Aqueous Humor Cytokines in Idiopathic Epiretinal Membrane: Correlation with Disease Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging and Disease Staging
2.2. Sample Collection and Preparation for Analysis
2.3. Glass-Chip Protein Array Analysis
2.4. Immunoprecipitation and SDS-PAGE Analysis
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinkmann, M.P.; Michels, S.; Brinkmann, C.; Rommel, F.; Ranjbar, M.; Graf Johansen, N.; Becker, M. Epiretinal Membrane Surgery Outcome in Eyes with Abnormalities of the Central Bouquet. Int. J. Retina Vitreous 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Cheung, N.; Wang, J.J.; Islam, A.F.M.; Kawasaki, R.; Meuer, S.M.; Cotch, M.F.; Klein, B.E.K.; Klein, R.; Wong, T.Y. Prevalence and Risk Factors for Epiretinal Membranes in a Multi-Ethnic United States Population. Ophthalmology 2011, 118, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Govetto, A.; Lalane, R.A.; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights Into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. Am. J. Ophthalmol. 2017, 175, 99–113. [Google Scholar] [CrossRef]
- Schumann, R.G.; Gandorfer, A.; Ziada, J.; Scheler, R.; Schaumberger, M.M.; Wolf, A.; Kampik, A.; Haritoglou, C. Hyalocytes in Idiopathic Epiretinal Membranes: A Correlative Light and Electron Microscopic Study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, A.; Shimada, Y.; Horiguchi, M. Comparison of Visual Acuity, Metamorphopsia, and Aniseikonia in Patients with an Idiopathic Epiretinal Membrane. Jpn. J. Ophthalmol. 2018, 62, 280–285. [Google Scholar] [CrossRef]
- Govetto, A.; Bhavsar, K.V.; Virgili, G.; Gerber, M.J.; Freund, K.B.; Curcio, C.A.; Burgoyne, C.F.; Hubschman, J.P.; Sarraf, D. Tractional Abnormalities of the Central Foveal Bouquet in Epiretinal Membranes: Clinical Spectrum and Pathophysiological Perspectives. Am. J. Ophthalmol. 2017, 184, 167–180. [Google Scholar] [CrossRef]
- Chen, H.; Chi, W.; Cai, X.; Deng, Y.; Jiang, X.; Wei, Y.; Zhang, S. Macular Microvasculature Features before and after Vitrectomy in Idiopathic Macular Epiretinal Membrane: An OCT Angiography Analysis. Eye 2019, 33, 619–628. [Google Scholar] [CrossRef]
- Fung, A.T.; Galvin, J.; Tran, T. Epiretinal Membrane: A Review. Clin. Exp. Ophthalmol. 2021, 49, 289–308. [Google Scholar] [CrossRef]
