Incidence of Carotid Blowout Syndrome in Patients with Head and Neck Cancer after Radiation Therapy: A Cohort Study
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Recruitment and Data
2.2. Grouping
2.3. Methods of RT
2.4. Follow-Up and Outcomes
2.5. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Suarez, C.; Fernandez-Alvarez, V.; Hamoir, M.; Mendenhall, W.M.; Strojan, P.; Quer, M.; Silver, C.E.; Rodrigo, J.P.; Rinaldo, A.; Ferlito, A. Carotid blowout syndrome: Modern trends in management. Cancer Manag. Res. 2018, 10, 5617–5628. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.C.; Lirng, J.F.; Luo, C.B.; Guo, W.Y.; Teng, M.M.; Tai, S.K.; Chang, C.Y. Carotid blowout syndrome in patients with head-and-neck cancers: Reconstructive management by self-expandable stent-grafts. AJNR Am. J. Neuroradiol. 2007, 28, 181–188. [Google Scholar] [PubMed]
- Leikensohn, J.; Milko, D.; Cotton, R. Carotid artery rupture. Management and prevention of delayed neurologic sequelae with low-dose heparin. Arch. Otolaryngol. 1978, 104, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Shumrick, D.A. Carotid artery rupture. Laryngoscope 1973, 83, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.A.; Oller, D.W.; Gee, W.; deFries, H.O. Elective carotid artery resection. Arch. Otolaryngol. 1975, 101, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Maran, A.G.; Amin, M.; Wilson, J.A. Radical neck dissection: A 19-year experience. J. Laryngol. Otol. 1989, 103, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.; Gahleitner, C.; Bier, H.; Knopf, A. Chemoradiation and local recurrence of head and neck squamous cell carcinoma and the risk of carotid artery blowout. Head Neck 2019, 41, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Myers, L.L.; Bradford, C.R.; Chepeha, D.B.; Hogikyan, N.D.; Teknos, T.N.; Terrell, J.E.; Wolf, G.T.; Eisbruch, A. Conformal re-irradiation of recurrent and new primary head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 377–385. [Google Scholar] [CrossRef]
- Tanvetyanon, T.; Padhya, T.; McCaffrey, J.; Zhu, W.; Boulware, D.; Deconti, R.; Trotti, A. Prognostic factors for survival after salvage reirradiation of head and neck cancer. J. Clin. Oncol. 2009, 27, 1983–1991. [Google Scholar] [CrossRef]
