An Innovative Virtual Reality System for Measuring Refractive Error
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
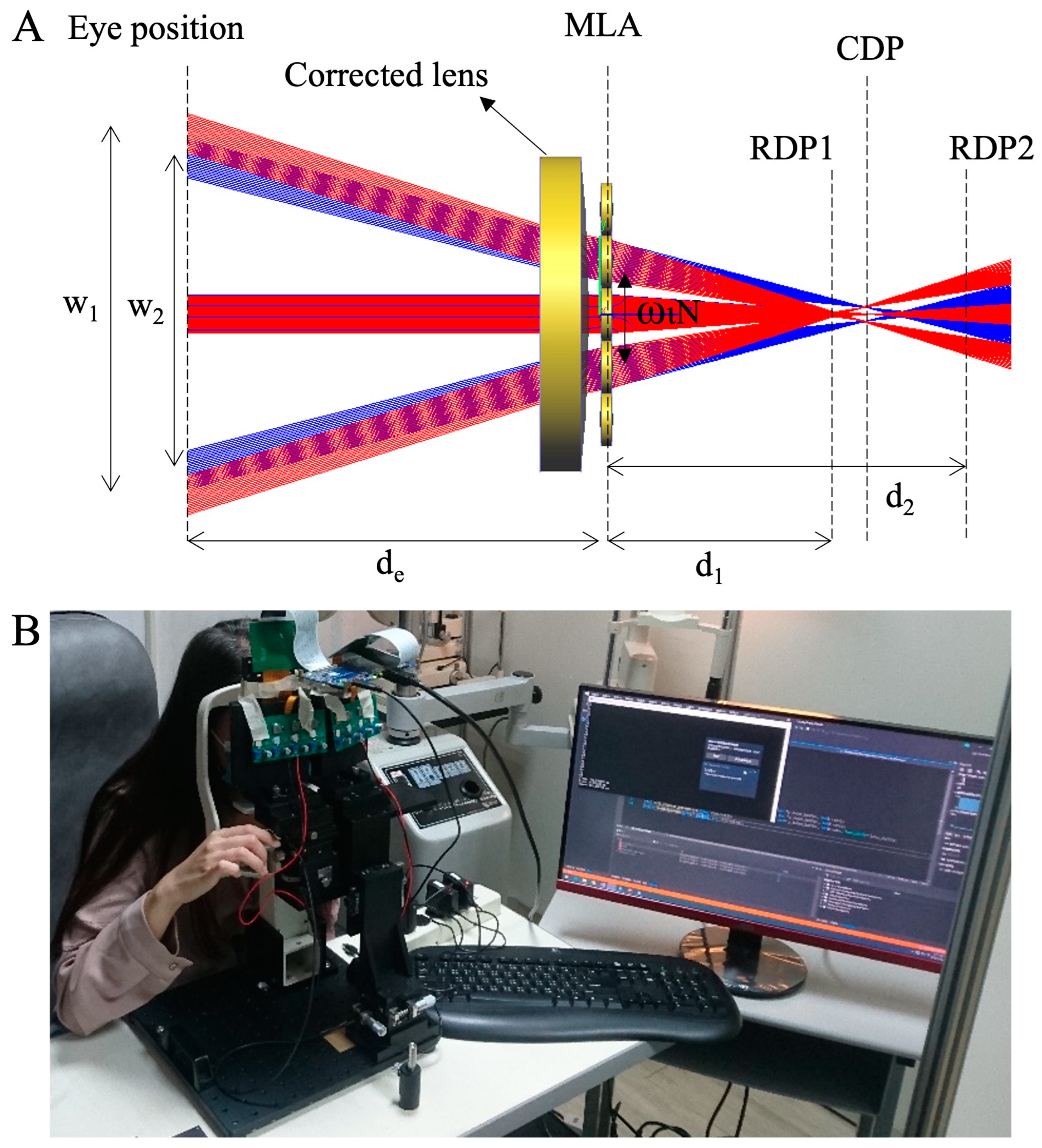
2.2. Light Field Virtual Reality
2.3. Testing Procedure
- (1)
- SE = S + (C/2)
- (2)
- J0 = −(C/2) × cos(2θ)
- (3)
- J45 = −(C/2) × sin(2θ)
2.4. Statistical Analysis
3. Results
3.1. Evaluation of Refractive Components via LFVR and Other Clinical Methods
3.2. Correlation and Repeatability of Refractive Components Measured by LFVR versus Other Clinical Methods
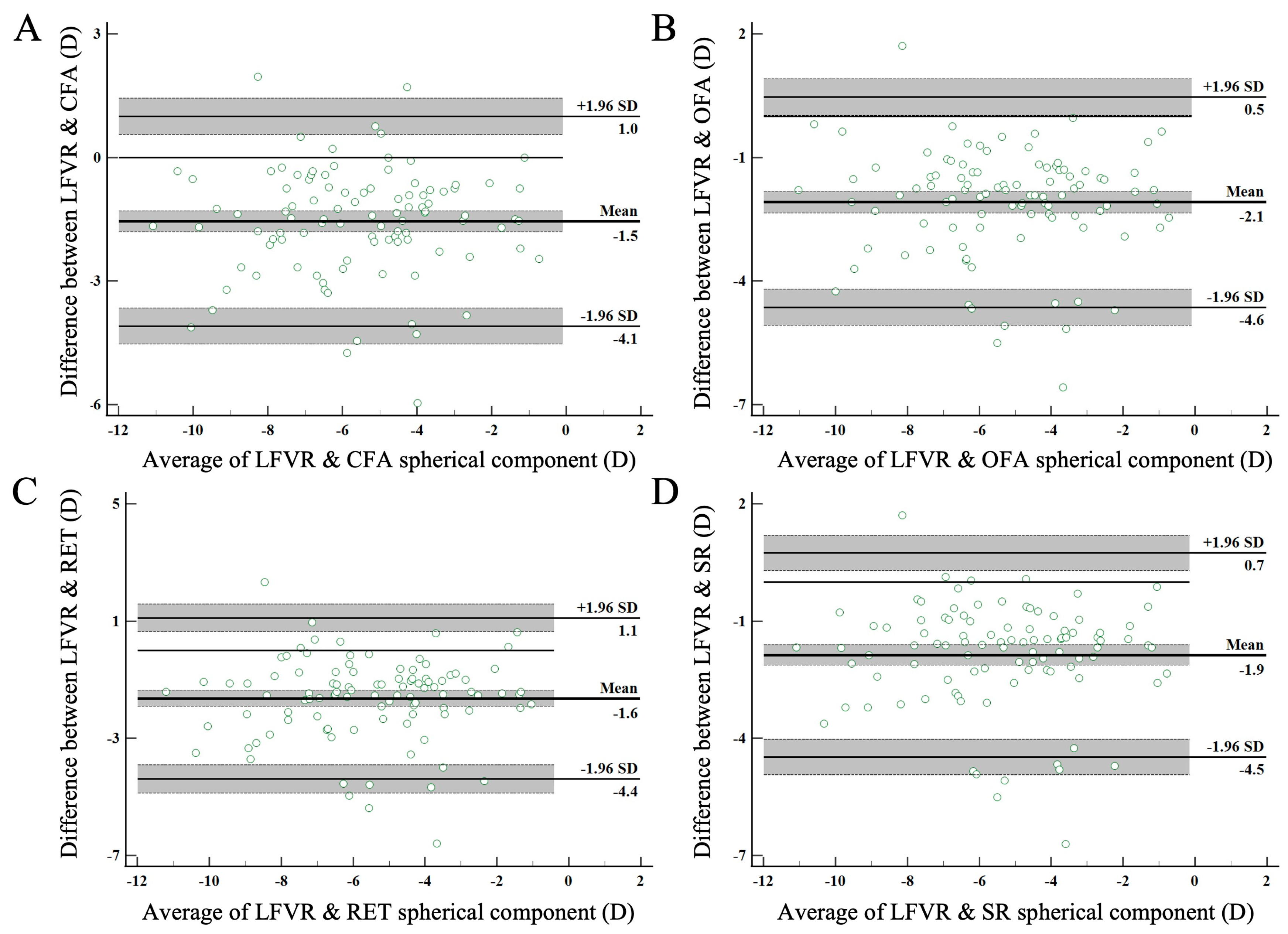
3.3. Agreement between LFVR and Other Clinical Methods
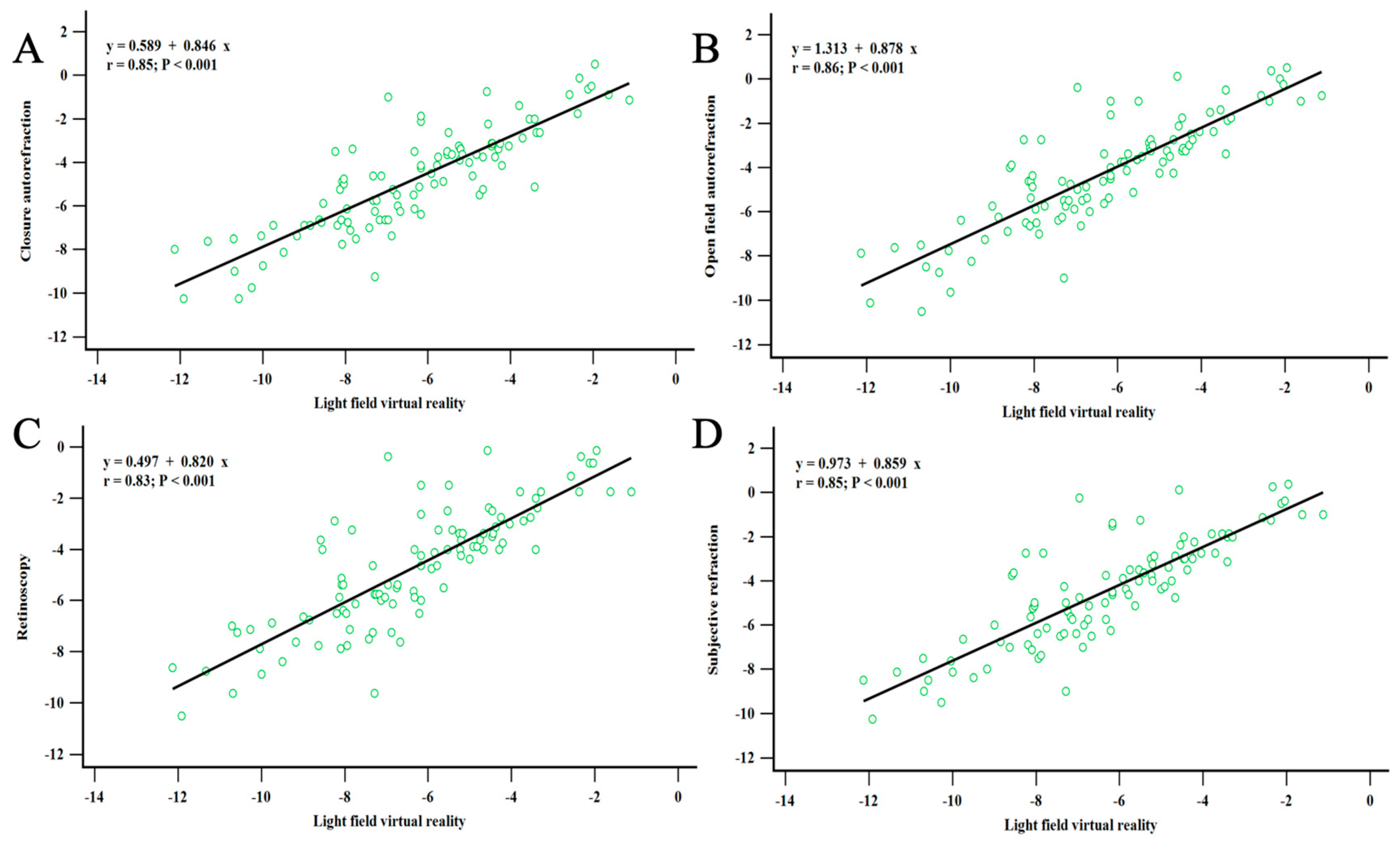
3.4. Correlation between Refractive Errors Measured by LFVR and Other Clinical Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashemi, H.; Fotouhi, A.; Yekta, A.; Pakzad, R.; Ostadimoghaddam, H.; Khabazkhoob, M. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J. Curr. Ophthalmol. 2018, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.S.; Jaggernath, J. Uncorrected refractive errors. Indian J. Ophthalmol. 2012, 60, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990-2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, J.H.; Kakizaki, H.; Jee, D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5579–5583. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Shih, Y.F.; Hsiao, C.K.; Chen, C.J. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann. Acad. Med. Singap. 2004, 33, 27–33. [Google Scholar] [PubMed]
- Smith, T.S.; Frick, K.D.; Holden, B.A.; Fricke, T.R.; Naidoo, K.S. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull. World Health Organ. 2009, 87, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, Y. Overview of the Complications of High Myopia. Retina 2017, 37, 2347–2351. [Google Scholar] [CrossRef] [PubMed]
- Dean, W.H.; Buchan, J.C.; Gichuhi, S.; Faal, H.; Mpyet, C.; Resnikoff, S.; Gordon, I.; Matende, I.; Samuel, A.; Visser, L.; et al. Ophthalmology training in sub-Saharan Africa: A scoping review. Eye 2021, 35, 1066–1083. [Google Scholar] [CrossRef]
