Differential Functional Changes in Visual Performance during Acute Exposure to Microgravity Analogue and Their Potential Links with Spaceflight-Associated Neuro-Ocular Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- -
- Any current or past health issue that could be a hazard for the participants;
- -
- Significant vision impairment defined as myopia over −6D, hypermetropia over +6D, or nystagmus, strabismus, or chromatic impairment of any type;
- -
- Any previous infectious diseases or other diseases, even resolved in the previous 2 weeks prior to the measurements;
- -
- Any current medication that could influence cognitive or motor performance;
- -
- Any previous participation in any kind of clinical study in the previous 4 weeks prior to the measurements.
2.2. Health Assessment
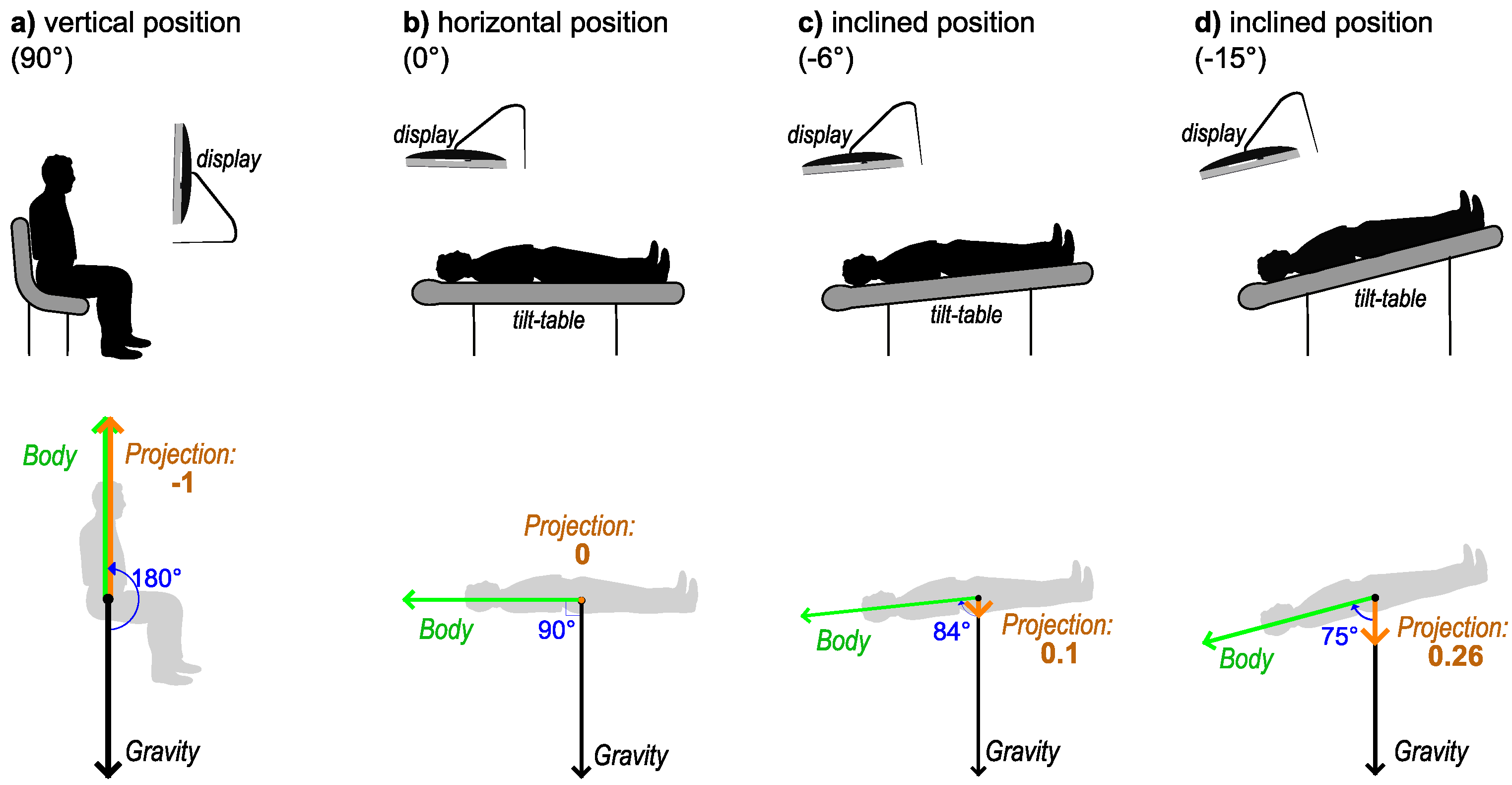
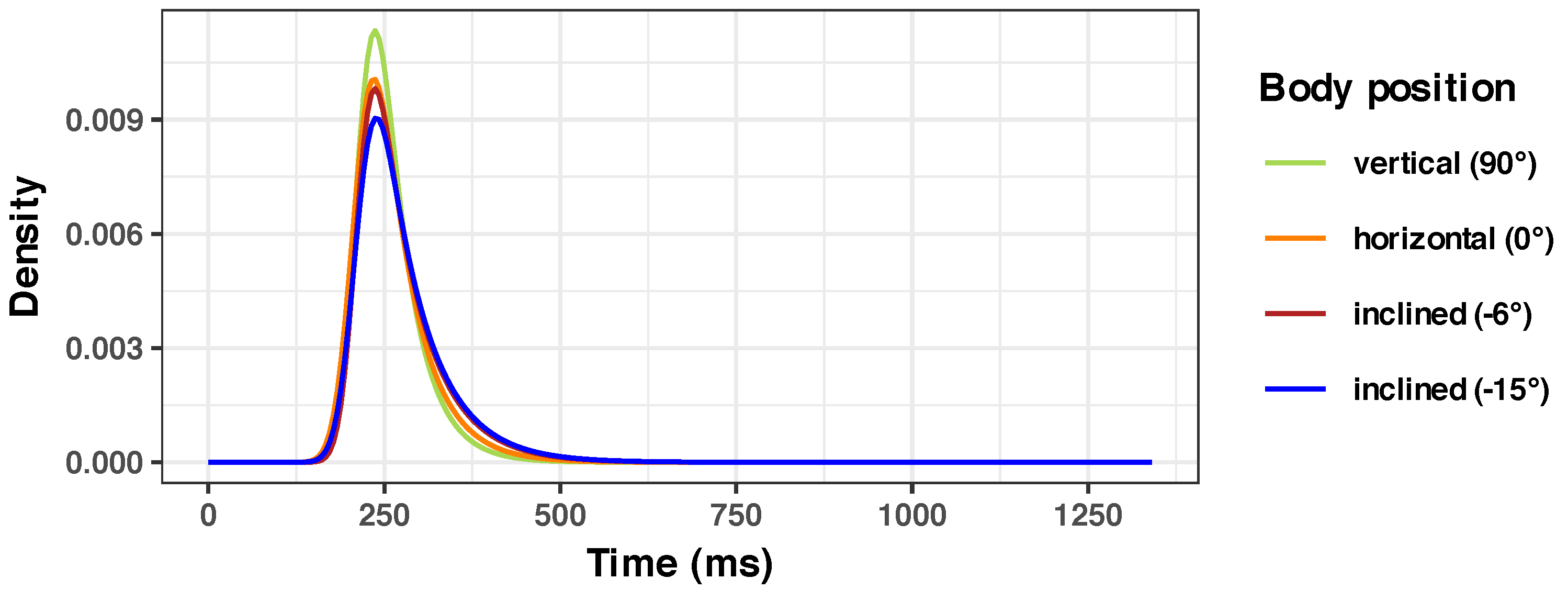
2.3. Body Positions
- (a)
- Vertical position (90° angle with the horizontal plane). The participant was seated on a regular office chair and observed the visual stimuli on a monitor that was positioned so that the normal line in the center of the monitor was aligned to the midpupillary point (the middle point between the eyes). This was considered the reference position for normal daily activities. In microgravity analogue studies, positions where the head is above the horizontal line are usually called “head-up tilt” (HUT) position. Therefore, this position would be 90° HUT;
- (b)
- Horizontal position (0° angle with the horizontal plane) on an adjustable bed (tilt table, see below);
- (c)