- Duprez, F.; Madani, I.; Bonte, K.; Boterberg, T.; Vakaet, L.; Derie, C.; De Gersem, W.; De Neve, W. Intensity-modulated radiotherapy for recurrent and second primary head and neck cancer in previously irradiated territory. Radiother. Oncol. 2009, 93, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Lartigau, E.F.; Tresch, E.; Thariat, J.; Graff, P.; Coche-Dequeant, B.; Benezery, K.; Schiappacasse, L.; Degardin, M.; Bondiau, P.Y.; Peiffert, D.; et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother. Oncol. 2013, 109, 281–285. [Google Scholar] [CrossRef] [PubMed]
- De Crevoisier, R.; Bourhis, J.; Domenge, C.; Wibault, P.; Koscielny, S.; Lusinchi, A.; Mamelle, G.; Janot, F.; Julieron, M.; Leridant, A.M.; et al. Full-dose reirradiation for unresectable head and neck carcinoma: Experience at the Gustave-Roussy Institute in a series of 169 patients. J. Clin. Oncol. 1998, 16, 3556–3562. [Google Scholar] [CrossRef] [PubMed]
- Salama, J.K.; Vokes, E.E.; Chmura, S.J.; Milano, M.T.; Kao, J.; Stenson, K.M.; Witt, M.E.; Haraf, D.J. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Iseli, T.A.; Iseli, C.E.; Rosenthal, E.L.; Caudell, J.J.; Spencer, S.A.; Magnuson, J.S.; Smith, A.N.; Carroll, W.R. Postoperative reirradiation for mucosal head and neck squamous cell carcinomas. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1158–1164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cengiz, M.; Özyiğit, G.; Yazici, G.; Doğan, A.; Yildiz, F.; Zorlu, F.; Gürkaynak, M.; Gullu, I.H.; Hosal, S.; Akyol, F. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ogita, M.; Kodani, N.; Nakamura, S.; Inoue, H.; Himei, K.; Kotsuma, T.; Yoshida, K.; Yoshioka, Y.; Yamashita, K.; et al. Frequency, outcome and prognostic factors of carotid blowout syndrome after hypofractionated re-irradiation of head and neck cancer using CyberKnife: A multi-institutional study. Radiother. Oncol. 2013, 107, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Yazici, G.; Sanlı, T.Y.; Cengiz, M.; Yuce, D.; Gultekin, M.; Hurmuz, P.; Yıldız, F.; Zorlu, F.; Akyol, F.; Gurkaynak, M.; et al. A simple strategy to decrease fatal carotid blowout syndrome after stereotactic body reirradiaton for recurrent head and neck cancers. Radiat. Oncol. 2013, 8, 242. [Google Scholar] [CrossRef]
