Application of Artificial Intelligence in Cone-Beam Computed Tomography for Airway Analysis: A Narrative Review
Abstract
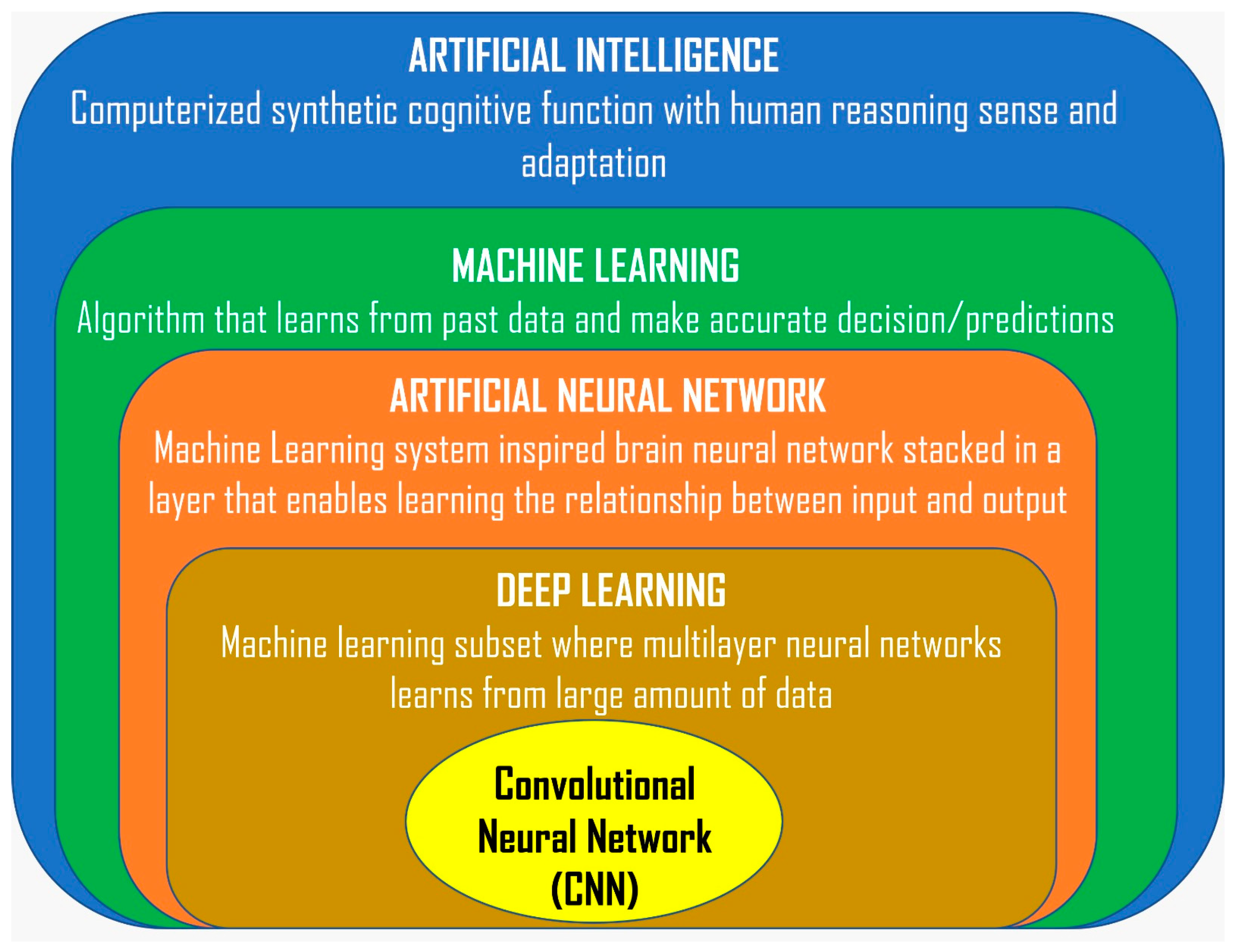
1. Introduction
2. Background
3. Application of AI in CBCT in Airway Analysis
3.1. 2D CNN Regression-Based Model
3.2. 3D CNN U-Net Resolution-Based Model
3.3. 3D CNN U-Net Threshold Value-Based Pipeline Model
3.4. Multivariate 3D CNN U-Net Resolution-Based Model
3.5. CNN U-Net Convolutional Long Short-Term Memory-Based Model
3.6. 2D CNN Minimal Cross-Sectional Area (MCSA) Localization Model
4. Benefits of AI in CBCT Airway Analysis
4.1. Enhanced Accuracy in Airway Volume Measurement
4.2. Improved Efficiency and Time Savings
4.3. Enhanced Diagnostic Capabilities
4.4. Standardization and Consistency
4.5. Integration with Treatment Planning
4.6. Continuous Learning and Improvement
4.7. Safety and Privacy-Preserved Information
5. Challenges and Limitations
5.1. Limited Data Availability
5.2. Lack of Standard Methodological Framework
5.3. Selection and Interpretation Bias
5.4. Accessibility and Transparency Issues
6. Future Direction
6.1. Enhance Collaboration and Data Sharing
6.2. Continuous Calibration and Validation
6.3. Integration with Clinical Workflows
6.4. Predictive Analytics
6.5. Ethical and Regulatory Advancements
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| CBCT | Cone-beam computed tomography |
| AI | Artificial intelligence |
| FOV | Field of view |
| ROI | Region of interest |
| MRI | Magnetic resonance imaging |
| ML | Machine learning |
| DL | Deep learning |
| ANN | Artificial neural network |
| CNN | Convolutional neural network |
| MCSA | Minimal cross-sectional area |
| PAS | Pharyngeal airway space |
| STL | Stereolithography |
| DICOM | Digital Imaging and Communications in Medicine |
| PROBAST | Prediction model Risk Of Bias Assessment Tool |
| MI-CLAIM | Minimum information about clinical artificial intelligence modelling |
| CLAIM | Checklist for Artificial Intelligence in Medical Imaging |
References
- Tsolakis, I.A.; Kolokitha, O.-E.; Papadopoulou, E.; Tsolakis, A.I.; Kilipiris, E.G.; Palomo, J.M. Artificial Intelligence as an Aid in CBCT Airway Analysis: A Systematic Review. Life 2022, 12, 1894. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.Y.; Yang, W.-F.; Leung, Y.Y. The Role of Cone Beam Computed Tomography (CBCT) in the Diagnosis and Clinical Management of Medication-Related Osteonecrosis of the Jaw (MRONJ). Diagnostics 2024, 14, 1700. [Google Scholar] [CrossRef]
- Abdelkarim, A.Z.; Almeshari, A.A.; Ozen, D.C.; Khalifa, A.R.; Rezallah, N.N.; Duman, S.B.; Khurana, S. Comparative Evaluation of Temporomandibular Joint Parameters in Unilateral and Bilateral Cleft Lip and Palate Patients Using Cone-Beam CT: Focus on Growing vs. Non-Growing Subjects. Healthcare 2024, 12, 1563. [Google Scholar] [CrossRef]
- Dostalova, T.; Eliasova, H.; Prochazka, A.; Nocar, A.; Urbanova, P. Imaging and 3D Analysis Based on Two or More Three-Dimensional CBCT Recordings before and after Orthodontic Treatment and Maxillofacial Therapy. Appl. Sci. 2024, 14, 4829. [Google Scholar] [CrossRef]
- Vasiljevic, M.; Selakovic, D.; Rosic, G.; Stevanovic, M.; Milanovic, J.; Arnaut, A.; Milanovic, P. Anatomical Factors of the Anterior and Posterior Maxilla Affecting Immediate Implant Placement Based on Cone Beam Computed Tomography Analysis: A Narrative Review. Diagnostics 2024, 14, 1697. [Google Scholar] [CrossRef] [PubMed]