- Oam, B.A.; Resnikoff, S. The role of optometry in vision 2020. Community Eye Health 2002, 15, 33–36. [Google Scholar]
- Cleary, G.; Spalton, D.J.; Patel, P.M.; Lin, P.F.; Marshall, J. Diagnostic accuracy and variability of autorefraction by the Tracey Visual Function Analyzer and the Shin-Nippon NVision-K 5001 in relation to subjective refraction. Ophthalmic Physiol. Opt. 2009, 29, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.N.; Mallen, E.A.; Wolffsohn, J.S.; Gilmartin, B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom. Vis. Sci. 2003, 80, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Shneor, E.; Millodot, M.; Avraham, O.; Amar, S.; Gordon-Shaag, A. Clinical evaluation of the L80 autorefractometer. Clin. Exp. Optom. 2012, 95, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Citek, K.; Feldman, J.M. Comparison of refractive error measurements in adults with Z-View aberrometer, Humphrey autorefractor, and subjective refraction. Optometry 2011, 82, 231–240. [Google Scholar] [CrossRef]
- Hennessy, R.T. Instrument myopia. J. Opt. Soc. Am. 1975, 65, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Cervino, A.; Hosking, S.L.; Rai, G.K.; Naroo, S.A.; Gilmartin, B. Wavefront analyzers induce instrument myopia. J. Refract. Surg. 2006, 22, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Goebels, S.; Käsmann-Kellner, B.; Eppig, T.; Seitz, B.; Langenbucher, A. Can retinoscopy keep up in keratoconus diagnosis? Cont. Lens Anterior Eye 2015, 38, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, W.J., 3rd; Pesudovs, K.; Marsack, J.D.; Sarver, E.J.; Applegate, R.A. Quantifying scatter in Shack-Hartmann images to evaluate nuclear cataract. J. Refract. Surg. 2004, 20, S515–S522. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahrouqi, H.; Oraba, S.B.; Al-Habsi, S.; Mundemkattil, N.; Babu, J.; Panchatcharam, S.M.; Al-Saidi, R.; Al-Raisi, A. Retinoscopy as a Screening Tool for Keratoconus. Cornea 2019, 38, 442–445. [Google Scholar] [CrossRef]
- Otero, C.; Vilaseca, M.; Arjona, M.; Martínez-Roda, J.A.; Pujol, J. Repeatability of aberrometric measurements with a new instrument for vision analysis based on adaptive optics. J. Refract. Surg. 2015, 31, 188–194. [Google Scholar] [CrossRef]
