Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Meta-Analysis of Randomized Controlled Trials and Trial Sequential Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Strategy
2.3. Outcomes
2.4. Statistical Analysis
2.5. Quality of Evidence Assessment
3. Results
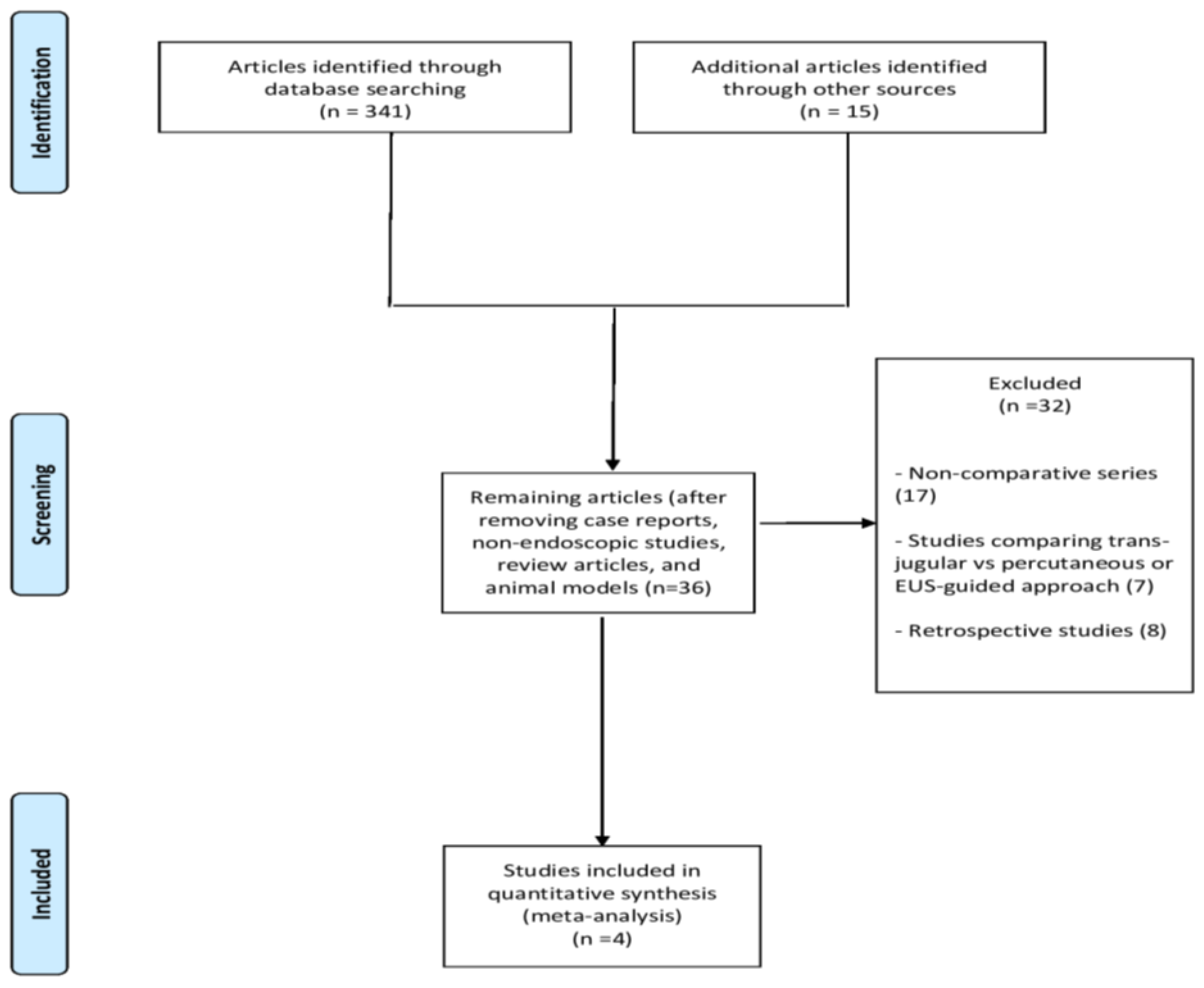
3.1. Included Studies
3.2. Sample Adequacy
3.3. Other Outcomes
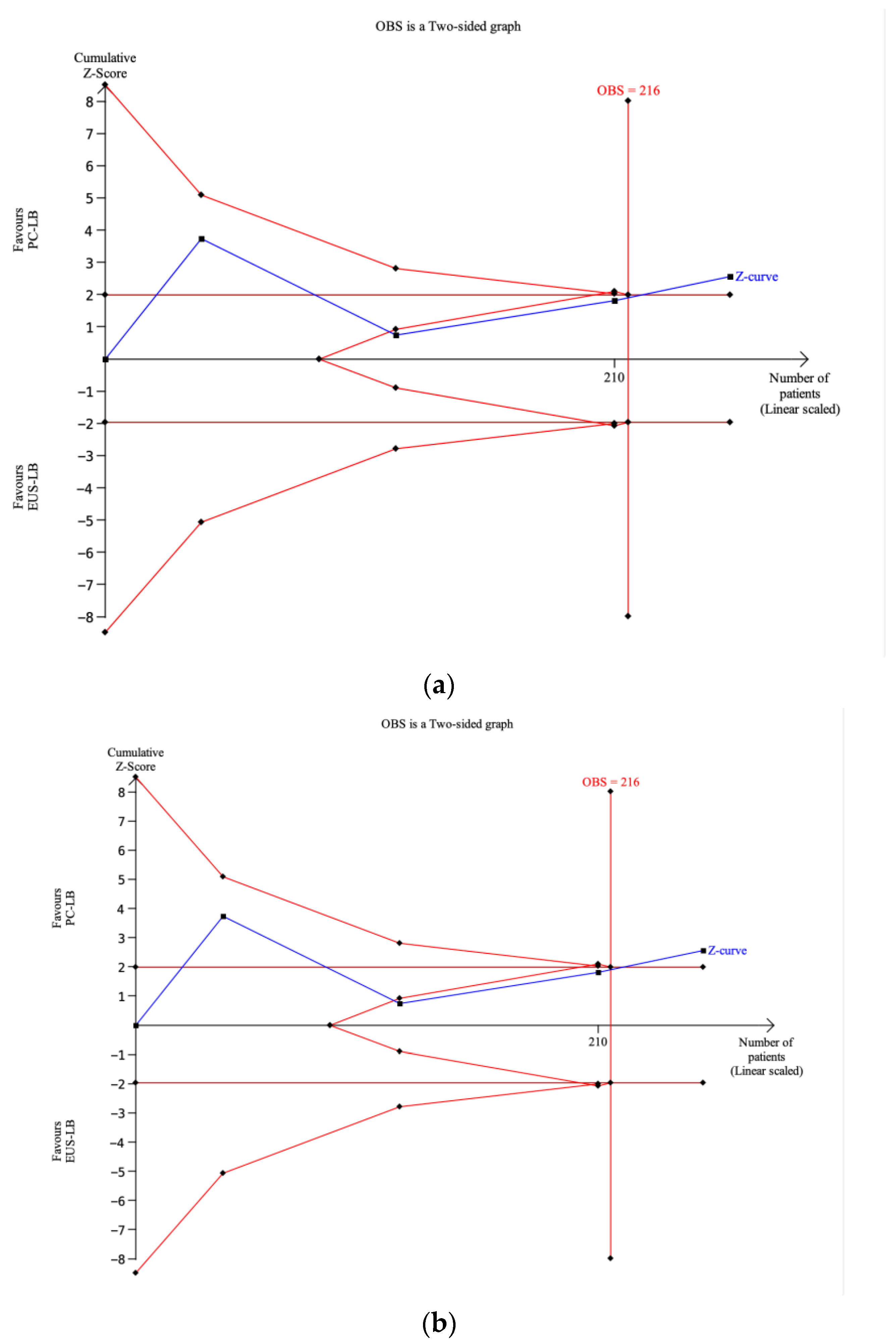
3.4. Trial Sequential Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Garcia Perdomo, H.A.; Muscatiello, N.; Buccino, R.V.; Wong, V.W.; Singh, S. Systematic review with meta-analysis: Change in liver stiffness during anti-viral therapy in patients with hepatitis B. Dig. Liver Dis. 2018, 50, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Thomaides-Brears, H.B.; Alkhouri, N.; Allende, D.; Harisinghani, M.; Noureddin, M.; Reau, N.S.; French, M.; Pantoja, C.; Mouchti, S.; Cryer, D.R. Incidence of Complications from Percutaneous Biopsy in Chronic Liver Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 3366–3394. [Google Scholar] [CrossRef]
- Lavian, J.D.; Thornton, L.M.; Zybulewski, A.; Kim, E.; Nowakowski, S.F.; Ranade, M.; Patel, R.S.; Lookstein, R.A.; Fischman, A.; Bishay, V. Safety of percutaneous versus transjugular liver biopsy: A propensity score matched analysis. Eur. J. Radiol. 2020, 133, 109399. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.W.; Qin, M.; Jiang, H.X.; Qin, S.Y. Comparison of EUS-FNA and EUS-FNB for diagnosis of solid pancreatic mass lesions: A meta-analysis of prospective studies. Scand. J. Gastroenterol. 2024, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Crinò, S.F.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Colombo, M.; Ramai, D.; Mangiavillano, B.; Chandan, S.; Gkolfakis, P.; et al. Comparative diagnostic yield of different endoscopic techniques for tissue sampling of upper gastrointestinal subepithelial lesions: A network meta-analysis. Endoscopy 2024, 56, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.P.; Shakhatreh, M.; Garg, R.; Ponnada, S.; Adler, D.G. Efficacy and safety of EUS-guided liver biopsy: A systematic review and meta-analysis. Gastrointest. Endosc. 