoiesis | |||
| Hb (g/dL) | 10.02 ± 1.46 | 10.29 ± 1.78 | >0.05 |
| Fe (μg/dL) | 62.09 ± 32.97 | 61.79 ± 29.41 | >0.05 |
| TIBC (μg/dL) | 222.34 ± 57.43 | 231.26 ± 57.36 | >0.05 |
| TSAT (%) | 28.29 ± 14.52 | 27.78 ± 13.64 | >0.05 |
| Ferritin (ng/mL) | 238.94 ± 245.46 | 274.31 ± 186.4 | >0.05 |
| Weekly dose of erytropoetin (U/week) | 403.51 ± 798.65 | 4914.89 ± 2253.79 | <0.01 |
| Iron supplementation (%) | 40 (35) | 41 (64) | <0.05 * |
| Clinical parameters | |||
| PWV (m/s) | 10.11 ± 2.38 | 9.64 ± 2.59 | >0.05 |
| CCI (point) | 6.46 ± 3.05 | 6.52 ± 3.21 | >0.05 |
| Smoking (pack-year) | 9.93 ± 15.19 | 14.01 ± 14.56 | >0.05 |
| The type of metabolic acidosis | |||
| non-HD, N = 115 | HD, N = 65 | p * | |
| pH-A < 7.33 (%) | 52 (45) | 11 (17) | <0.01 |
| Non-MA-A (%) | 24 (21) | 42 (62) | <0.01 |
| AGMA-A (%) | 49 (43) | 8 (12) | <0.01 |
| HAGMA-A (%) | 42 (37) | 15 (23) | >0.05 |
| Anion gap-A ≥ 10mmo/L (%) | 53 (46) | 35 (54) | >0.05 |
| HCO3−-A ≤ 22 mmol/L (%) | 91 (79) | 23 (35) | <0.01 |
| Non-MA-V (%) | 16 (14) | 22 (34) | <0.05 |
| AGMA-V (%) | 28 (24) | 0 (0) | <0.01 |
| HAGMA-V (%) | 71 (62) | 43 (66) | >0.05 |
| Anion gap V ≥ 10 mmol/L (%) | 82 (71) | 65 (100) | >0.05 |
| HCO3−-V ≤ 22 mmol/L (%) | 91 (86) | 43 (66) | >0.05 |
| non-HD | A | V | p |
| p50 (mmHg) | 26.89 ± 2.02 | 26.81 ± 1.83 | 0.66 |
| pH | 7.33 ± 0.06 | 7.27 ± 0.07 | <0.01 |
| pCO2 (mmHg) | 34.44 ± 4.98 | 37.06 ± 5.04 | <0.01 |
| HCO3− (mmol/L) | 18.49 ± 3.99 | 17.62 ± 4.02 | <0.01 |
| Anion gap (mmol/L) | 9.78 ± 2.33 | 11.17 ± 2.49 | <0.01 |
| ABE (mmol/L) | −6.62 ± 4.34 | −8.47 ± 4.67 | <0.01 |
| SBE (mmol/L) | −7.37 ± 4.78 | −9.14 ± 5.06 | <0.01 |
| HD | A | V | p |
| p50 (mmHg) | 26.68 ± 1.99 | 27.34 ± 1.84 | <0.01 |
| pH | 7.38 ± 0.05 | 7.32 ± 0.06 | <0.01 |
| pCO2 (mmHg) | 37.06 ± 4.66 | 41.09 ± 4.65 | <0.01 |
| HCO3− (mmol/L) | 22.70 ± 2.95 | 20.68 ± 2.93 | <0.01 |
| Anion gap (mmol/L) | 10.49 ± 2.34 | 12.39 ± 2.37 | <0.01 |
| ABE (mmol/L) | −2.07 ± 3.64 | −4.41 ± 3.79 | <0.01 |
| SBE (mmol/L) | −2.39 ± 4.00 | −4.61 ± 4.07 | <0.01 |
| HD | non-HD | |||||
|---|---|---|---|---|---|---|
| p50 < 27.01 mmHg, N = 38 | p50 > 27.01 mmHg, N = 27 | p | p50 < 26.85 mmHg, N = 63 | p50 > 26.85 mmHg, N = 52 | p | |
| pH-A | 7.41 ± 0.04 | 7.35 ± 0.05 | <0.01 | 7.35 ± 0.04 | 7.30 ± 0.06 | <0.01 |
| pCO2-A (mmHg) | 37.07 ± 5.01 | 37.05 ± 4.17 | 0.99 | 35.34 ± 4.20 | 33.36 ± 5.64 | <0.05 |