- Inclined at a −6° degree angle from the horizontal (−6° HDT, “head-down tilt”, as the head is below the horizontal line);
- (d)
- Inclined at −15° degrees from the horizontal (−15° HDT).
2.4. Instrumentation
2.4.1. Tilt Table
2.4.2. Displays
2.4.3. Computer Setup
2.4.4. Laboratory
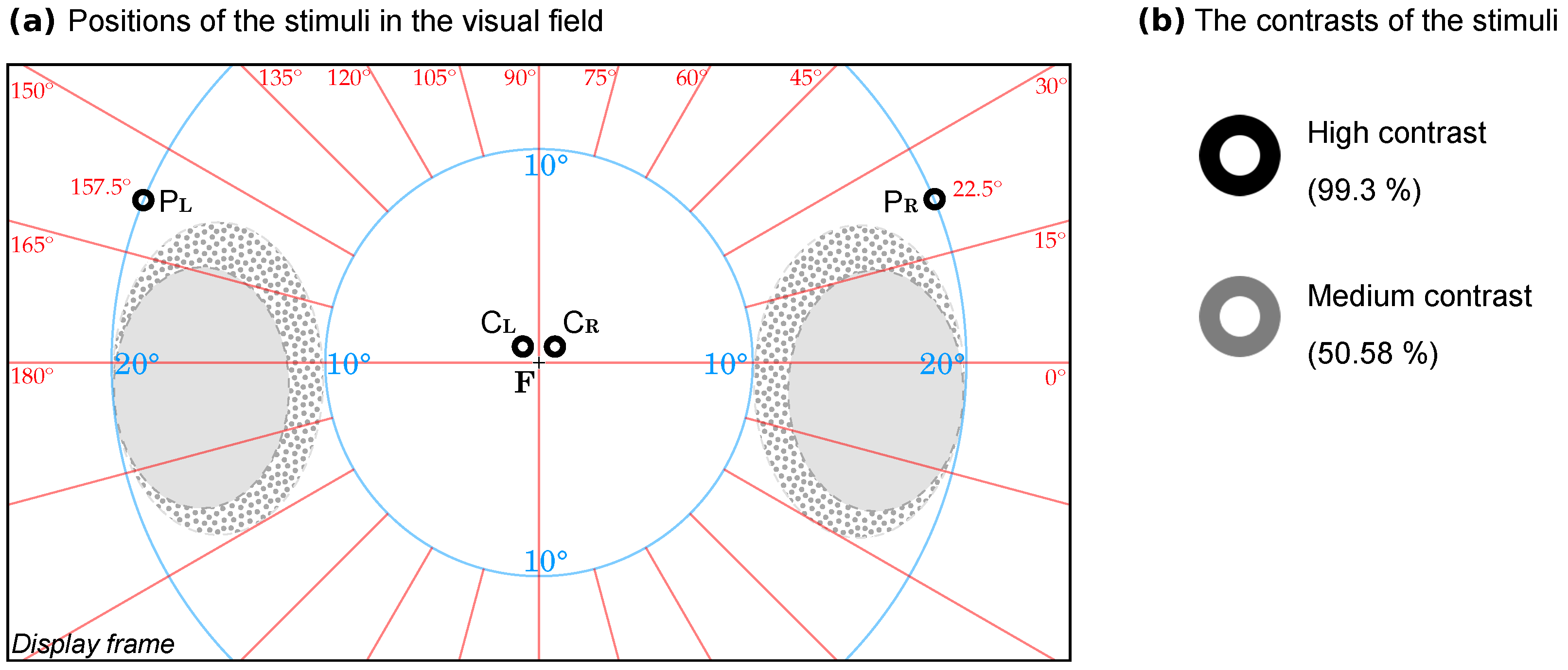
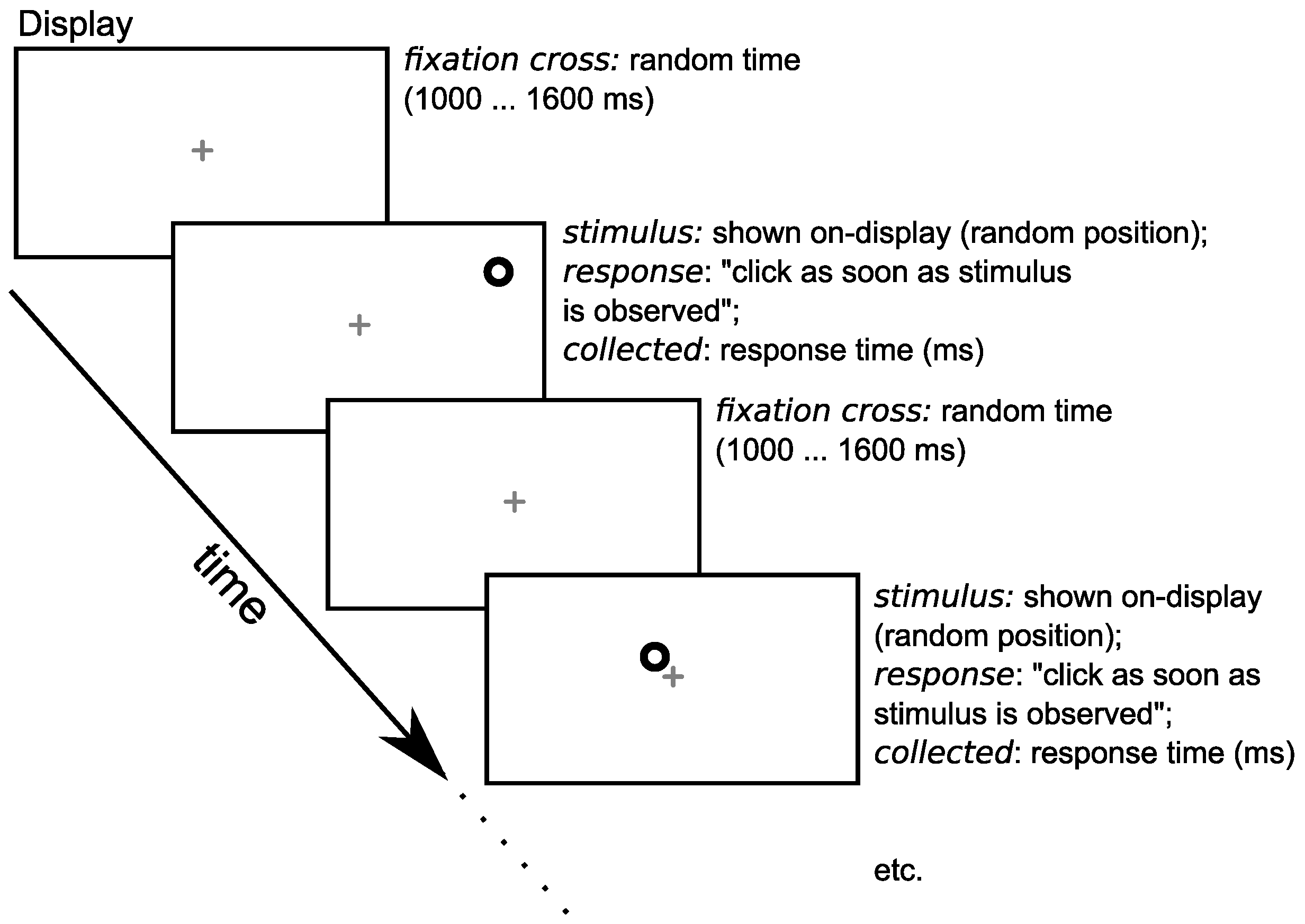
2.5. The Visual Stimuli
- -
- In the central visual field, parafoveal position at a 0.5° elevation from the horizontal meridian and a 0.5° radial angle from the vertical meridian, to the left () and, respectively, to the right ();
- -
- In the perimacular visual field, at a 22.5° elevation from the horizontal meridian and at a 20° radial angle from the horizontal meridian, to the left () and, respectively, to the right ().