- Lebow, K.A.; Campbell, C.E. A comparison of a traditional and wavefront autorefraction. Optom. Vis. Sci. 2014, 91, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Courtney-Harris, M.; Jolly, N.; Rowe, F.; Rose, K. Validation of a vision-screening tool for use by nurses and other non-eye care health practitioners on stroke survivors. Contemp. Nurse 2022, 58, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.L.; Dukhovny, D.; Chan, R.V.P.; Chiang, M.F.; Campbell, J.P. Cost-effectiveness of Artificial Intelligence-Based Retinopathy of Prematurity Screening. JAMA Ophthalmol. 2022, 140, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ma, Y.; Gao, L. Compact light field photography towards versatile three-dimensional vision. Nat. Commun. 2022, 13, 3333. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M. Amblyopia (lazy eye) in children. CMAJ 2014, 186, 292. [Google Scholar] [CrossRef] [PubMed]
- Garnham, L.; Sloper, J.J. Effect of age on adult stereoacuity as measured by different types of stereotest. Br. J. Ophthalmol. 2006, 90, 91–95. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, J.; Ma, Q.; Wu, J.; Du, B.; Zhang, H. Hybrid Computational Near-Eye Light Field Display. IEEE Photonics J. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Yao, C.; Cheng, D.; Yang, T.; Wang, Y. Design of an optical see-through light-field near-eye display using a discrete lenslet array. Opt. Express 2018, 26, 18292–18301. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Wu, J.-Y.; Chou, P.-Y.; Xie, J.; Huang, C.-T.; Chen, J.-L.; Shieh, H.-P.; Huang, Y.-P. 68-1: Investigation on Defocusing-Induced Accommodation Shift in Microlens Array-Based Near-Eye Light Field Displays. SID Symp. Dig. Tech. Pap. 2020, 51, 1009–1012. [Google Scholar] [CrossRef]
- Qin, Z.; Chou, P.-Y.; Wu, J.-Y.; Chen, Y.T.; Huang, C.T.; Balram, N.; Huang, Y.-P. Image formation modeling and analysis of near-eye light field displays. J. Soc. Inf. Disp. 2019, 27, 238–250. [Google Scholar] [CrossRef]