- Karam, I.; Yao, M.; Heron, D.E.; Poon, I.; Koyfman, S.A.; Yom, S.S.; Siddiqui, F.; Lartigau, E.; Cengiz, M.; Yamazaki, H.; et al. Survey of current practices from the International Stereotactic Body Radiotherapy Consortium (ISBRTC) for head and neck cancers. Future Oncol. 2017, 13, 603–613. [Google Scholar] [CrossRef]
- Ling, D.C.; Vargo, J.A.; Gebhardt, B.J.; Grimm, R.J.; Clump, D.A.; Ferris, R.L.; Ohr, J.P.; Heron, D.E. Dose-response modeling the risk of carotid bleeding events after stereotactic body radiation therapy for previously irradiated head and neck cancer. J. Radiosurg SBRT 2019, 6, 83–89. [Google Scholar]
- Aihara, T.; Hiratsuka, J.; Ishikawa, H.; Kumada, H.; Ohnishi, K.; Kamitani, N.; Suzuki, M.; Sakurai, H.; Harada, T. Fatal carotid blowout syndrome after BNCT for head and neck cancers. Appl. Radiat. Isot. 2015, 106, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Padua, P.F.; Fang, H.Y.; Young, C.K.; Yeh, C.H.; Lin, C.C.; Liao, C.T.; Chang, T.J.; Tsao, C.K.; Huang, S.F. Carotid arterial blowout after organ preserving chemoradiation therapy in hypopharyngeal cancer. Medicine 2022, 101, e31391. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Paulson, E.; Li, X.A.; Erickson, B.; Schultz, C.; Tree, A.; Awan, M.; Low, D.A.; McDonald, B.A.; Salzillo, T.; et al. Magnetic resonance linear accelerator technology and adaptive radiation therapy: An overview for clinicians. CA Cancer J. Clin. 2022, 72, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.J.; Chen, K.W.; Chen, M.H.; Chu, P.Y.; Tai, S.K.; Wang, L.W.; Chang, P.M.; Yang, M.H. Predisposing factors, management, and prognostic evaluation of acute carotid blowout syndrome. J. Vasc. Surg. 2013, 58, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D., Jr.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Lin, C.Y.; Hsieh, C.H.; Hsu, C.L.; Fan, K.H.; Chang, J.T.; Huang, S.F.; Kang, C.J.; Liao, C.T.; Ng, S.H.; et al. Induction chemotherapy with dose-modified docetaxel, cisplatin, and 5-fluorouracil in Asian patients with borderline resectable or unresectable head and neck cancer. J. Formos. Med. Assoc. 