- Joshi, M.; Agrawal, S.; Christoforidis, J.B. Inflammatory Mechanisms of Idiopathic Epiretinal Membrane Formation. Mediat. Inflamm. 2013, 2013, 192582. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Wiedemann, P. Involvement of Müller Glial Cells in Epiretinal Membrane Formation. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Tsotridou, E.; Loukovitis, E.; Zapsalis, K.; Pentara, I.; Asteriadis, S.; Tranos, P.; Zachariadis, Z.; Anogeianakis, G. A Review of Last Decade Developments on Epiretinal Membrane Pathogenesis. Med. Hypothesis Discov. Innov. Ophthalmol. 2020, 9, 91–110. [Google Scholar]
- Kohno, R.I.; Hata, Y.; Kawahara, S.; Kita, T.; Arita, R.; Mochizuki, Y.; Aiello, L.P.; Ishibashi, T. Possible Contribution of Hyalocytes to Idiopathic Epiretinal Membrane Formation and Its Contraction. Br. J. Ophthalmol. 2009, 93, 1020–1026. [Google Scholar] [CrossRef]
- Wei, Q.; Zhuang, X.; Fan, J.; Jiang, R.; Chang, Q.; Xu, G.; Yu, Z. Proinflammatory and Angiogenesis-Related Cytokines in Vitreous Samples of Highly Myopic Patients. Cytokine 2021, 137, 155308. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Phone, A.; Lamy, R.; Ma, D.; Laotaweerungsawat, S.; Chen, Y.; Zhao, T.; Ma, W.; Zhang, F.; Psaras, C.; et al. Correlation of Aqueous, Vitreous, and Plasma Cytokine Levels in Patients with Proliferative Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Pollreisz, A.; Funk, M.; Breitwieser, F.P.; Parapatics, K.; Sacu, S.; Georgopoulos, M.; Dunavoelgyi, R.; Zlabinger, G.J.; Colinge, J.; Bennett, K.L.; et al. Quantitative Proteomics of Aqueous and Vitreous Fluid from Patients with Idiopathic Epiretinal Membranes. Exp. Eye Res. 2013, 108, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Yamasaki, M.; Tsukamoto, H.; Mimura, T.; Sone, T.; Hirayama, T.; Tamura, H.; Yamashita, H.; Minamoto, A.; et al. Aqueous Humour Levels of Cytokines Are Correlated to Vitreous Levels and Severity of Macular Oedema in Branch Retinal Vein Occlusion. Eye 2008, 22, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hu, L.; Li, W.; Xu, G.; Xu, L.; Zhang, C.; Wang, F. Epo Inhibits the Fibrosis and Migration of Müller Glial Cells Induced by TGF-β and High Glucose. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.M.; Foos, R.Y. Surface Wrinkling Retinopathy in Eyes Enucleated at Autopsy. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1971, 75, 1047–1058. [Google Scholar]
- Yu, J.; Feng, L.; Wu, Y.; Wang, H.; Ba, J.; Zhu, W.; Xie, C. Vitreous Proteomic Analysis of Idiopathic Epiretinal Membranes. Mol. Biosyst. 2014, 10, 2558–2566. [Google Scholar] [CrossRef]