2.6. The Experimental Protocol for Each Participant
- -
- In the initial presentation day in the laboratory, we explained the procedure and showed a short demonstration of the measurement equipment, obtained consent, asked for the self-assessment questionnaire for personal medical history and handedness, and performed an in-laboratory optometric screening, followed by a break (~10 min);
- -
- Two measurements (in two different body positions) in random order. Between them, there was a break of at least 30 min when the participant rested vertically (on a chair or walked for a break);
- -
- Followed by a break of 5–14 days (depending on the schedule of the participants);
- -
- Followed by the comprehensive ophthalmic investigation (in the hospital);
- -
- Followed by a break of 5–14 days (depending on the schedule of the participants);
- -
- Followed by a day in the laboratory when the last two measurements were performed (in two different body positions), in random order, with a break of 30 min (as in the first day).
2.7. The Measurement Session for Each Participant
- -
- The participants seated themselves on the tilted table (or chair);
- -
- A 2 min initial adaptation time: the table was adjusted to the desired angle, and then we waited for a maximum of 2 min to check the stability of the position of the participants, to check for any issues reported by the participants (if any), and to perform a quick verification of the response time experiment (8 practice trials, which were discarded and not included in the analysis);
- -
- A 6.2–8 min time to perform the reaction time measurement (SRT trials, see below);
- -
- Coming back to the initial position.
2.7.1. The Timeline of a Measurement
2.7.2. Time
2.8. Statistics
2.8.1. At the Participant Level
2.8.2. At the Group Level
2.8.3. Software
3. Results
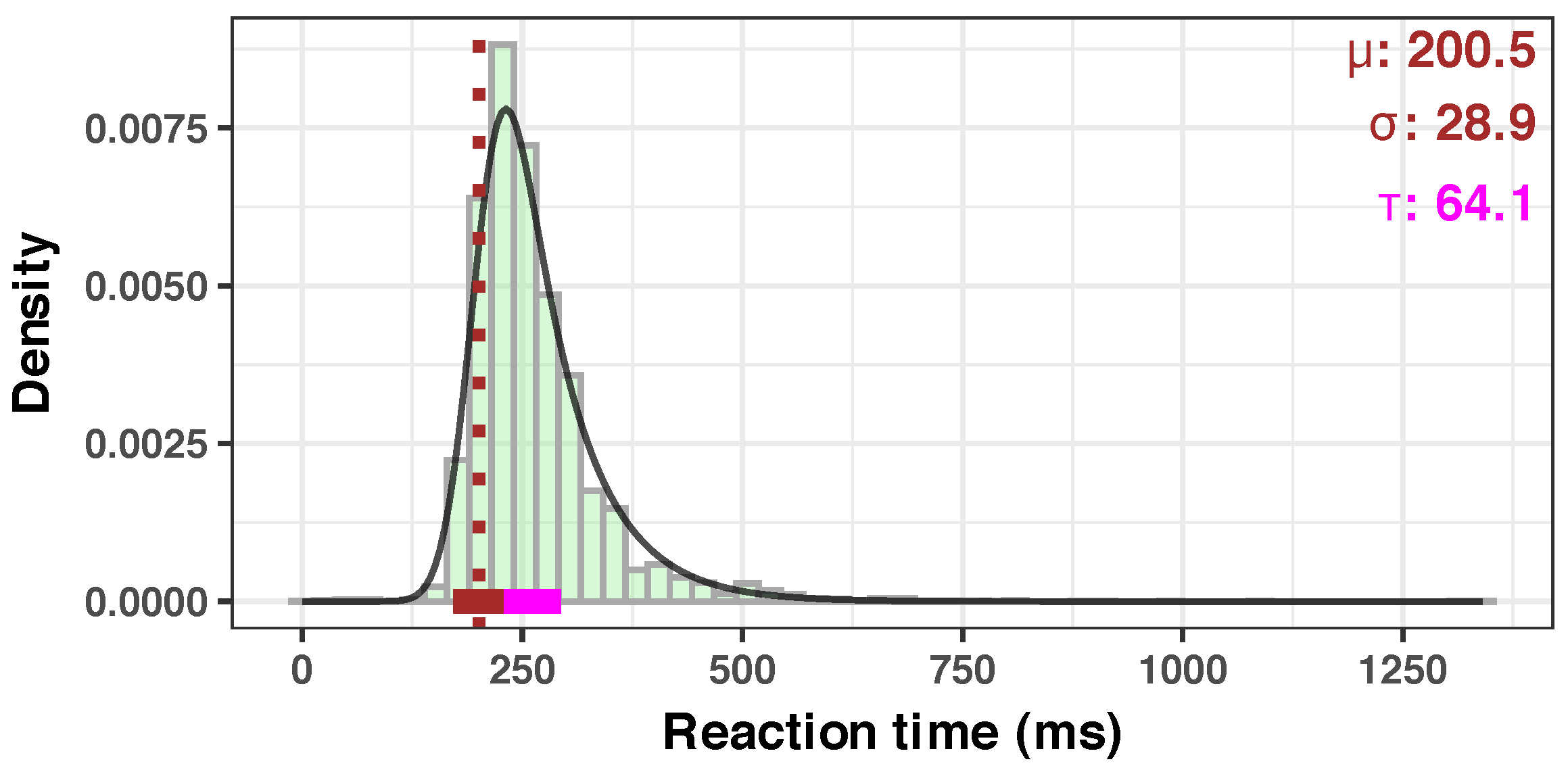
3.1. Quality of the Measurements
3.2. Overall Group Results
- (a) Mean RT at the group level (Figure 6a) The LME model of the mean RT as a function of the body position has the following R formula: Mean ~ bs(BodyPosition, knots = c(0), degree = 1) + (1|ID). The model included ID as a random effect (formula: ~1|ID).