2017, 116, 185–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weinberg, J.H.; Sweid, A.; Joffe, D.; Piper, K.; Abbas, R.; Hussain, Z.; Anderson, B.; Gooch, M.R.; Herial, N.; Tjoumakaris, S.; et al. Carotid Blowout Management in the Endovascular Era. World Neurosurg. 2020, 141, e1010–e1016. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, N.F.; Rezaee, R.P.; Ray, A.; Wick, C.C.; Blackham, K.; Stepnick, D.; Lavertu, P.; Zender, C.A. Contemporary management of carotid blowout syndrome utilizing endovascular techniques. Laryngoscope 2017, 127, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.L.; Guedes, B.D.; Duvvuri, U.; Singh, M.J.; Chaer, R.A.; Makaroun, M.S.; Sachdev, U. Outcomes of interventions for carotid blowout syndrome in patients with head and neck cancer. J. Vasc. Surg. 2016, 63, 1525–1530. [Google Scholar] [CrossRef]
- Chang, F.C.; Luo, C.B.; Lirng, J.F.; Lin, C.J.; Lee, H.J.; Wu, C.C.; Hung, S.C.; Guo, W.Y. Endovascular Management of Post-Irradiated Carotid Blowout Syndrome. PLoS ONE 2015, 10, e0139821. [Google Scholar] [CrossRef]
- Bond, K.M.; Brinjikji, W.; Murad, M.H.; Cloft, H.J.; Lanzino, G. Endovascular treatment of carotid blowout syndrome. J. Vasc. Surg. 2017, 65, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Rueda Domínguez, A.; Cirauqui, B.; García Castaño, A.; Alvarez Cabellos, R.; Carral Maseda, A.; Castelo Fernández, B.; Iglesias Rey, L.; Rubió-Casadevall, J.; Arrazubi, V.; Mesía, R. SEOM-TTCC clinical guideline in nasopharynx cancer (2021). Clin. Transl. Oncol. 2022, 24, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Board, P.D.Q.A.T.E. Laryngeal Cancer Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Herrmann, J.; Yang, E.H.; Iliescu, C.A.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.; Leesar, M.A.; Grines, C.L.; Marmagkiolis, K. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation 2016, 133, 1272–1289. [Google Scholar] [CrossRef] [PubMed]
- Mikus, E.; Zucchetta, F.; Carigi, S.; Tripodi, A. Asymptomatic giant circumflex aneurysm in patient with a cisplatin treatment history. J. Card. Surg. 2021, 36, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Leopardi, M.; Di Marco, E.; Musilli, A.; Ricevuto, E.; Bruera, G.; Ventura, M. Effects of Chemotherapy in Patients with Concomitant Aortic Aneurysm and Malignant Disease. Ann. Vasc. Surg. 2017, 45, 268.e13–268.e20. [Google Scholar] [CrossRef] [PubMed]