| pO2-A (mmHg) | 83.10 ± 17.60 | 82.13 ± 16.12 | 0.82 | 82.66 ± 12.95 | 89.25 ± 19.19 | <0.05 |
| HCO3−-A (mmo/L) | 23.79 ± 2.70 | 21.16 ± 2.64 | <0.01 | 20.03 ± 3.16 | 16.63 ± 4.12 | <0.01 |
| ABE-A (mmo/L) | −0.73 ± 3.26 | −3.95 ± 3.36 | <0.01 | −4.80 ± 3.26 | −8.84 ± 4.49 | <0.01 |
| SBE-A (mmo/L) | −0.96 ± 3.62 | −4.41 ± 3.67 | <0.01 | −5.43 ± 3.65 | −9.72 ± 4.96 | <0.01 |
| sO2-A (%) | 95.80 ± 2.67 | 94.41 ± 3.22 | 0.06 | 96.11 ± 1.71 | 95.45 ± 4.23 | 0.26 |
| K+-A (mmo/L) | 4.62 ± 0.73 | 4.97 ± 0.74 | 0.06 | 4.37 ± 0.72 | 4.45 ± 0.85 | 0.56 |
| Na+-A (mmo/L) | 138.97 ± 3.20 | 139.18 ± 2.90 | 0.79 | 140.38 ± 3.15 | 140.78 ± 3.21 | 0.50 |
| Ca2+-A (mmo/L) | 1.10 ± 0.10 | 1.08 ± 0.09 | 0.59 | 1.15 ± 0.11 | 1.12 ± 0.10 | 0.23 |
| Cl−-A (mg/dL) | 105.00 ± 4.03 | 106.85 ± 4.19 | 0.08 | 110.61 ± 4.66 | 114.36 ± 5.89 | <0.01 |
| AG-A (mmo/L) | 9.87 ± 2.20 | 11.35 ± 2.30 | 0.01 | 9.74 ± 2.15 | 9.82 ± 2.55 | 0.84 |
| pH-V | 7.35 ± 0.05 | 7.27 ± 0.05 | <0.01 | 7.30 ± 0.06 | 7.24 ± 0.07 | <0.01 |
| pCO2-V (mmHg) | 40.67 ± 3.82 | 41.69 ± 5.67 | 0.39 | 37.90 ± 4.47 | 36.04 ± 5.54 | <0.05 |
| pO2-V (mmHg) | 56.82 ± 11.16 | 55.29 ± 11.20 | 0.59 | 58.75 ± 10.93 | 62.65 ± 15.50 | 0.12 |
| HCO3−-V (mmo/L) | 21.94 ± 2.44 | 18.90 ± 2.66 | <0.01 | 19.14 ± 3.41 | 15.78 ± 3.97 | <0.01 |
| ABE-V (mmo/L) | −2.80 ± 3.09 | −6.67 ± 3.57 | <0.01 | −6.61 ± 3.86 | −10.72 ± 4.60 | <0.01 |
| SBE-V (mmo/L) | −2.92 ± 3.32 | −6.98 ± 3.89 | <0.01 | −7.15 ± 4.20 | −11.55 ± 5.01 | <0.01 |
| sO2-V (%) | 87.45 ± 6.93 | 84.21 ± 9.11 | 0.11 | 89.03 ± 6.49 | 88.00 ± 7.64 | 0.44 |
| K+-V (mmo/L) | 4.77 ± 0.65 | 5.07 ± 0.70 | 0.08 | 4.43 ± 0.66 | 4.52 ± 0.86 | 0.52 |
| Na+-V (mmo/L) | 139.39 ± 2.85 | 139.77 ± 2.78 | 0.59 | 141.06 ± 2.96 | 141.05 ± 3.13 | 0.99 |
| Ca2+-V (mmo/L) | 1.12 ± 0.09 | 1.12 ± 0.09 | 0.83 | 1.17 ± 0.11 | 1.15 ± 0.10 | 0.27 |
| Cl−-V (mg/dL) | 105.05 ± 3.73 | 106.44 ± 4.12 | 0.16 | 110.60 ± 4.70 | 114.46 ± 5.47 | <0.01 |
| AG-V (mmo/L) | 11.58 ± 1.89 | 13.53 ± 2.53 | <0.01 | 11.36 ± 2.26 | 10.94 ± 2.74 | 0.37 |
| TSH (iuU/mL) | 1.67 ± 1.92 | 1.71 ± 2.10 | 0.95 | 1.59 ± 1.07 | 2.12 ± 1.56 | 0.06 |
| Total protein (g/dL) | 6.18 ± 0.85 | 6.50 ± 0.68 | 0.15 | 6.17 ± 0.86 | 5.68 ± 0.94 | <0.01 |
| Albumin (g/dL) | 3.47 ± 0.47 | 3.52 ± 0.50 | 0.69 | 3.50 ± 0.51 | 3.20 ± 0.64 | <0.01 |
| TC (mg/dL) | 193.10 ± 60.27 | 155.54 ± 37.74 | 0.01 | 194.69 ± 67.63 | 189.10 ± 68.20 | 0.68 |
| TG (mg/dL) | 158.91 ± 108.45 | 111.81 ± 50.84 | 0.06 | 167.82 ± 82.68 | 143.91 ± 78.29 | 0.14 |