- Pop, S.I.; Cerghizan, D.; Mițariu, L.; Jánosi, K.M.; D’Andrea, A. CBCT Evaluation of Alveolar Bone Change and Root Resorption after Orthodontic Treatment: A Retrospective Study. Diagnostics 2024, 14, 1757. [Google Scholar] [CrossRef]
- Fagundes, N.C.F.; Loliencar, P.; MacLean, J.E.; Flores-Mir, C.; Heo, G. Characterization of craniofacial-based clinical phenotypes in children with suspected obstructive sleep apnea. J. Clin. Sleep Med. 2023, 19, 1857–1865. [Google Scholar] [CrossRef]
- Alsufyani, N.; Flores-Mir, C.; Major, P. Three-dimensional segmentation of the upper airway using cone beam CT: A systematic review. Dentomaxillofac. Radiol. 2012, 41, 276–284. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Ronsivalle, V.; Gastaldi, G.; Leonardi, R. Assessment of the accuracy of imaging software for 3D rendering of the upper airway, usable in orthodontic and craniofacial clinical settings. Prog. Orthod. 2022, 23, 22. [Google Scholar] [CrossRef]
- Monill-González, A.; Rovira-Calatayud, L.; d’Oliveira, N.G.; Ustrell-Torrent, J.M. Artificial intelligence in orthodontics: Where are we now? A scoping review. Orthod. Craniofac. Res. 2021, 24, 6–15. [Google Scholar] [CrossRef]
- Schwendicke, F.; Golla, T.; Dreher, M.; Krois, J. Convolutional neural networks for dental image diagnostics: A scoping review. J. Dent. 2019, 91, 103226. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Urbanová, W.; Novák, B.; Czako, L.; Siebert, T.; Stano, P.; Mareková, S.; Fountoulaki, G.; Kosnáčová, H.; Varga, I. Where Is the Artificial Intelligence Applied in Dentistry? Systematic Review and Literature Analysis. Healthcare 2022, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Patcas, R.; Bernini, D.A.J.; Volokitin, A.; Agustsson, E.; Rothe, R.; Timofte, R. Applying artificial intelligence to assess the impact of orthognathic treatment on facial attractiveness and estimated age. Int. J. Oral Maxillofac. Surg. 2019, 48, 77–83. [Google Scholar] [CrossRef]
- Shujaat, S.; Jazil, O.; Willems, H.; Van Gerven, A.; Shaheen, E.; Politis, C.; Jacobs, R. Automatic segmentation of the pharyngeal airway space with convolutional neural network. J. Dent. 2021, 111, 103705. [Google Scholar] [CrossRef] [PubMed]
- Sin, Ç.; Akkaya, N.; Aksoy, S.; Orhan, K.; Öz, U. A deep learning algorithm proposal to automatic pharyngeal airway detection and segmentation on CBCT images. Orthod. Craniofac. Res. 2021, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Su, Y.; Bornstein, M.M. CT Scan vs. Cone Beam CT: An Overview. Available online: https://blog.iti.org/clinical-insights/ct-scan-vs-cone-beam-ct/ (accessed on 27 July 2024).
- Gaêta-Araujo, H.; Leite, A.F.; Vasconcelos, K.d.F.; Jacobs, R. Two decades of research on CBCT imaging in DMFR—An appraisal of scientific evidence. Dentomaxillofac. Radiol. 2021, 50, 20200367. [Google Scholar] [CrossRef]
- Venkatesh, E.; Elluru, S.V. Cone beam computed tomography: Basics and applications in dentistry. J. Istanb. Univ. Fac. Dent. 2017, 51, S102–S121. [Google Scholar] [CrossRef]
- Olch, A.J.; Alaei, P. How low can you go? A CBCT dose reduction study. J. Appl. Clin. Med. Phys. 2021, 22, 85–89. [Google Scholar] [CrossRef]
- Nasseh, I.; Al-Rawi, W. Cone Beam Computed Tomography. Dent. Clin. N. Am. 2018, 62, 361–391. [Google Scholar] [CrossRef]