- Cannavale, A.; Corona, M.; Nardis, P.; De Rubeis, G.; Cannavale, G.; Santoni, M.; De Gyurgyokai, S.Z.; Catalano, C.; Bezzi, M. Computed Tomography Angiography findings can predict massive bleeding in head and neck tumours. Eur. J. Radiol. 2020, 125, 108910. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hu, J.; Huang, Q.; Cai, W.; Zhuang, Z.; Liu, H.; Hou, J.; Liu, X.; Wang, C. Risk factors and nomogram for predicting carotid blowout syndrome based on computed tomography angiography. Oral. Dis. 2022, 28, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wang, C.P.; Wang, C.C.; Jiang, R.S.; Lin, J.C.; Liu, S.A. Carotid blowout in patients with head and neck cancer: Associated factors and treatment outcomes. Head Neck 2015, 37, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Xirasagar, S.; Cheng, Y.F.; Chen, C.S.; Chang, W.P.; Lin, H.C. Trends in the incidence of head and neck cancer: A nationwide population-based study. Oral. Oncol. 2023, 140, 106391. [Google Scholar] [CrossRef]
- Yamazaki, H.; Suzuki, G.; Aibe, N.; Nakamura, S.; Yoshida, K. Fractionation or tumor factors-what matters in carotid blowout syndrome? Strahlenther. Onkol. 2021, 197, 744–745. [Google Scholar] [CrossRef]
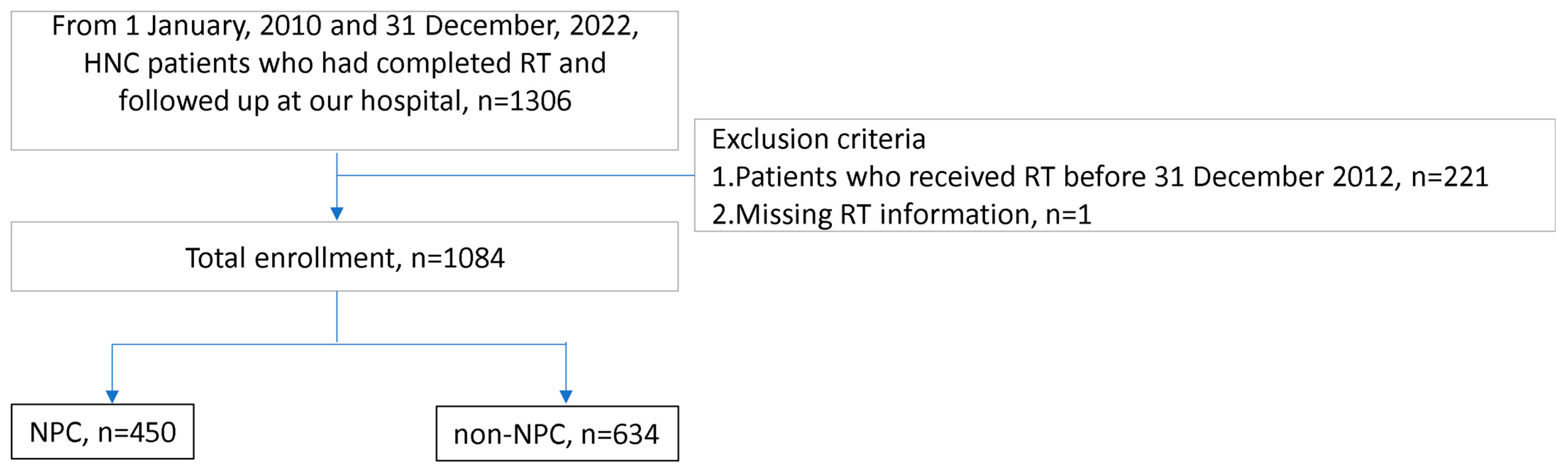

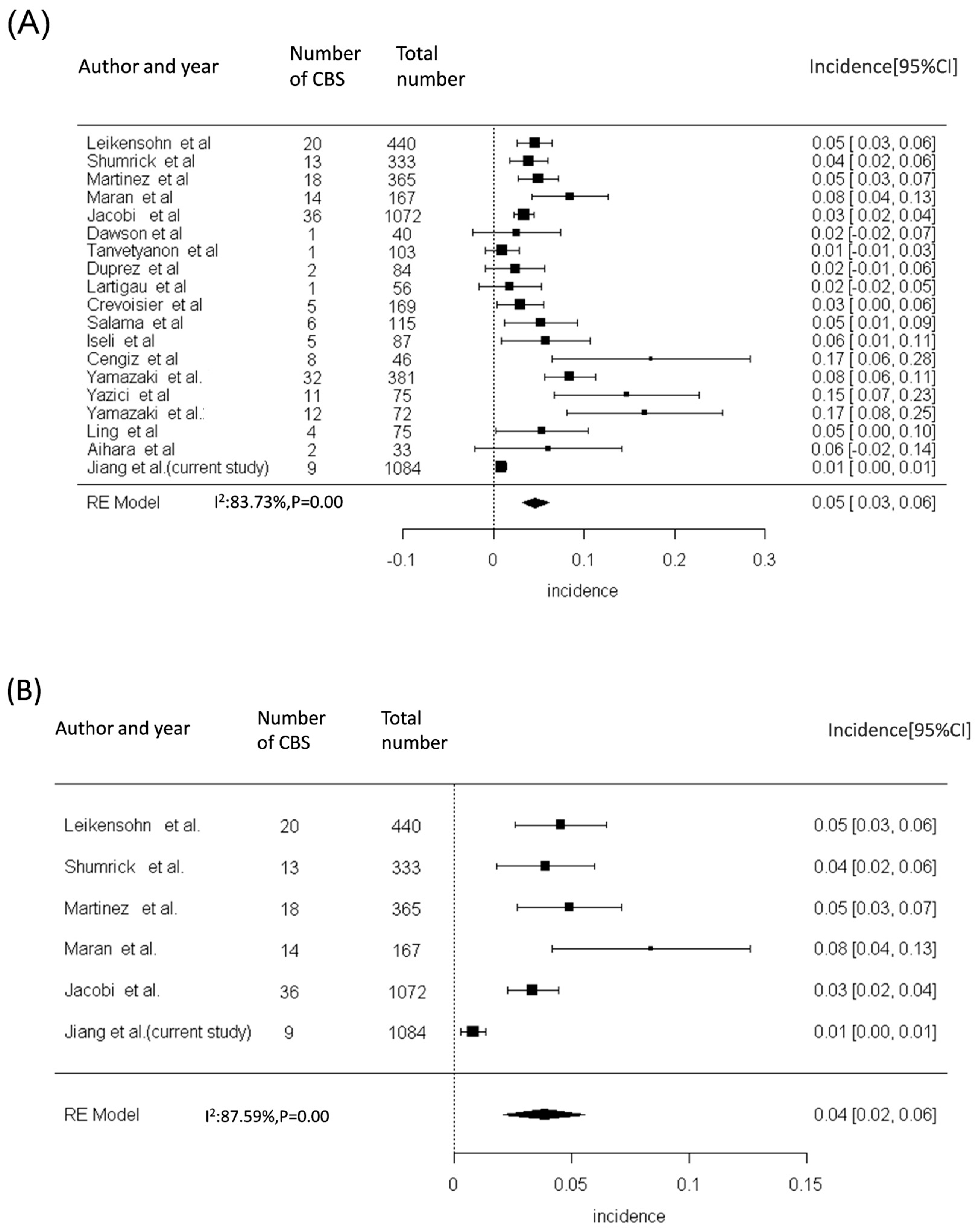
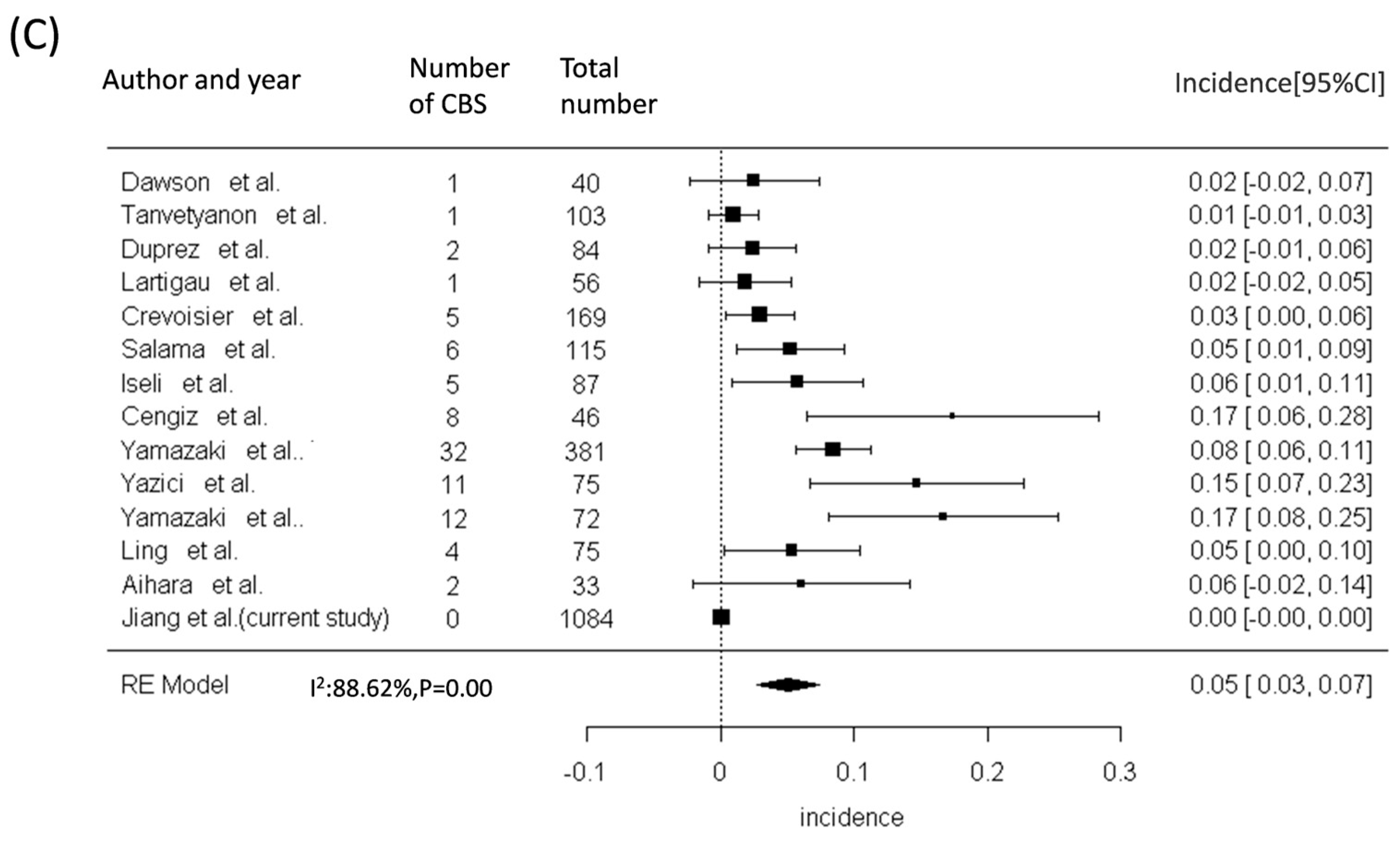

| Total, n = 1084 | Non-NPC, n = 634 | NPC, n = 450 | p-Value | |
|---|---|---|---|---|
| Gender, male (%) | 915 (84%) | 555 (88%) | 360 (80%) | <0.001 |
| Age, years | 56 (48, 63) | 59 (51, 65) | 51 (43, 59) | <0.001 |
| Diabetes mellitus (%) | 123 (11%) | 76 (12%) | 47 (10%) | 0.430 |
| Hypertension (%) | 198 (18%) | 127 (20%) | 71 (16%) | 0.074 |
| Dyslipidemia (%) | 150 (14%) | 90 (14%) | 60 (13%) | 0.685 |
| Smoking | <0.001 | |||
| Never | 619 (57%) | 306 (48%) | 313 (70%) | |
| Current | 137 (13%) | 102 (16%) | 35 (7%) | |
| Former | 328 (30%) | 226 (36%) | 102 (23%) | |
| Cancer type | <0.001 | |||
| NPC | 450(41.5%) | 0 | 450(100%) | |
| Oral cancer | 448(41.3%) | 448(70.7%) | 0 | |
| Hypopharyngeal cancer | 98(9%) | 98(15.5%) | 0 | |
| Laryngeal cancer | 62(5.7%) | 62(9.8%) | 0 | |