- -
- The effect of body position [HUT] is statistically non-significant and positive ( = 6.91, 95% CI [−8.94, 22.76], t(27) = 0.89, p = 0.379; Std. beta = −0.02, 95% CI [−0.53, 0.50]).
- -
- The effect of Body Position [HDT] is statistically significant and positive ( = 19.40, 95% CI [3.00, 35.79], t(27) = 2.43, p = 0.022; Std. beta = 0.44, 95% CI [0.07, 0.81]).
- (b) RT standard deviation at the group level (Figure 6b)
- -
- The effect of body position [HUT] is statistically non-significant and positive ( = 6.75, 95% CI [−8.35, 21.85], t(27) = 0.92, p = 0.367; Std. beta = −0.15, 95% CI [−0.92, 0.63]).
- -
- The effect of body position [HDT] is statistically significant and positive ( = 24.39, 95% CI [8.77, 40.01], t(27) = 3.20, p = 0.003; Std. beta = 0.87, 95% CI [0.31, 1.43]).
- (c) Tau parameter at the group level (Figure 6c)
- -
- The effect of body position in HUT is statistically non-significant and positive ( = 10.31, 95% CI [−3.60, 24.22], t(27) = 1.52, p = 0.140; Std. beta = 0.14, 95% CI [−0.69, 0.98]).
- -
- The effect of body position in HDT is statistically significant and positive ( = 21.46, 95% CI [7.07, 35.85], t(27) = 3.06, p = 0.005; Std. beta = 0.89, 95% CI [0.29, 1.49]).
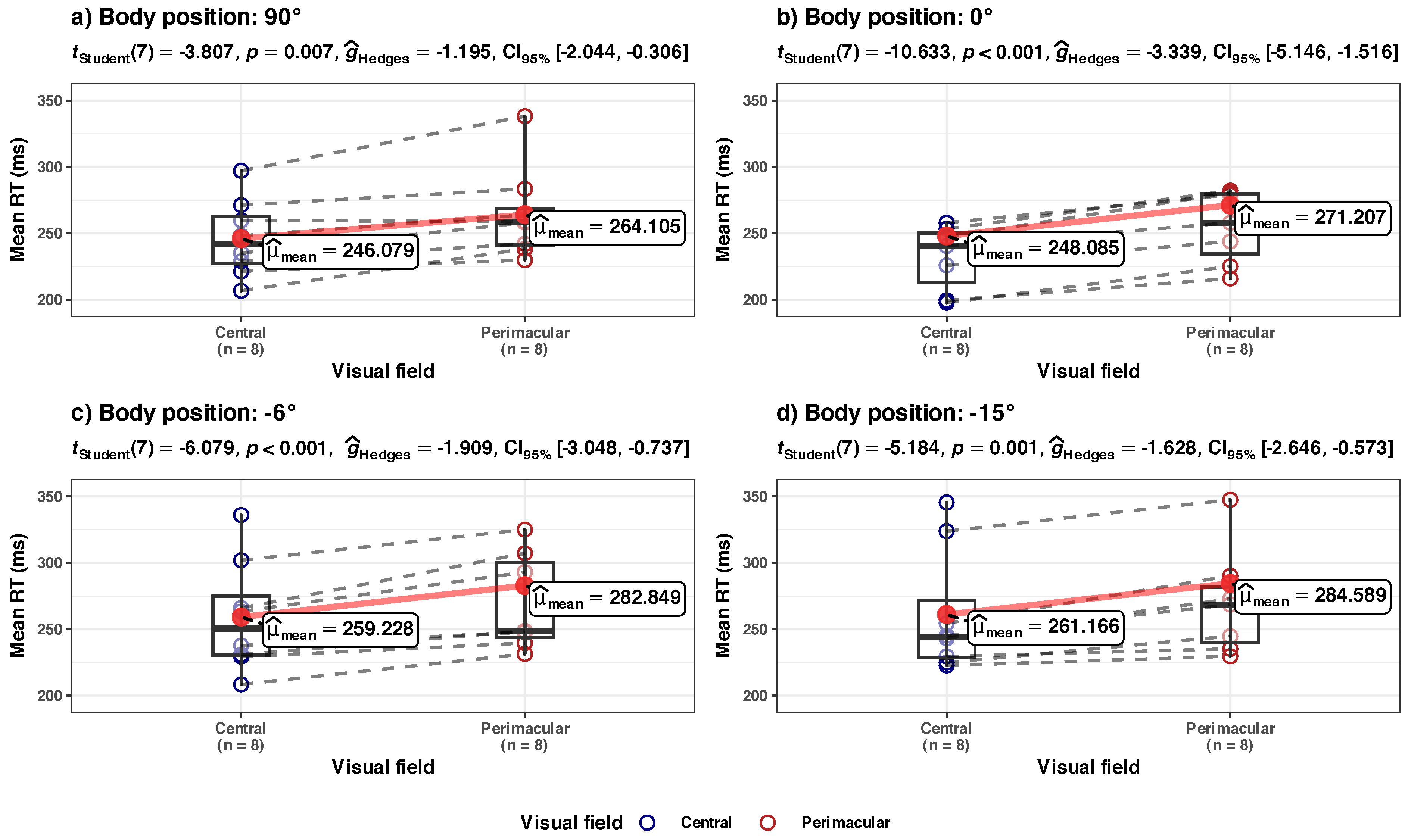
3.3. Central vs. Perimacular Field of View