- American Association of Endodontists, American Academy of Oral and Maxillofacial Radiology. Use of cone-beam computed tomography in endodontics Joint Position Statement of the American Association of Endodontists and the American Academy of Oral and Maxillofacial Radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 234–237. [Google Scholar] [CrossRef]
- American Dental Association Council on Scientific Affairs. The use of cone-beam computed tomography in dentistry: An advisory statement from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2012, 143, 899–902. [Google Scholar] [CrossRef]
- Horner, K.; O’Malley, L.; Taylor, K.; Glenny, A.-M. Guidelines for clinical use of CBCT: A review. Dentomaxillofac. Radiol. 2015, 44, 20140225. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, F.; Dagassan-Berndt, D.; Patcas, R.; Mak, W.-S.; Kanavakis, G.; Verna, C.; Gu, M.; Bornstein, M.M. The use of CBCT in orthodontics with special focus on upper airway analysis in patients with sleep-disordered breathing. Dentomaxillofac. Radiol. 2024, 53, 178–188. [Google Scholar] [CrossRef]
- Bronoosh, P.; Khojastepour, L. Analysis of Pharyngeal Airway Using Lateral Cephalogram vs CBCT Images: A Cross-sectional Retrospective Study. Open Dent. J. 2015, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Ashique Abdulhameed, S.; Riyaz SS, M.A.; Almutairy, M.; Khan, N.S.; Jayakumar, S.; Gaonkar, P. Assessing the Accuracy of Lateral Cephalogram in Quantifying Three-Dimensional Pharyngeal Airway Morphology Compared to Cone-Beam Computed Tomography. Cureus 2024, 16, e57301. [Google Scholar] [CrossRef]
- Fonseca, C.; Cavadas, F.; Fonseca, P. Upper Airway Assessment in Cone-Beam Computed Tomography for Screening of Obstructive Sleep Apnea Syndrome: Development of an Evaluation Protocol in Dentistry. JMIR Res. Protoc. 2023, 12, e41049. [Google Scholar] [CrossRef]
- Schendel, S.A.; Hatcher, D. Automated 3-dimensional airway analysis from cone-beam computed tomography data. J. Oral Maxillofac. Surg. 2010, 68, 696–701. [Google Scholar] [CrossRef]
- Tarce, M.; Zhou, Y.; Antonelli, A.; Becker, K. The Application of Artificial Intelligence for Tooth Segmentation in CBCT Images: A Systematic Review. Appl. Sci. 2024, 14, 6298. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Wajer, R.; Wajer, A.; Kiian, V.; Kloska, A.; Kazimierczak, N.; Janiszewska-Olszowska, J.; Serafin, Z. Periapical Lesions in Panoramic Radiography and CBCT Imaging—Assessment of AI’s Diagnostic Accuracy. J. Clin. Med. 2024, 13, 2709. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, W.; Kazimierczak, N.; Issa, J.; Wajer, R.; Wajer, A.; Kalka, S.; Serafin, Z. Endodontic Treatment Outcomes in Cone Beam Computed Tomography Images—Assessment of the Diagnostic Accuracy of AI. J. Clin. Med. 2024, 13, 4116. [Google Scholar] [CrossRef]
- Wajer, R.; Wajer, A.; Kazimierczak, N.; Wilamowska, J.; Serafin, Z. The Impact of AI on Metal Artifacts in CBCT Oral Cavity Imaging. Diagnostics 2024, 14, 1280. [Google Scholar] [CrossRef]
- Chahal, A.; Gulia, P. Machine Learning and Deep Learning. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 4910–4914. [Google Scholar] [CrossRef]