| CRP (mg/L) | 10.26 ± 14.37 | 20.75 ± 25.19 | 0.04 | 8.90 ± 17.24 | 9.02 ± 11.24 | 0.97 |
| Pi (mg/dL) | 5.57 ± 1.62 | 5.61 ± 1.58 | 0.93 | 5.26 ± 1.22 | 5.73 ± 1.29 | <0.05 |
| AP (UI/L) | 94.94 ± 73.92 | 85.19 ± 51.94 | 0.60 | 73.20 ± 28.68 | 77.27 ± 29.16 | 0.48 |
| BNP (pg/mL) | 2752.62 ± 6919 | 904.26 ± 1305 | 0.39 | 532.02 ± 859 | 600.78 ± 662 | 0.71 |
| Hb (g/dL) | 9.95 ± 1.61 | 10.76 ± 1.93 | 0.07 | 10.48 ± 1.51 | 9.44 ± 1.18 | <0.01 |
| sCr (mg/dL) | 6.46 ± 2.40 | 6.89 ± 2.53 | 0.48 | 5.37 ± 2.15 | 5.41 ± 1.43 | 0.90 |
| eGFR (mL/min/1.75 m2) | 10.32 ± 3.81 | 9.56 ± 5.46 | 0.51 | 12.22 ± 4.73 | 10.88 ± 3.46 | 0.09 |
| Urea (mg/dL) | 96.21 ± 33.48 | 113.38 ± 36.25 | 0.05 | 143.88 ± 45.28 | 150.69 ± 37.95 | 0.39 |
| PTH (pg/mL) | 427.67 ± 392.02 | 384.27 ± 251.59 | 0.69 | 311.69 ± 194.62 | 392.77 ± 259.20 | 0.09 |
| p50(act)-A (mmHg) | 25.46 ± 1.13 | 28.39 ± 1.67 | <0.01 | 25.67 ± 1.23 | 28.37 ± 1.79 | <0.01 |
| p50(act)-V (mmHg) | 26.26 ± 1.39 | 28.85 ± 1.23 | <0.01 | 25.71 ± 1.36 | 28.13 ± 1.41 | <0.01 |
| Δp50(act) (mmHg) | −0.80 ± 1.96 | −0.46 ± 1.91 | 0.49 | −0.04 ± 1.97 | 0.24 ± 2.16 | 0.45 |
| Δp50(act) (%) | −3.38 ± 7.79 | −1.91 ± 6.63 | 0.43 | −0.44 ± 7.52 | 0.51 ± 7.38 | 0.49 |
| Mean p50(act) (mmHg) | 25.86 ± 0.79 | 28.62 ± 1.11 | <0.01 | 25.69 ± 0.84 | 28.25 ± 1.19 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korus, J.; Wydro, M.; Gołębiowski, M.; Krakowska, K.; Poznański, P.; Musiał, K.; Konieczny, A.; Augustyniak-Bartosik, H.; Stojanowski, J.; Kusztal, M.A.; et al. Changes of Dissociative Properties of Hemoglobin in Patients with Chronic Kidney Disease. Diagnostics 2024, 14, 1219. https://doi.org/10.3390/diagnostics14121219
Korus J, Wydro M, Gołębiowski M, Krakowska K, Poznański P, Musiał K, Konieczny A, Augustyniak-Bartosik H, Stojanowski J, Kusztal MA, et al. Changes of Dissociative Properties of Hemoglobin in Patients with Chronic Kidney Disease. Diagnostics. 2024; 14(12):1219. https://doi.org/10.3390/diagnostics14121219
Chicago/Turabian StyleKorus, Justyna, Maria Wydro, Maciej Gołębiowski, Kornelia Krakowska, Paweł Poznański, Kinga Musiał, Andrzej Konieczny, Hanna Augustyniak-Bartosik, Jakub Stojanowski, Mariusz Andrzej Kusztal, and et al. 2024. "Changes of Dissociative Properties of Hemoglobin in Patients with Chronic Kidney Disease" Diagnostics 14, no. 12: 1219. https://doi.org/10.3390/diagnostics14121219