- (a) Mean RT, in central vs. perimacular fields of view, at the group level (Figure 8a)
- (b) Std.Dev. RT in central vs. perimacular fields of viewat the group level (Figure 8b)
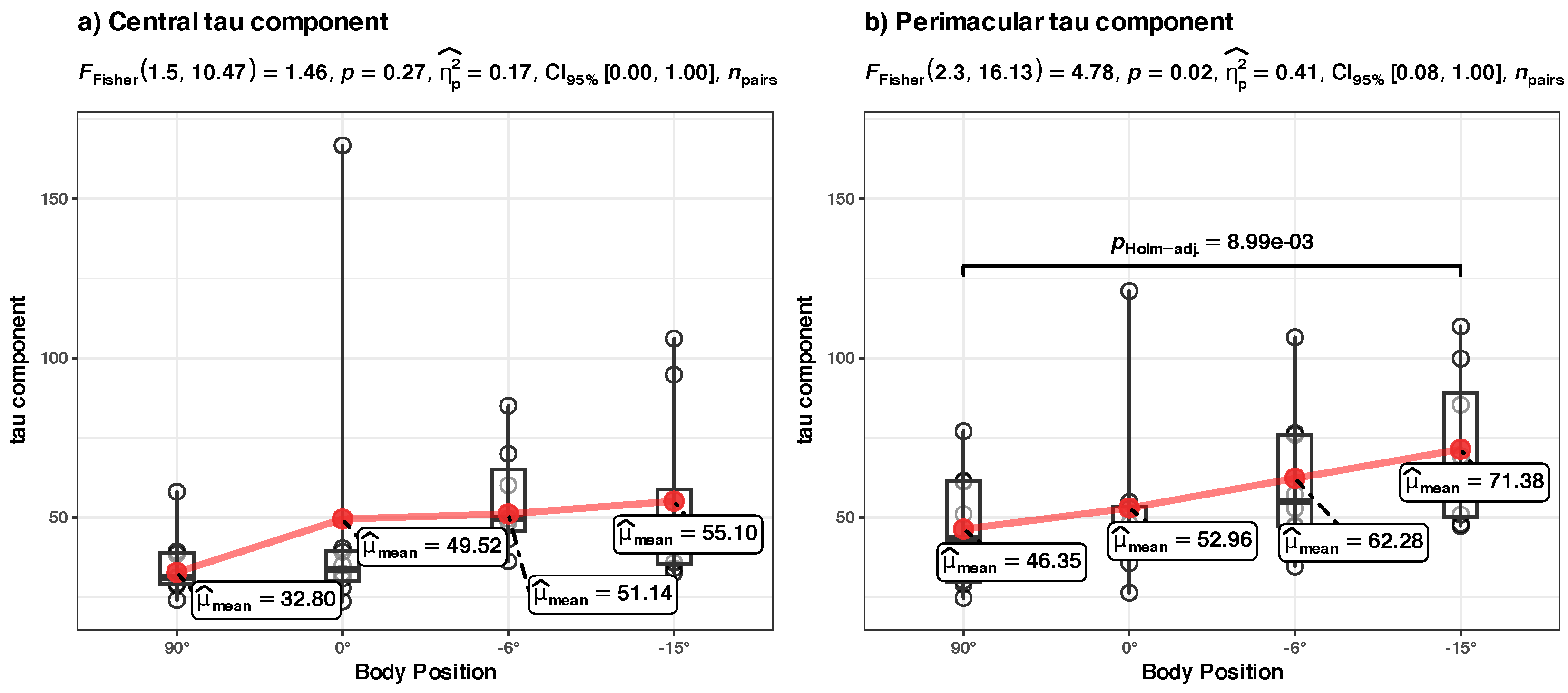
- (c) Tau component in central vs. perimacular fields of view at the group level (Figure 8c)
3.4. Visual-Motor Integration
3.5. High vs. Medium Visual Contrast
3.6. Other Anthropological Parameters
4. Discussion
4.1. Quality of the Measurements
4.2. Overall Group Results
Mean RT
- The experimental protocols are different; RTs are very sensitive to other confounding factors (discussed in Methods), and due to logistic and timing constraints on spaceflights, some of these factors were perhaps unaccounted for;
- The sample sizes are inconsistent, and there is a lack of standardization of the equipment used (with the associate timing errors discussed in Methods). All these factors diminish the signal-to-noise ratio of the measurements;
- A difficulty of comparison arises because of the different meanings of the term “acute” (referring to the time of exposure to microgravity). In the studies cited in this paper, we observed that the same “acute” term was given to very different time scales: seconds (in parabolic flight studies), minutes (in HDT), first 2 h, first 4 h or the first day (in HDT or immersion studies).