- Campesato, O. Artificial Intelligence, Machine Learning, and Deep Learning; Mercury Learning and Information; Walter de Gruyter GmbH: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Sharifani, K.; Amini, M. Machine Learning and Deep Learning: A Review of Methods and Applications; Social Science Research Network: Rochester, NY, USA, 2023. [Google Scholar]
- Shanmuganathan, S. Artificial Neural Network Modelling: An Introduction. In Artificial Neural Network Modelling; Shanmuganathan, S., Samarasinghe, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–14. [Google Scholar] [CrossRef]
- You, J.; Leskovec, J.; He, K.; Xie, S. Graph Structure of Neural Networks. In Proceedings of the 37th International Conference on Machine Learning, Virtual, 13–18 July 2020; PMLR: New York, NY, USA, 2020; pp. 10881–10891. [Google Scholar]
- Fan, W.; Zhang, J.; Wang, N.; Li, J.; Hu, L. The Application of Deep Learning on CBCT in Dentistry. Diagnostics 2023, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef]
- Purwono, P.; Ma’Arif, A.; Rahmaniar, W.; Fathurrahman, H.I.K.; Frisky, A.Z.K.; Haq, Q.M.U. Understanding of Convolutional Neural Network (CNN): A Review. Int. J. Robot. Control Syst. 2023, 2, 739–748. [Google Scholar] [CrossRef]
- Park, J.; Hwang, J.; Ryu, J.; Nam, I.; Kim, S.-A.; Cho, B.-H.; Shin, S.-H.; Lee, J.-Y. Deep Learning Based Airway Segmentation Using Key Point Prediction. Appl. Sci. 2021, 11, 3501. [Google Scholar] [CrossRef]
- Nogueira-Reis, F.; Morgan, N.; Suryani, I.R.; Tabchoury, C.P.M.; Jacobs, R. Full virtual patient generated by artificial intelligence-driven integrated segmentation of craniomaxillofacial structures from CBCT images. J. Dent. 2024, 141, 104829. [Google Scholar] [CrossRef]
- Leonardi, R.; Giudice, A.L.; Farronato, M.; Ronsivalle, V.; Allegrini, S.; Musumeci, G.; Spampinato, C. Fully automatic segmentation of sinonasal cavity and pharyngeal airway based on convolutional neural networks. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 824–835.e1. [Google Scholar] [CrossRef]
- Chu, G.; Zhang, R.; He, Y.; Ng, C.H.; Gu, M.; Leung, Y.Y.; He, H.; Yang, Y. Deep Learning Models for Automatic Upper Airway Segmentation and Minimum Cross-Sectional Area Localisation in Two-Dimensional Images. Bioengineering 2023, 10, 915. [Google Scholar] [CrossRef]
- Orhan, K.; Shamshiev, M.; Ezhov, M.; Plaksin, A.; Kurbanova, A.; Ünsal, G.; Gusarev, M.; Golitsyna, M.; Aksoy, S.; Mısırlı, M.; et al. AI-based automatic segmentation of craniomaxillofacial anatomy from CBCT scans for automatic detection of pharyngeal airway evaluations in OSA patients. Sci. Rep. 2022, 12, 11863. [Google Scholar] [CrossRef]
- Hatcher, D.C. Cone Beam Computed Tomography: Craniofacial and Airway Analysis. Sleep Med. Clin. 2010, 5, 59–70. [Google Scholar] [CrossRef]
- Tingelhoff, K.; Moral, A.I.; Kunkel, M.E.; Rilk, M.; Wagner, I.; Eichhorn, K.W.; Wahl, F.M.; Bootz, F. Comparison between manual and semi-automatic segmentation of nasal cavity and paranasal sinuses from CT images. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 5505–5508. [Google Scholar] [CrossRef]
- Krishna, U.V.; G, S.R.; Addepalli, L.; M, B.; Sd, V.S.; Jaime, L.M. Enhancing Airway Assessment with a Secure Hybrid Network-Blockchain System for CT & CBCT Image Evaluation. Int. Res. J. Multidiscip. Technovation 2024, 6, 51–69. [Google Scholar] [CrossRef]