- Smith, L.E.H.; Shen, W.; Perruzzi, C.; Soker, S.; Kinose, F.; Xu, X.; Robinson, G.; Driver, S.; Bischoff, J.; Zhang, B.; et al. Regulation of Vascular Endothelial Growth Factor-Dependent Retinal Neovascularization by Insulin-like Growth Factor-1 Receptor. Nat. Med. 1999, 5, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.A.; Roda, V.M.d.P.; Matsuda, M.; Siqueira, P.V.; Lustoza-Costa, G.J.; Wu, D.C.; Hamassaki, D.E. Cellular Components of the Idiopathic Epiretinal Membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1435–1444. [Google Scholar] [CrossRef]
- Minchiotti, S.; Stampachiacchiere, B.; Micera, A.; Lambiase, A.; Ripandelli, G.; Billi, B.; Bonini, S. Human Idiopathic Epiretinal Membranes Express NGF and NGF Receptors. Retina 2008, 28, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Harada, C.; Mitamura, Y.; Akazawa, C.; Ohtsuka, K.; Ohno, S.; Takeuchi, S.; Wada, K. Neurotrophic Factor Receptors in Epiretinal Membranes after Human Diabetic Retinopathy. Diabetes Care 2002, 25, 1060–1065. [Google Scholar] [CrossRef][Green Version]
- Vishwakarma, S.; Gupta, R.K.; Jakati, S.; Tyagi, M.; Pappuru, R.R.; Reddig, K.; Hendricks, G.; Volkert, M.R.; Khanna, H.; Chhablani, J.; et al. Molecular Assessment of Epiretinal Membrane: Activated Microglia, Oxidative Stress and Inflammation. Antioxidants 2020, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, P.; Geng, W.; Qin, M.; Su, S.; Zhou, T.; Yuan, Y.; Zhang, G.; Wu, J.; Ji, M.; et al. Cytokines Possibly Involved in Idiopathic Epiretinal Membrane Progression after Uncomplicated Cataract Surgery. Exp. Eye Res. 2022, 217, 108957. [Google Scholar] [CrossRef]
- Zandi, S.; Tappeiner, C.; Pfister, I.B.; Despont, A.; Rieben, R.; Garweg, J.G. Vitreal Cytokine Profile Differences between Eyes with Epiretinal Membranes or Macular Holes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6320–6326. [Google Scholar] [CrossRef] [PubMed]
- Iannetti, L.; Accorinti, M.; Malagola, R.; Bozzoni-Pantaleoni, F.; da Dalt, S.; Nicoletti, F.; Gradini, R.; Traficante, A.; Campanella, M.; Pivetti-Pezzi, P. Role of the Intravitreal Growth Factors in the Pathogenesis of Idiopathic Epiretinal Membrane. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5786–5789. [Google Scholar] [CrossRef] [PubMed]
- Krishna Chandran, A.M.; Coltrini, D.; Belleri, M.; Rezzola, S.; Gambicorti, E.; Romano, D.; Morescalchi, F.; Calza, S.; Semeraro, F.; Presta, M. Vitreous from Idiopathic Epiretinal Membrane Patients Induces Glial-to-Mesenchymal Transition in Müller Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166181. [Google Scholar] [CrossRef]