4.3. Central vs. Perimacular Field of View
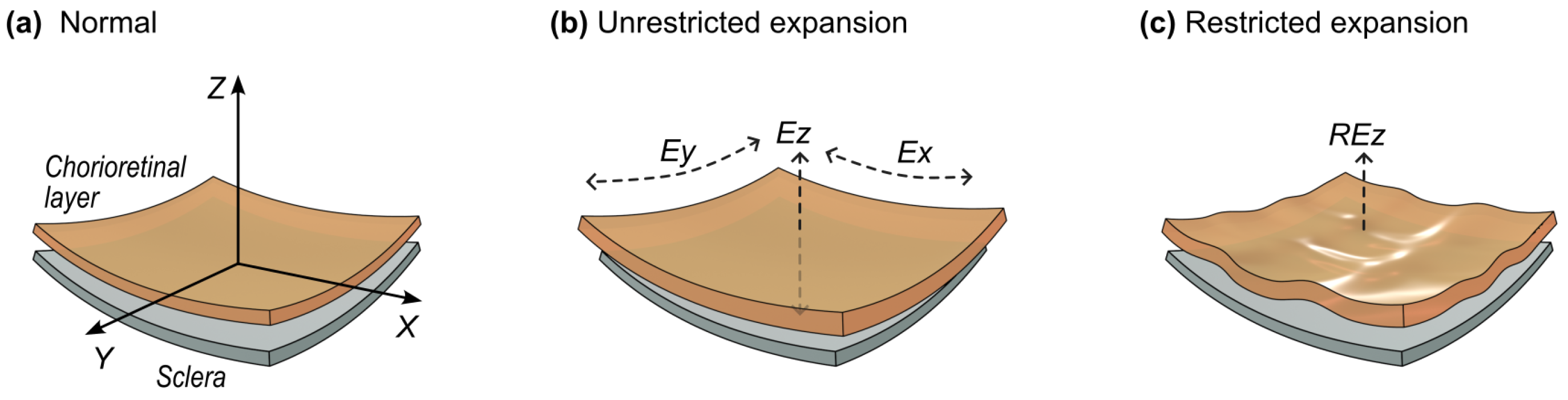
4.3.1. Mechanical Stress Dynamics in Time
4.3.2. The Distribution of This Mechanical Stress in the Chorioretinal Layer as a Whole
4.4. Visual-Motor Integration
4.5. High vs. Medium Visual Contrast
4.6. Implications
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CI | confidence interval |
| DC | direct current |
| HDMI | High-Definition Multimedia Interface |
| HDT | head-down tilt |
| HUT | head-up tilt |
| ID | the unique identifier of a participant’s responses in the dataset |
| IPS | in-plane switching |
| ISS | International Space Station |
| LCD | liquid-crystal display |
| LED | light-emitting diode |
| LME | linear mixed effects |
| PNS | parasympathetic nervous system |
| SANS | Spaceflight-Associated Neuro-Ocular Syndrome |
| SRT | simple reaction time |
| Std. beta | standardized beta |
| Std. Dev. | standard deviation |
| RT | reaction time |
| OCT | optical coherence tomography |
Appendix A. Statistical Details
Appendix A.1. Descriptive Group Statistics
| Characteristic | Body Position | |||
|---|---|---|---|---|
| Vertical (90°), N = 8 | Horizontal (0°), N = 8 | Inclined (−6°), N = 8 | Inclined (−15°), N = 8 | |
| Median | ||||
| (ms) | 246.84 | 245.64 | 255.72 | 255.55 |
| Mean | ||||
| (ms) | 255.09 | 259.64 | 271.04 | 272.88 |
| SD | 52.79 | 58.68 | 68.14 | 76.59 |
| Skewness | 1.93 | 1.83 | 2.54 | 2.64 |
| Kurtosis | 11.16 | 7.52 | 11.27 | 16.46 |
| mu | 215.29 | 211.03 | 212.98 | 212.81 |
| sigma | 21.86 | 22.96 | 19.48 | 22.52 |
| tau | 40.07 | 48.46 | 58.13 | 60.22 |
| logLIK | −578.69 | −582.53 | −595.76 | −613.66 |
| AIC | 1163.38 | 1171.05 | 1197.52 | 1233.31 |
| BIC | 1171.54 | 1179.21 | 1205.68 | 1241.47 |
Appendix A.2. Overall Group Models
| Dependent Variable: | |||
|---|---|---|---|
| Mean RT | RT St.Dev. | tau | |
| 6.91 | 6.75 | 10.31 | |
| (7.72) | (7.36) | (6.78) | |
| 19.40 * | 24.39 ** | 21.46 ** | |
| (7.99) | (7.61) | (7.01) | |
| Constant | 255.09 *** | 52.79 *** | 40.07 *** |
| (16.17) | (9.75) | (8.38) | |
| Observations | 32 | 32 | 32 |
| Conditional | 0.87 | 0.71 | 0.67 |
| Marginal | 0.02 | 0.10 | 0.10 |
| Log Likelihood | −137.32 | −132.10 | −129.07 |
| Akaike Inf. Crit. | 284.64 | 274.20 | 268.14 |
| Bayesian Inf. Crit. | 291.97 | 281.53 | 275.47 |
- (a) LME model for mean RT at the group level: Figure 6a
- -
- The effect of Body Position [HUT] is statistically non-significant and positive ( = 6.91, 95% CI [−8.94, 22.76], t(27) = 0.89, p = 0.379; Std. beta = −0.02, 95% CI [−0.53, 0.50]).
- -
- The effect of Body Position [HDT] is statistically significant and positive ( = 19.40, 95% CI [3.00, 35.79], t(27) = 2.43, p = 0.022; Std. beta = 0.44, 95% CI [0.07, 0.81]).
- (b) LME model for RT standard deviation at the group level: Figure 6b
- -
- The effect of Body Position [HUT] is statistically non-significant and positive ( = 6.75, 95% CI [−8.35, 21.85], t(27) = 0.92, p = 0.367; Std. beta = −0.15, 95% CI [−0.92, 0.63]).