- Koul, A.; Bawa, R.K.; Kumar, Y. Artificial Intelligence Techniques to Predict the Airway Disorders Illness: A Systematic Review. Arch. Comput. Methods Eng. 2023, 30, 831–864. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chuang, L.-C.; Guilleminault, C. 0726 Changes in Craniofacial and Airway Morphology As Well As Quality of Life After Passive Myofunctional Therapy in Children with Obstructive Sleep Apnea: A Comparative Cohort Study Quality of Life After Passive Myofunctional Therapy in Children with Obstructive Sleep Apnea: A Comparative Cohort Study. Sleep 2019, 42, A291–A292. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Saif, M.; Batool, A.; Sohail, A.; Khan, M. A Survey of Deep Learning Techniques for the Analysis of COVID-19 and Their Usability for Detecting Omicron. J. Exp. Theor. Artif. Intell. 2023, 1–43. [Google Scholar] [CrossRef]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial Intelligence in Dentistry: Chances and Challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Pandian, A.P. Performance Evaluation and Comparison using Deep Learning Techniques in Sentiment Analysis. J. Soft Comput. Paradig. 2021, 3, 123–134. [Google Scholar]
- Lotan, E.; Tschider, C.; Sodickson, D.K.; Caplan, A.L.; Bruno, M.; Zhang, B.; Lui, Y.W. Medical Imaging and Privacy in the Era of Artificial Intelligence: Myth, Fallacy, and the Future. J. Am. Coll. Radiol. 2020, 17, 1159–1162. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Wong, N.S.M. Reject Rates of Radiographic Images in Dentomaxillofacial Radiology: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 8076. [Google Scholar] [CrossRef]
- van Eijnatten, M.; Wolff, J.; Pauwels, R.; Karhu, K.; Hietanen, A.; der Sarkissian, H.; Koivisto, J.H. Influence of head positioning during cone-beam CT imaging on the accuracy of virtual 3D models. Dentomaxillofac. Radiol. 2022, 51, 20220104. [Google Scholar] [CrossRef] [PubMed]
- Nagarajappa, A.K.; Dwivedi, N.; Tiwari, R. Artifacts: The downturn of CBCT image. J. Int. Soc. Prev. Community Dent. 2015, 5, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Moratin, J.; Berger, M.; Rückschloss, T.; Metzger, K.; Berger, H.; Gottsauner, M.; Engel, M.; Hoffmann, J.; Freudlsperger, C.; Ristow, O. Head motion during cone-beam computed tomography: Analysis of frequency and influence on image quality. Imaging Sci. Dent. 2020, 50, 227–236. [Google Scholar] [CrossRef]
- Brennan, H.L.; Kirby, S.D. The role of artificial intelligence in the treatment of obstructive sleep apnea. J. Otolaryngol.—Head. Neck Surg. 2023, 52, 7. [Google Scholar] [CrossRef]
- Geis, J.R.; Brady, A.P.; Wu, C.C.; Spencer, J.; Ranschaert, E.; Jaremko, J.L.; Langer, S.G.; Kitts, A.B.; Birch, J.; Shields, W.F.; et al. Ethics of Artificial Intelligence in Radiology: Summary of the Joint European and North American Multisociety Statement. J. Am. Coll. Radiol. 2019, 16, 1516–1521. [Google Scholar] [CrossRef]
- Brady, A.P.; Neri, E. Artificial Intelligence in Radiology-Ethical Considerations. Diagnostics 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Vokinger, K.N.; Feuerriegel, S.; Kesselheim, A.S. Mitigating bias in machine learning for medicine. Commun. Med. 2021, 1, 25. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.; Riley, R.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; PROBAST Group. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Norgeot, B.; Quer, G.; Beaulieu-Jones, B.K.; Torkamani, A.; Dias, R.; Gianfrancesco, M.; Arnaout, R.; Kohane, I.S.; Saria, S.; Topol, E.; et al. Minimum information about clinical artificial intelligence modeling: The MI-CLAIM checklist. Nat. Med. 2020, 26, 1320–1324. [Google Scholar] [CrossRef]