- Mandelcorn, E.; Khan, Y.; Javorska, L.; Cohen, J.; Howarth, D.; Mandelcorn, M. Idiopathic Epiretinal Membranes: Cell Type, Growth Factor Expression, and Fluorescein Angiographic and Retinal Photographic Correlations. Can. J. Ophthalmol. 2003, 38, 457–463. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hackett, S.F.; Schoenfeld, C.L.; Vinores, M.A.; Vinores, S.A.; Campochiaro, P.A. Localisation of Vascular Endothelial Growth Factor and Its Receptors to Cells of Vascular and Avascular Epiretinal Membranes. Br. J. Ophthalmol. 1997, 81, 919–926. [Google Scholar] [CrossRef]
- Kanda, A.; Noda, K.; Hirose, I.; Ishida, S. TGF-β-SNAIL Axis Induces Müller Glial-Mesenchymal Transition in the Pathogenesis of Idiopathic Epiretinal Membrane. Sci. Rep. 2019, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.C.; Kuijer, R.; van der Worp, R.J.; Postma, G.; Renardel de Lavalette, V.W.; Li, X.R.; Hooymans, J.M.M.; Los, L.I. Immunohistochemical Evaluation of Idiopathic Epiretinal Membranes and in Vitro Studies on the Effect of TGF-β on Müller Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6506–6514. [Google Scholar] [CrossRef]
- Ferrer-Martín, R.M.; Martín-Oliva, D.; Sierra-Martín, A.; Carrasco, M.C.; Martín-Estebané, M.; Calvente, R.; Martín-Guerrero, S.M.; Marín-Teva, J.L.; Navascués, J.; Cuadros, M.A. Microglial Activation Promotes Cell Survival in Organotypic Cultures of Postnatal Mouse Retinal Explants. PLoS ONE 2015, 10, e0135238. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular Fluid Biomarkers (Liquid Biopsy) in Human Diabetic Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Parrozzani, R.; Midena, G.; Trainiti, S.; Marchione, G.; Cosmo, E.; Londei, D.; Frizziero, L. In Vivo Intraocular Biomarkers. Medicine 2020, 99, e22091. [Google Scholar] [CrossRef]
- Abcouwer, S.F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J. Clin. Cell Immunol. 2013, 1 (Suppl. S1), 1–12. [Google Scholar] [CrossRef]
- Russo, A.; Ragusa, M.; Barbagallo, C.; Longo, A.; Avitabile, T.; Uva, M.G.; Bonfiglio, V.; Toro, M.D.; Caltabiano, R.; Mariotti, C.; et al. MiRNAs in the Vitreous Humor of Patients Affected by Idiopathic Epiretinal Membrane and Macular Hole. PLoS ONE 2017, 12, e0174297. [Google Scholar] [CrossRef] [PubMed]
- Feugate, J.E.; Li, Q.J.; Wong, L.; Martins-Green, M. The Cxc Chemokine CCAF Stimulates Differentiation of Fibroblasts into Myofibroblasts and Accelerates Wound Closure. J. Cell Biol. 2002, 156, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kawaji, T.; Inatani, M.; Kameda, T.; Yoshimura, N.; Tanihara, H. Simultaneous Increases in Multiple Proinflammatory Cytokines in the Aqueous Humor in Pseudophakic Glaucomatous Eyes. J. Cataract. Refract. Surg. 2012, 38, 1389–1397. [Google Scholar] [CrossRef]