- Thibos, L.N.; Wheeler, W.; Horner, D. Power vectors: An application of Fourier analysis to the description and statistical analysis of refractive error. Optom. Vis. Sci. 1997, 74, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Carkeet, A. A Review of the Use of Confidence Intervals for Bland-Altman Limits of Agreement in Optometry and Vision Science. Optom. Vis. Sci. 2020, 97, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Carkeet, A. Exact parametric confidence intervals for Bland-Altman limits of agreement. Optom. Vis. Sci. 2015, 92, e71–e80. [Google Scholar] [CrossRef]
- Zou, G.Y. Confidence interval estimation for the Bland-Altman limits of agreement with multiple observations per individual. Stat. Methods Med. Res. 2013, 22, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Ondategui-Parra, J.C.; Badiella, L.; Otero, C.; Vilaseca, M.; Aldaba, M. Spherical subjective refraction with a novel 3D virtual reality based system. J. Optom. 2017, 10, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.W.; Moon, N.J.; Lee, W.K.; Jeon, S. Ophthalmological factors influencing visual asthenopia as a result of viewing 3D displays. Br. J. Ophthalmol. 2012, 96, 1391–1394. [Google Scholar] [CrossRef]
- Goyal, A.; Bopardikar, A.; Tiwari, V.N. Estimation of Spherical Refractive Errors Using Virtual Reality Headset. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4973–7976. [Google Scholar]
- Wesner, M.F.; Miller, R.J. Instrument myopia conceptions, misconceptions, and influencing factors. Doc. Ophthalmol. 1986, 62, 281–308. [Google Scholar] [CrossRef]
- Salmon, T.O.; van de Pol, C. Evaluation of a clinical aberrometer for lower-order accuracy and repeatability, higher-order repeatability, and instrument myopia. Optometry 2005, 76, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Richards, O.W.; Mathews, S.M.; Shaffer, S.M. Focused apparent image position with convergent, parallel binocular and monocular microscopes. J. Microsc. 1981, 122, 187–191. [Google Scholar] [CrossRef]
- Schober, H.A.W.; Dehler, H.; Kassel, R. Accommodation During Observations with Optical Instruments. J. Opt. Soc. Am. 1970, 60, 103–106. [Google Scholar] [CrossRef]