- Mongan, J.; Moy, L.; Kahn, C.E. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A Guide for Authors and Reviewers. Radiol. Artif. Intell. 2020, 2, e200029. [Google Scholar] [CrossRef]
- Flory, M.N.; Napel, S.; Tsai, E.B. Artificial Intelligence in Radiology: Opportunities and Challenges. Semin. Ultrasound CT MR 2024, 45, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Miragall, M.F.; Knoedler, S.; Kauke-Navarro, M.; Saadoun, R.; Grabenhorst, A.; Grill, F.D.; Ritschl, L.M.; Fichter, A.M.; Safi, A.-F.; Knoedler, L. Face the Future—Artificial Intelligence in Oral and Maxillofacial Surgery. J. Clin. Med. 2023, 12, 6843. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Nikhade, P. Artificial Intelligence in Dentistry: Past, Present, and Future. Cureus 2022, 14, e27405. [Google Scholar] [CrossRef] [PubMed]

| Author, Year, Location | CBCT Machine (Technical Specification) | No. of CBCT Data | AI Application | Image Processing Method—Software Used | AI Modelling—Software and/or Hardware Used | Beneficial Results |
|---|---|---|---|---|---|---|
| Shujaat et al., 2021, Belgium [14] | Promax 3D Max (Planmeca, Helsinki, Finland) (96 kV, 216 mAs, slice thickness: 0.6 mm, field of view: 230 × 260 mm2) Newtom VGi evo (Cefla, Imola, Italy) (110 kV, 15.3 mAs, slice thickness: 0.3, field of view: 240 × 190 mm2) | 103 | 3D CNN U-Net resolution-based model | Segmentation of PAS volume limited by the nasal cavity, oral cavity, pharyngeal border until the limit of the scan either at 2nd, 3rd, or 4th cervical vertebrae. Delineation was based on resolution of Hounsfield unit to create mask in axial, sagittal, and coronal plane to convert to STL file format
| Own model Online customized user-interactive cloud-based platform (version 1.0, Toothflow, Relu, Inc., Leuven. Belgium) |
|
| Sin et al., 2021, Turkey [15] | Newtom 3G (Quantitative Radiology srl, Verona, Italy) (120 kVp and 3–5 mA, 12in, 13.48 cm imaging field, axial slice thickness 0.3 mm, isotropic voxels) | 306 | 3D CNN U-Net threshold value-based pipeline model | Semi-auto segmentation by determining thresholding values to isolate the anatomic region, then placement of seed regions for active contour model
| Own model
|
|
| Park et al., 2021, South Korea [41] | PaX-i3D (Vatech Co., Hwaseong-si, South Korea) (105–114 KVP, 5.6–6.5 mA with 160 mm × 160 mm field of view, and 0.3 mm in voxel size) | 315 | 2D CNN Regression-based models | 5 coordinates predicted for airway segmentation in sagittal plane includes posterior palate, vomer, 1st, 2nd, or 3rd cervical vertebrae
| Own model
|
|
| Nogueira-Reis et al., 2024, Belgium [42] | 3D Accuitomo 170 (J. Morita, Kyoto, Japan) (90 kVp, 5 mA, 0.2–0.25 mm voxel size, FOV 17 × 12 cm, 14 × 10 cm, 10 × 10 cm) Newtom VGi evo (Cefla, Imola, Italy) (110 kB, 6–12 mA, 0.25–0.3 mm Voxel size, FOV 24 × 19 cm) | 30 | Multivariate 3D CNN U-Net resolution-based model | Six craniofacial structures, encompassing the maxillofacial complex bones, maxillary sinus, dentition, mandible, mandibular canal, and pharyngeal airway space, were segmented. Minor refinements were manually corrected. Refined segmentation served as reference for comparison.