- Agrawal, R.; Balne, P.K.; Wei, X.; Bijin, V.A.; Lee, B.; Ghosh, A.; Narayanan, R.; Agrawal, M.; Connolly, J. Cytokine Profiling in Patients with Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 376–382. [Google Scholar] [CrossRef]
- Jing, R.; Qi, T.; Wen, C.; Yue, J.; Wang, G.; Pei, C.; Ma, B. Interleukin-2 Induces Extracellular Matrix Synthesis and TGF-Β2 Expression in Retinal Pigment Epithelial Cells. Dev. Growth Differ. 2019, 61, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.J.; Chen, Q.; Wang, L.; Yang, X.; Cun, Q.; Yang, W.Y.; Zhong, H. Pirfenidone Suppresses the Abnormal Activation of Human Müller Cells after Platelet-Derived Growth Factor-BB Stimulation. Int. J. Ophthalmol. 2019, 12, 1075–1082. [Google Scholar] [CrossRef]
- Yoneyama, H.; Matsuno, K.; Zhang, Y.; Murai, M.; Itakura, M.; Ishikawa, S.; Hasegawa, G.; Naito, M.; Asakura, H.; Matsushima, K. Regulation by Chemokines of Circulating Dendritic Cell Precursors, and the Formation of Portal Tract-Associated Lymphoid Tissue, in a Granulomatous Liver Disease. J. Exp. Med. 2001, 193, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A New Class of Membrane-Bound Chemokine with a CX3C Motif. Nature 1997, 385, 640–642. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and Unique Mechanisms Regulate Fibrosis in Various Fibroproliferative Diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Nakagawa, K.; Sueishi, K.; Ishibashi, T.; Inomata, H.; Ueno, H. Hypoxia-Induced Expression of Vascular Endothelial Growth Factor by Retinal Glial Cells Promotes in Vitro Angiogenesis. Virchows Arch. 1995, 426, 479–486. [Google Scholar] [CrossRef]
- Behzadian, M.A.; Wang, X.-L.; Jiang, B.; Caldwell, R.B. Angiostatic Role or Astrocytes: Suppression of Vascular Endothelial Cell Growth by TGF-β and Other Inhibitory Factor(s). Glia 1995, 15, 480–490. [Google Scholar] [CrossRef]
- Guidry, C.; Bradley, K.M.; King, J.L. Tractional Force Generation by Human Müller Cells: Growth Factor Responsiveness and Integrin Receptor Involvement. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1355–1363. [Google Scholar] [CrossRef]
- Romaniuk, D.; Kimsa, M.W.M.C.; Strzalka-Mrozik, B.; Kimsa, M.W.M.C.; Kabiesz, A.; Romaniuk, W.; Mazurek, U. Gene Expression of IGF1, IGF1R, and IGFBP3 in Epiretinal Membranes of Patients with Proliferative Diabetic Retinopathy: Preliminary Study. Mediat. Inflamm. 2013, 2013, 986217. [Google Scholar] [CrossRef] [PubMed]
- Hollborn, M.; Jahn, K.; Limb, G.A.; Kohen, L.; Wiedemann, P.; Bringmann, A. Characterization of the Basic Fibroblast Growth Factor-Evoked Proliferation of the Human Müller Cell Line, MIO-M1. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 414–422. [Google Scholar] [CrossRef]