- Richards, O.W. Instrument myopia--microscopy. Am. J. Optom. Physiol. Opt. 1976, 53, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.W.; Schmid, K.L.; Lam, C.S.; Edwards, M.H. Objective real-time measurement of instrument myopia in microscopists under different viewing conditions. Vis. Res. 2006, 46, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Duane, A. Studies in Monocular and Binocular Accommodation with their Clinical Applications. Am. J. Ophthalmol. 1922, 5, 865–877. [Google Scholar] [CrossRef]
- Charman, W.N. Near vision, lags of accommodation and myopia. Ophthalmic Physiol. Opt. 1999, 19, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Momeni-Moghaddam, H.; Goss, D.A.; Sobhani, M. Accommodative response under monocular and binocular conditions as a function of phoria in symptomatic and asymptomatic subjects. Clin. Exp. Optom. 2014, 97, 36–42. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, J.; Park, S.W.; Heo, H. Influence of virtual reality on visual parameters: Immersive versus non-immersive mode. BMC Ophthalmol. 2020, 20, 200. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, H.S.; Sung, M.S.; Park, S.W.; Heo, H. Effects of prolonged use of virtual reality smartphone-based head-mounted display on visual parameters: A randomised controlled trial. Sci. Rep. 2021, 11, 15382. [Google Scholar] [CrossRef]
- Hoffman, D.M.; Girshick, A.R.; Akeley, K.; Banks, M.S. Vergence-accommodation conflicts hinder visual performance and cause visual fatigue. J. Vis. 2008, 8, 33. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.; Kim, H.; Park, C.Y. Visual fatigue induced by watching virtual reality device and the effect of anisometropia. Ergonomics 2021, 64, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Triantaphillidou, S.; Jarvis, J.; Psarrou, A.; Gupta, G. Contrast sensitivity in images of natural scenes. Signal Process. Image Commun. 2019, 75, 64–75. [Google Scholar] [CrossRef]
- Watson, A.B.; Ahumada, A.J., Jr. A standard model for foveal detection of spatial contrast. J. Vis. 2005, 5, 717–740. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, L.; Hibbard, P.B. Visual discomfort and blur. J. Vis. 2013, 13, 7. [Google Scholar] [CrossRef]