| Own model
|
|
| Leonardi et al., 2021, Italy [43] | iCAT Next Generation CBCT unit (Imaging Sciences International, Hatfield, Pa) (120 kVp; 48 mA; 0.3 mm voxel size; scan time, 26 s; field view of 17 cm in height × 23 cm in depth) | 40 | CNN U-Net Convolutional Long Short-Term Memory-based model | Landmarks and boundaries used include Nasion, second and third cervical vertebrae, porion, and orbitale. Segmentation mask of the sino-nasal cavity and pharyngeal subregion after the enhancement of boundaries performed by manually erasing the parts outsides the region of interest
| Own model
|
|
| Chu et al., 2023, Hong Kong [44] | ProMax 3D Mid (Planmeca Oy, Helsinki, Finland) (96 kV, 216 mAs, slice thickness: 0.6 mm, field of view: 230 × 260 mm2) | 201 | 2D CNN Minimal Cross-Sectional Area (MCSA) localization model | MCSA at three different levels using midsagittal plane: nasopharynx, retropalatal pharynx, retroglossal pharynx
| Own model Model training based on Adam optimization algorithm and Pytorch framework using Intel i7-8700 CPU, 32GB RAM and a single Nvidia RTX 2080 Ti GPU with 12G VRAM (Jumbo computer supplies, Hong Kong, China) |
|
| Orhan et al., 2022, Denmark [45] | Pax-i3D Smart PHT-30LFO0 (Vatech, Gyeonggi-do, South Korea) Carestream Health CS 8100 3D (Kodak, Rochester, NY, USA), Orthophos XG 3D (Sirona, Germany) isotropic voxels which differ between 0.1 and 0.2 mm3 | 200 | 3D CNN U-Net resolution-based model | Automatic segmentation focusing on external surface of bones, teeth, and airways:
| Diagnocat (DGNCT LLC, Miami, FL, USA) Training using NVIDIA GeForce RTX A100 GPU |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, I.N.; Subramaniam, P.K.; Chi Adam, K.B.; Ghazali, A.B. Application of Artificial Intelligence in Cone-Beam Computed Tomography for Airway Analysis: A Narrative Review. Diagnostics 2024, 14, 1917. https://doi.org/10.3390/diagnostics14171917
Ismail IN, Subramaniam PK, Chi Adam KB, Ghazali AB. Application of Artificial Intelligence in Cone-Beam Computed Tomography for Airway Analysis: A Narrative Review. Diagnostics. 2024; 14(17):1917. https://doi.org/10.3390/diagnostics14171917
Chicago/Turabian StyleIsmail, Izzati Nabilah, Pram Kumar Subramaniam, Khairul Bariah Chi Adam, and Ahmad Badruddin Ghazali. 2024. "Application of Artificial Intelligence in Cone-Beam Computed Tomography for Airway Analysis: A Narrative Review" Diagnostics 14, no. 17: 1917. https://doi.org/10.3390/diagnostics14171917
APA StyleIsmail, I. N., Subramaniam, P. K., Chi Adam, K. B., & Ghazali, A. B. (2024). Application of Artificial Intelligence in Cone-Beam Computed Tomography for Airway Analysis: A Narrative Review. Diagnostics, 14(17), 1917. https://doi.org/10.3390/diagnostics14171917