2019, 89, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ching-Companioni, R.A.; Diehl, D.L.; Johal, A.S.; Confer, B.D.; Khara, H.S. 19G aspiration needle versus 19G core biopsy needle for endoscopic ultrasound-guided liver biopsy: A prospective randomized trial. Endoscopy 2019, 51, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Ward, T.J.; Guirguis, S.; Krall, K.; Contreras, F.; Jhala, N.; Navaneethan, U.; Hawes, R.H.; Varadarajulu, S. Radiology-guided percutaneous approach is superior to EUS for performing liver biopsies. Gut 2021, 70, 2224–2226. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Shah, B.; Jagtap, N.; Angadi, S.; Jain, A.K.; Afzalpurkar, S.; Giri, S. Diagnostic efficacy of endoscopic ultrasound-guided liver biopsy for diffuse liver diseases and its predictors—A multicentric retrospective analysis. Clin. Exp. Hepatol. 2023, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lariño-Noia, J.; Fernández-Castroagudín, J.; de la Iglesia-García, D.; Lázare, H.; Nieto, L.; Porto, S.; Vallejo-Senra, N.; Molina, E.; San Bruno, A.; Martínez-Seara, X.; et al. Quality of Tissue Samples Obtained by Endoscopic Ultrasound-Guided Liver Biopsy: A Randomized, Controlled Clinical Trial. Am. J. Gastroenterol. 2023, 118, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Chavan, R.; Gupta, P.; Chalamarla, L.K.; Dhar, J.; Choudhoury, A.; Panda, I.; Mitra, S.; Sekaran, A.; Das, A.; et al. Eus-guided liver biopsy scores over radiology guided percutaneous liver biopsy: A multicenter randomised controlled trial. Gastrointest. Endosc. 