- Blackwell, H.R. Contrast Thresholds of the Human Eye. J. Opt. Soc. Am. 1946, 36, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Nasrabadi, H.; Jin, J.; Mazade, R.; Pons, C.; Najafian, S.; Alonso, J.-M. Image luminance changes contrast sensitivity in visual cortex. Cell Rep. 2021, 34, 108692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davé, S.B.; Wang, J.; Subramanian, P.S. Clinical color vision testing and correlation with visual function. Am. J. Ophthalmol. 2015, 160, 547–552.e541. [Google Scholar] [CrossRef]
- Singh, G.; Ellis, S.R.; Swan, J.E. The Effect of Focal Distance, Age, and Brightness on Near-Field Augmented Reality Depth Matching. IEEE Trans. Vis. Comput. Graph. 2020, 26, 1385–1398. [Google Scholar] [CrossRef]
- Maiello, G.; Chessa, M.; Solari, F.; Bex, P.J. The (In)Effectiveness of Simulated Blur for Depth Perception in Naturalistic Images. PLoS ONE 2015, 10, e0140230. [Google Scholar] [CrossRef]
- Armbrüster, C.; Wolter, M.; Kuhlen, T.; Spijkers, W.; Fimm, B. Depth perception in virtual reality: Distance estimations in peri- and extrapersonal space. Cyberpsychol. Behav. 2008, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.M.; Hussen, M.S.; Mersha, G.A.; Eticha, B.L. Stereoacuity Among Patients with Refractive Error at University of Gondar, Northwest Ethiopia. Clin. Optom. 2021, 13, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Smith, C.; Magoz, Z.; Sears, J. Screen door effect reduction using mechanical shifting for virtual reality displays. In Proceedings of the Optical Architectures for Displays and Sensing in Augmented, Virtual, and Mixed Reality (AR, VR, MR), San Francisco, CA, USA, 19 February 2020; pp. 200–210. [Google Scholar]
- Joungmin, C.; Kim, Y.; Hee, J.S.; Hyunchang, S.; Kim, T. 78-4: Screen Door Effect Mitigation and Its Quantitative Evaluation in VR Display. SID Symp. Dig. Tech. Pap. 2017, 48, 1154–1156. [Google Scholar] [CrossRef]
- Bonders, F.C. On the Anomalies of Accommodation and Refraction of the Eye. Arch. Ophthalmol. 1986, 104, 1281. [Google Scholar] [CrossRef]