- Cheng, T.; Cao, W.; Wen, R.; Steinberg, R.H.; LaVail, M.M. Prostaglandin E2 Induces Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor MRNA Expression in Cultured Rat Muller Cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 581–591. [Google Scholar]
- Wu, D.; Kanda, A.; Liu, Y.; Noda, K.; Murata, M.; Ishida, S. Involvement of Müller Glial Autoinduction of TGF-β in Diabetic Fibrovascular Proliferation via Glial-Mesenchymal Transition. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
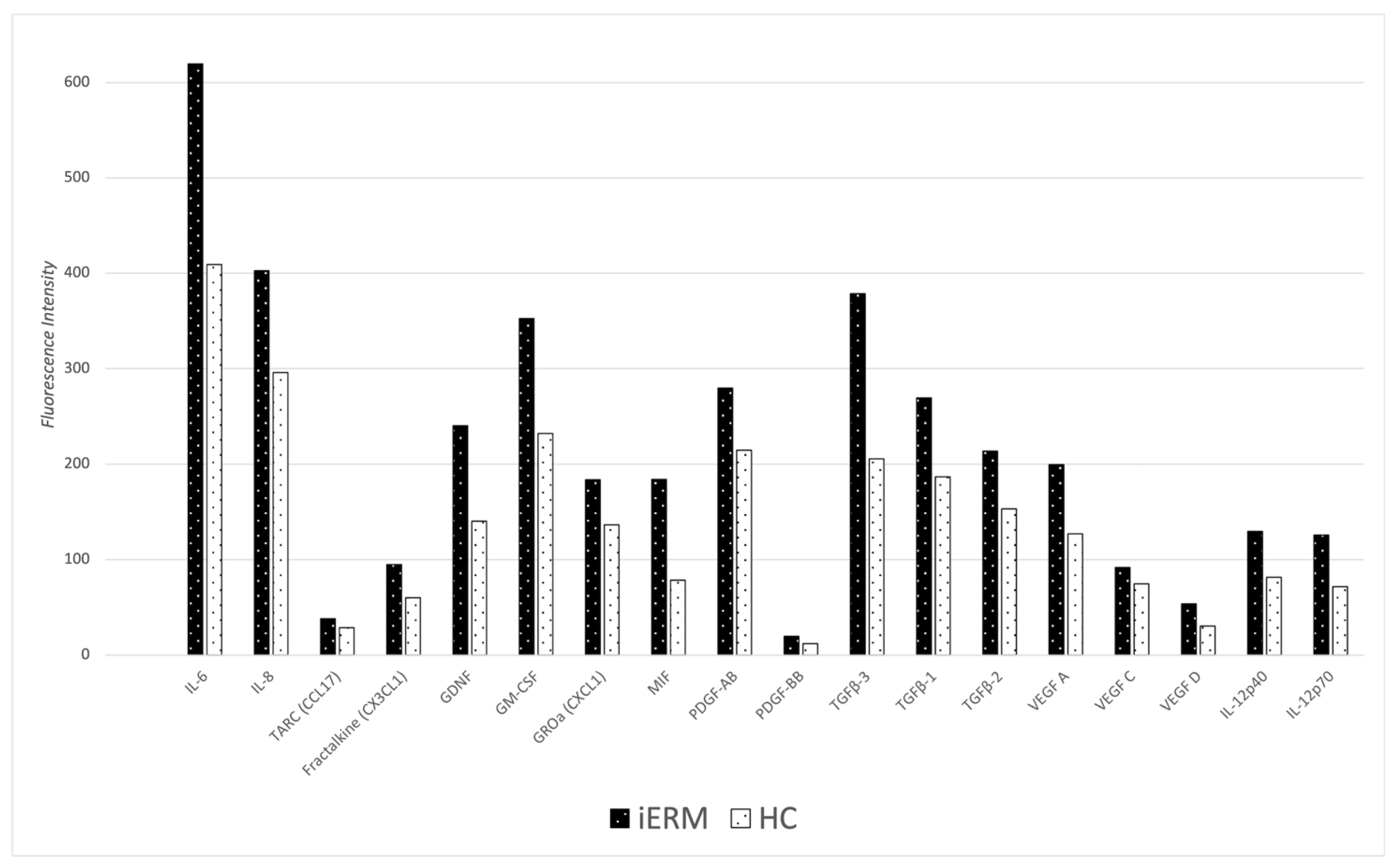

| n | Age Mean ± SD (years) | Gender M/F | Mean Visual Acuity (LogMAR) | |
|---|---|---|---|---|
| HC group | 14 | 72.2 ± 9.5 | 7/7 | 0.37 ± 0.17 |
| iERM subgroup 1 | 4 | 73.0 ± 6.2 | 2/2 | 0.20 ± 0.00 |
| iERM subgroup 2 | 4 | 74 ± 1.2 | 3/1 | 0.40 ± 0.35 |
| iERM subgroup 3 | 4 | 76.2 ± 1.0 | 1/3 | 0.43 ± 0.22 |
| iERM subgroup 4 | 4 | 77 ± 3.8 | 2/2 | 0.50 ± 0.16 |
| Cytokine | Cytokine Primary Function | iERM Group Mean ± SD (16 Eyes) | HC Group Mean ± SD (14 Eyes) | p-Value | Statistical Power Value |
|---|---|---|---|---|---|
| IL-6 (FI) | PI | 619.50 ± 280.17 | 408.93 ± 206.39 | 0.026 † | 0.76 |
| IL-8 (FI) | PI | 402.72 ± 117.33 | 296.07 ± 100.39 | 0.012 † | 0.79 |
| MCP-1 (FI) | PI | 572.69 ± 229.93 | 506.36 ± 252.15 | 0.461 † | 0.10 |
| MDC (FI) | C | 16.03 ± 3.64 | 15.00 ± 4.21 | 0.482 † | 0.10 |
| TARC (CCL17) (FI) | C | 38.09 ± 7.72 | 28.79 ± 8.75 | 0.005 † | 0.85 |
| MIP-3a (FI) | PI | 61.28 ± 26.27 | 54.36 ± 19.09 | 0.412 † | 0.11 |
| CXCL16 (FI) | C | 440.19 ± 285.44 | 619.29 ± 457.81 | 0.220 † | 0.001 |
| FRACTALKINE (CX3CL1) (FI) | C | 94.56 ± 24.81 | 60.07 ± 23.13 | <0.001 † | 0.96 |
| GDNF (FI) | GF | 239.97 ± 108.01 | 140.43 ± 73.91 | 0.006 † | 0.80 |
| GM-CSF (FI) | PI | 352.38 ± 128.33 | 231.79 ± 100.53 | 0.008 † | 0.78 |
| GROa (CXCL1) (FI) | C | 183.56 ± 49.93 | 136.29 ± 38.92 | 0.007 † | 0.79 |
| MIF (FI) | PI | 183.81 ± 91.86 | 78.42 ± 80.14 | 0.031 † | 0.89 |
| PDGF-AA (FI) | GF | 81.47 ± 37.72 | 107.79 ± 78.40 | 0.267 † | 0.002 |
| PDGF-AB (FI) | GF | 279.72 ± 72.35 | 214.29 ± 63.06 | 0.013 † | 0.81 |
| PDGF-BB (FI) | GF | 19.91 ± 5.98 | 12.07 ± 2.39 | <0.001 † | 0.99 |
| OPN (FI) | PI | 4983.16 ± 3762.79 | 5618.36 ± 1828.35 | 0.555 † | 0.007 |
| TGFβ-3 (FI) | PF | 378.69 ± 126.18 | 205.57 ± 105.72 | <0.001 † | 0.97 |
| TGFβ-1 (FI) | PF | 269.09 ± 76.93 | 186.71 ± 70.87 | 0.005 † | 0.83 |
| TGFβ-2 (FI) | PF | 213.34 ± 53.04 | 153.07 ± 66.89 | 0.012 † | 0.76 |