2022, 95, AB485–AB486. [Google Scholar] [CrossRef]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Fabbri, C.; Mangiavillano, B.; Lisotti, A.; Muscatiello, N.; Cotsoglou, C.; Fusaroli, P. Diagnostic yield of endoscopic ultrasound-guided liver biopsy in comparison to percutaneous liver biopsy: A systematic review and meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chandan, S.; Deliwala, S.; Khan, S.R.; Mohan, B.P.; Dhindsa, B.S.; Bapaye, J.; Goyal, H.; Kassab, L.L.; Kamal, F.; Sayles, H.R.; et al. EUS-guided versus percutaneous liver biopsy: A comprehensive review and meta-analysis of outcomes. Endosc. Ultrasound. 2023, 12, 171–180. [Google Scholar] [PubMed]
- Ali, A.H.; Nallapeta, N.S.; Yousaf, M.N.; Petroski, G.F.; Sharma, N.; Rao, D.S.; Yin, F.; Davis, R.M.; Bhat, A.; Swi, A.I.A.; et al. EUS-guided versus percutaneous liver biopsy: A prospective randomized clinical trial. Endosc. Ultrasound. 2023, 12, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wetterslev, J.; Jakobsen, J.C.; Gluud, C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D.; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Claire, R.; Gluud, C.; Berlin, I.; Coleman, T.; Leonardi-Bee, J. Using trial sequential analysis for estimating the sample sizes of further trials: Example using smoking cessation intervention. BMC Med. Res. Methodol. 2020, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Bignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Dargère, D.; Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, F.; Sagnelli, E.; Pasquale, G.; Giusti, G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J. Hepatol. 1986, 2, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, C.; Hocke, M.; Fusaroli, P.; Gilja, O.H.; Buscarini, E.; Havre, R.F.; Ignee, A.; Saftoiu, A.; Vilmann, P.; Burmester, E.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV—EUS-guided interventions: General Aspects and EUS-guided Sampling (Short Version). Ultraschall. Med. 2016, 37, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Diehl, D.L.; Sangwan, V.; Johal, A.S.; Khara, H.S.; Conver, B. A 19-gauge fine-needle biopsy needle compared with a 22-gauge fine-needle biopsy needle for EUS-guided liver biopsy: A prospective randomized study. Gastrointest. Endosc. 