| Others | 26(2.5%) | 26(4%) | 0 | |
| Proton therapy (%) | 241 (22%) | 82 (13%) | 159 (35%) | <0.001 |
| Reirradiation (%) | 63 (5.8%) | 43 (6.8%) | 20 (4.4%) | 0.105 |
| Radiation dose (Mean ± standard deviation, Centi-gray) | 6996 ± 749 | 6600 ± 692 | 6996 ± 785 | <0.001 |
| Chemotherapy | 992 (92%) | 567 (89%) | 425 (94%) | 0.004 |
| Surgery (%) | 271 (25%) | 246 (39%) | 7 (1.6%) | <0.001 |
| Follow-up duration, month | 52.3 (30.3, 80) | 52 (29.8, 80.5) | 52.5 (30.75, 79.3) | 0.711 |
| Characteristic | Total, n = 1084 | Non-NPC, n = 634 | NPC, n = 450 | p-Value |
|---|---|---|---|---|
| Primary outcome | ||||
| Carotid blowout syndrome | 9 (0.8%) | 6 (0.9%) | 3 (0.7%) | 0.743 |
| Secondary outcomes | ||||
| Pseudoaneurysm | 6 (0.6%) | 3 (0.5%) | 3(0.7%) | 0.697 |
| Mortality | 2 (0.2%) | 2 (0.3%) | 0 (0%) | 0.514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.-L.; Chang, J.T.-C.; Yeh, C.-H.; Chang, T.-Y.; Huang, B.-S.; Sung, P.-S.; Lin, C.-Y.; Fan, K.-H.; Wei, Y.-C.; Liu, C.-H. Incidence of Carotid Blowout Syndrome in Patients with Head and Neck Cancer after Radiation Therapy: A Cohort Study. Diagnostics 2024, 14, 1222. https://doi.org/10.3390/diagnostics14121222
Jiang J-L, Chang JT-C, Yeh C-H, Chang T-Y, Huang B-S, Sung P-S, Lin C-Y, Fan K-H, Wei Y-C, Liu C-H. Incidence of Carotid Blowout Syndrome in Patients with Head and Neck Cancer after Radiation Therapy: A Cohort Study. Diagnostics. 2024; 14(12):1222. https://doi.org/10.3390/diagnostics14121222
Chicago/Turabian StyleJiang, Jian-Lin, Joseph Tung-Chieh Chang, Chih-Hua Yeh, Ting-Yu Chang, Bing-Shen Huang, Pi-Shan Sung, Chien-Yu Lin, Kang-Hsing Fan, Yi-Chia Wei, and Chi-Hung Liu. 2024. "Incidence of Carotid Blowout Syndrome in Patients with Head and Neck Cancer after Radiation Therapy: A Cohort Study" Diagnostics 14, no. 12: 1222. https://doi.org/10.3390/diagnostics14121222
APA StyleJiang, J.-L., Chang, J. T.-C., Yeh, C.-H., Chang, T.-Y., Huang, B.-S., Sung, P.-S., Lin, C.-Y., Fan, K.-H., Wei, Y.-C., & Liu, C.-H. (2024). Incidence of Carotid Blowout Syndrome in Patients with Head and Neck Cancer after Radiation Therapy: A Cohort Study. Diagnostics, 14(12), 1222. https://doi.org/10.3390/diagnostics14121222