- Carnegie, K.; Rhee, T. Reducing Visual Discomfort with HMDs Using Dynamic Depth of Field. IEEE Comput. Graph. Appl. 2015, 35, 34–41. [Google Scholar] [CrossRef]
| SE (D) | J0 (D) | J45 (D) | F1 (D) | F2 (D) | |
|---|---|---|---|---|---|
| LFVR | −6.33 ± 2.39 | 0.27 ± 0.59 | −0.02 ± 0.32 | −5.96 ± 2.29 | −6.75 ± 2.69 |
| CFA | −4.77 ± 2.37 | 0.36 ± 0.50 | −0.03 ± 0.31 | −4.20 ± 2.26 | −5.34 ± 2.54 |
| OFA | −4.25 ± 2.45 | 0.35 ± 0.47 | −0.04 ± 0.30 | −3.70 ± 2.33 | −4.80 ± 2.61 |
| RET | −4.70 ± 2.37 | 0.45 ± 0.57 | 0.00 ± 0.23 | −4.10 ± 2.21 | −5.29 ± 2.60 |
| SR | −4.46 ± 2.42 | 0.43 ± 0.54 | −0.04 ± 0.29 | −3.87 ± 2.22 | −5.06 ± 2.68 |
| ICC | 95% CI | p-Value | ||
|---|---|---|---|---|
| LFVR-CFA | SE | 0.852 | 0.79 to 0.90 | <0.01 |
| F1 | 0.839 | 0.77 to 0.89 | <0.01 | |
| F2 | 0.861 | 0.80 to 0.90 | <0.01 | |
| LFVR-OFA | SE | 0.855 | 0.79 to 0.90 | <0.01 |
| F1 | 0.838 | 0.77 to 0.89 | <0.01 | |
| F2 | 0.867 | 0.81 to 0.91 | <0.01 | |
| LFVR-RET | SE | 0.827 | 0.75 to 0.88 | <0.01 |
| F1 | 0.807 | 0.73 to 0.87 | <0.01 | |
| F2 | 0.843 | 0.77 to 0.89 | <0.01 | |
| LFVR-SR | SE | 0.847 | 0.78 to 0.89 | <0.01 |
| F1 | 0.820 | 0.74 to 0.88 | <0.01 | |
| F2 | 0.864 | 0.80 to 0.91 | <0.01 |
| First Focal Line | Second Focal Line | |||
|---|---|---|---|---|
| ICC (%) | 95% CI | ICC (%) | 95% CI | |
| LFVR | 88.8 | 0.85 to 0.92 | 97.5 | 0.97 to 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-T.; Lin, C.-N.; Chen, S.-T.; Kuo, H.-Y.; Sun, H.-Y. An Innovative Virtual Reality System for Measuring Refractive Error. Diagnostics 2024, 14, 1633. https://doi.org/10.3390/diagnostics14151633
Huang C-T, Lin C-N, Chen S-T, Kuo H-Y, Sun H-Y. An Innovative Virtual Reality System for Measuring Refractive Error. Diagnostics. 2024; 14(15):1633. https://doi.org/10.3390/diagnostics14151633
Chicago/Turabian StyleHuang, Chin-Te, Chien-Nien Lin, Shyan-Tarng Chen, Hui-Ying Kuo, and Han-Yin Sun. 2024. "An Innovative Virtual Reality System for Measuring Refractive Error" Diagnostics 14, no. 15: 1633. https://doi.org/10.3390/diagnostics14151633