2024, 99, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Ching-Companioni, R.A.; Johal, A.S.; Confer, B.D.; Forster, E.; Khara, H.S.; Diehl, D.L. Single-pass 1-needle actuation versus single-pass 3-needle actuation technique for EUS-guided liver biopsy sampling: A randomized prospective trial (with video). Gastrointest. Endosc. 2021, 94, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Saraireh, H.; Abdelfattah, T.; Hassouneh, R.; Lippman, R.; Puri, P.; Mutha, P.; Singh, S.; Fuchs, M.; Shah, T. “Wet Heparin” and “Wet Saline” EUS-Guided Liver Biopsy Techniques Both Provide High Rates of Specimen Adequacy for Benign Parenchymal Liver Disease. Dig. Dis. Sci. 2022, 67, 5256–5261. [Google Scholar] [CrossRef]

| Study | Country | Study Period | Sample Size | Age | Gender Male | Indication (Abnormal Liver Function Tests) | Number of Needle Passes | Needle Used | Definition of Adequate Samples |
|---|---|---|---|---|---|---|---|---|---|
| Bang 2021 [11] | USA | 2019–2020 | EUS-LB: 21 PC-LB: 19 | 55 ± 15.9 53.7 ± 14.6 | 8 (38.1%) 6 (31.6%) | NR | 2 1 | 19 G Acquire® 16 G Biopince® | Specimen length ≥ 25 mm and number of CPTs ≥ 11 |
| Ali 2023 [17] | USA | 2020–2021 | EUS-LB: 40 PC-LB: 40 | 52.5 (19–76) 53 (25–75) | 13 (32.5%) 13 (32.5%) | 25 (62.5%) 27 (67.5%) | 2 (1–3) 3 (1–7) | 19 G Acquire® or Sharcore® 18 G needle | Number of CPTs ≥ 11 |
| Larino-Noia 2023 [13] | Spain | 2022 | EUS-LB: 44 PC-LB: 46 | 60.8 ± 8 58.8 ± 11.2 | 22 (50%) 21 (45.7%) | NR | 1 (1–2) 1 (1–2) | 19 G Acquire® 16 G Biopince® | Specimen length ≥ 20 mm and number of CPTs ≥ 11 |
| Samanta 2023 [14] a | India | 2020–2021 | EUS-LB: 24 PC-LB: 24 | 37.04 ± 11.6 37.46 ± 13.9 | 18 (75%) 12 (50%) | 9 (37.5%) 6 (25%) | NR | 19 G FNA 18 G needle | Specimen length ≥ 20 mm and number of CPTs ≥ 11 |