| VEFG A (FI) | PA | 199.56 ± 53.97 | 127.14 ± 48.84 | 0.001 † | 0.96 |
| VEGF C (FI) | PA | 91.78 ± 22.35 | 74.50 ± 20.29 | 0.035 † | 0.76 |
| VEGF D (FI) | PA | 53.66 ± 16.09 | 30.57 ± 10.82 | <0.001 † | 0.99 |
| IL-12p40 (FI) | PI | 129.56 ± 45.91 | 81.64 ± 33.55 | 0.003 † | 0.87 |
| PEDF (FI) | GF | 140.03 ± 32.88 | 121.21 ± 36.96 | 0.155 † | 0.29 |
| EPO R (FI) | GF | 85.50 ± 31.59 | 50.79 ± 17.64 | 0.001 † | 0.94 |
| IL-12p70 (FI) | PI | 125.59 ± 45.28 | 71.43 ± 36.25 | 0.001 † | 0.93 |
| Kir 4.1 (OD) | NA | 101.90 ± 5.60 | 98.99 ± 5.49 | 0.207 † | 0.27 |
| AQP1 (pg/mg) | NA | 153.64 ± 75.33 | 135.12 ± 59.44 | 0.474 † | 0.10 |
| AQP4 (pg/mg) | NA | 47.30 ± 21.27 | 39.31 ± 16.99 | 0.280 † | 0.18 |
| AQP9 (pg/mg) | NA | 360.64 ± 185,06 | 281.13 ± 86.31 | 0.144 † | 0.28 |
| GFAP (pg/mg) | NA | 69.77 ± 28.62 | 90.35 ± 35.13 | 0.093 † | 0.0003 |
| Cytokine | Cytokine Primary Function | Correlation Coefficient |
|---|---|---|
| IL-6 | PI | 0.85 † |
| IL-8 | PI | 0.82 † |
| TARC (CCL17) | C | 0.64 † |
| FRACTALKINE (CX3CL1) | C | 0.84 † |
| GDNF | GF | 0.87 † |
| GM-CSF | PI | 0.86 † |
| GROa (CXCL1) | C | 0.86 † |
| MIF | PI | 0.81 † |
| PDGF-AB | GF | 0.81 † |
| PDGF-BB | GF | 0.63 † |
| TGF-β3 | PF | 0.85 † |
| TGF-β1 | PF | 0.78 † |
| TGF-β2 | PF | 0.55 † |
| VEGF A | PA | 0.79 † |
| VEGF C | PA | 0.76 † |
| VEGF D | PA | 0.89 † |
| IL-12p40 | PI | 0.87 † |
| EPO R | GF | 0.03 † |
| IL-12p70 | PI | 0.91 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torresin, T.; Greggio, A.; Frisina, R.; Motta, L.; Gius, I.; Midena, G.; Midena, E. Aqueous Humor Cytokines in Idiopathic Epiretinal Membrane: Correlation with Disease Severity. Diagnostics 2024, 14, 1797. https://doi.org/10.3390/diagnostics14161797
Torresin T, Greggio A, Frisina R, Motta L, Gius I, Midena G, Midena E. Aqueous Humor Cytokines in Idiopathic Epiretinal Membrane: Correlation with Disease Severity. Diagnostics. 2024; 14(16):1797. https://doi.org/10.3390/diagnostics14161797
Chicago/Turabian StyleTorresin, Tommaso, Angelo Greggio, Rino Frisina, Lorenzo Motta, Irene Gius, Giulia Midena, and Edoardo Midena. 2024. "Aqueous Humor Cytokines in Idiopathic Epiretinal Membrane: Correlation with Disease Severity" Diagnostics 14, no. 16: 1797. https://doi.org/10.3390/diagnostics14161797
APA StyleTorresin, T., Greggio, A., Frisina, R., Motta, L., Gius, I., Midena, G., & Midena, E. (2024). Aqueous Humor Cytokines in Idiopathic Epiretinal Membrane: Correlation with Disease Severity. Diagnostics, 14(16), 1797. https://doi.org/10.3390/diagnostics14161797