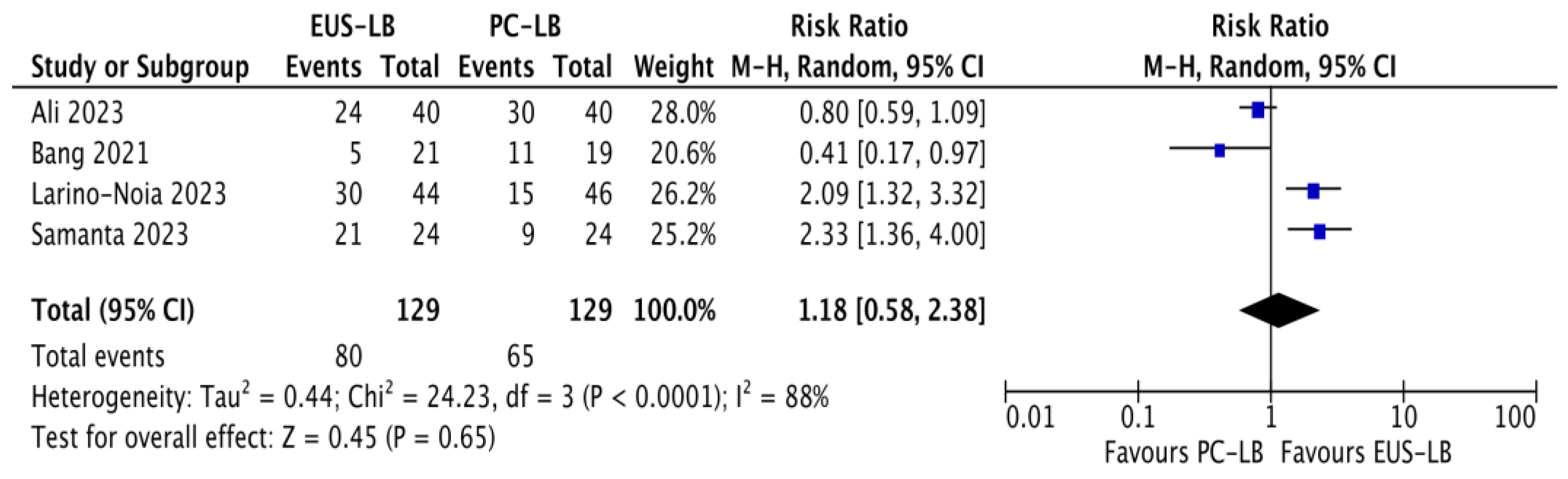
| Outcome | Interventions | No. of Studies | No. of Patients | Risk Ratio (95% CI) | Within-Group Heterogeneity (I2) |
|---|---|---|---|---|---|
| Optimal core procurement rate | EUS-LB PC-LB | 4 | 129 129 | 1.18 (0.58–2.38) p = 0.65 | 88% |
| Diagnostic accuracy | EUS-LB PC-LB | 4 | 129 129 | 1.0 (0.95–1.05) p = 0.88 | 0% |
| Outcome | Interventions | No. of Studies | No. of Patients | Mean Difference (95% CI) | Within-group heterogeneity (I2) |
| Number of CPTs | EUS-LB PC-LB | 3 | 108 110 | 2.29 (−4.08 to 8.66) p = 0.48 | 90% |
| Max specimen length | EUS-LB PC-LB | 4 | 129 129 | −3.11 (−5.51 to −0.71) p = 0.01 | 75% |
| Total specimen length | EUS-LB PC-LB | 3 | 108 110 | −0.51 (−20.92 to 19.90) p = 0.96 | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, M.; Samanta, J.; Spadaccini, M.; Fugazza, A.; Crinò, S.F.; Gkolfakis, P.; Triantafyllou, K.; Dhar, J.; Maida, M.; Pugliese, N.; et al. Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Meta-Analysis of Randomized Controlled Trials and Trial Sequential Analysis. Diagnostics 2024, 14, 1238. https://doi.org/10.3390/diagnostics14121238
Bhandari M, Samanta J, Spadaccini M, Fugazza A, Crinò SF, Gkolfakis P, Triantafyllou K, Dhar J, Maida M, Pugliese N, et al. Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Meta-Analysis of Randomized Controlled Trials and Trial Sequential Analysis. Diagnostics. 2024; 14(12):1238. https://doi.org/10.3390/diagnostics14121238
Chicago/Turabian StyleBhandari, Megha, Jayanta Samanta, Marco Spadaccini, Alessandro Fugazza, Stefano Francesco Crinò, Paraskevas Gkolfakis, Konstantinos Triantafyllou, Jahnvi Dhar, Marcello Maida, Nicola Pugliese, and et al. 2024. "Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Meta-Analysis of Randomized Controlled Trials and Trial Sequential Analysis" Diagnostics 14, no. 12: 1238. https://doi.org/10.3390/diagnostics14121238