- -
- The effect of Body Position [HDT] is statistically significant and positive ( = 24.39, 95% CI [8.77, 40.01], t(27) = 3.20, p = 0.003; Std. beta = 0.87, 95% CI [0.31, 1.43]).
- (c) Tau parameter at the group level (Figure 6c)
- -
- The effect of BodyPosition [HUT] is statistically non-significant and positive ( = 10.31, 95% CI [−3.60, 24.22], t(27) = 1.52, p = 0.140; Std. beta = 0.14, 95% CI [−0.69, 0.98]).
- -
- The effect of Body Position [HDT] is statistically significant and positive ( = 21.46, 95% CI [7.07, 35.85], t(27) = 3.06, p = 0.005; Std. beta = 0.89, 95% CI [0.29, 1.49]).
Appendix A.3. Central vs. Perimacular Fields of View Models
| Dependent Variable: | ||||||
|---|---|---|---|---|---|---|
| Mean RT | RT Std. Dev. | tau | ||||
| Central | Peri. | Central | Peri. | Central | Peri. | |
| 4.29 | 9.54 | 16.90 | −0.47 | 16.47 | 7.34 | |
| (7.97) | (8.20) | (10.24) | (8.31) | (10.59) | (6.50) | |
| 16.65 | 22.14 * | 21.02 | 28.12 ** | 22.13 | 25.52 *** | |
| (8.24) | (8.48) | (10.59) | (8.60) | (10.96) | (6.73) | |
| Constant | 246.08 *** | 264.11 *** | 39.39 *** | 59.87 *** | 32.80 ** | 46.35 *** |
| (15.31) | (17.21) | (10.20) | (10.95) | (10.66) | (8.48) | |
| Obs. | 32 | 32 | 32 | 32 | 32 | 32 |
| Cond. | 0.85 | 0.88 | 0.47 | 0.72 | 0.48 | 0.71 |
| Margin. | 0.02 | 0.03 | 0.08 | 0.13 | 0.08 | 0.14 |
| LogLIK | −137.54 | −139.07 | −138.70 | −135.57 | −139.81 | −128.37 |
| AIC | 285.09 | 288.15 | 287.40 | 281.13 | 289.61 | 266.75 |
| BIC | 292.42 | 295.48 | 294.72 | 288.46 | 296.94 | 274.07 |
- -
- The effect of Body Position [HUT] is statistically non-significant and positive (= 4.29, 95% CI [−12.05, 20.64], t(27). = 0.54, p = 0.594; Std. beta = −0.08, 95% CI [−0.64, 0.48])
- -
- The effect of Body Position [HDT] is statistically non-significant and positive ( = 16.65, 95% CI [−0.26, 33.56], t(27) = 2.02, p = 0.053; Std. beta = 0.40, 95% CI [−0.0063, 0.80]).
- -
- The effect of Body Position [HUT] is statistically non-significant and positive ( = 9.54, 95% CI [−7.29, 26.36], t(27) = 1.16, p = 0.255; Std. beta = 0.04, 95% CI [−0.48, 0.55]).
- -
- The effect of Body Position [HDT] is statistically significant and positive ( = 22.14, 95% CI [4.74, 39.55], t(27) = 2.61, p = 0.015; Std. beta = 0.47, 95% CI [0.10, 0.84]).
- -
- The effect of BodyPosition [HUT] is statistically non-significant and positive ( = 16.90, 95% CI [−4.11, 37.91], t(27) = 1.65, p = 0.111; Std. beta = 0.50, 95% CI [−0.55, 1.54]).
- -
- The effect of Body Position [HDT] is statistically non-significant and positive ( = 21.02, 95% CI [−0.71, 42.76], t(27) = 1.98, p = 0.057; Std. beta = 0.73, 95% CI [−0.02, 1.48]).
- -
- The effect of Body Position [in HUT] is statistically non-significant and negative ( = −0.47, 95% CI [−17.52, 16.57], t(27) = −0.06, p = 0.955; Std. beta = −0.57, 95% CI [−1.33, 0.20]).
- -
- The effect of Body Position [in HDT] is statistically significant and positive ( = 28.12, 95% CI [10.48, 45.76], t(27) = 3.27, p = 0.003; Std. beta = 0.88, 95% CI [0.33, 1.44]).
- -
- The effect of Body Position [HUT] is statistically non-significant and positive ( = 16.47, 95% CI [−5.27, 38.21], t(27) = 1.55, p = 0.132; Std. beta = 0.43, 95% CI [−0.60, 1.46]).
- -
- The effect of Body Position [HDT] is statistically non-significant and positive ( = 22.13, 95% CI [−0.36, 44.62], t(27) = 2.02, p = 0.054; Std. beta = 0.73, 95% CI [−0.01, 1.48]).
- Tau component in perimacular field of view at the group level
- -
- The effect of Body Position [HUT] is statistically non-significant and positive ( = 7.34, 95% CI [−6.01, 20.68], t(27) = 1.13, p = 0.269; Std. beta = −0.15, 95% CI [−0.93, 0.62]).
- -
- The effect of Body Position [HDT] is statistically significant and positive ( = 25.52, 95% CI [11.72, 39.33], t(27) = 3.79, p < 0.001; Std. beta = 1.03, 95% CI [0.47, 1.58]).
Appendix B. Miscellaneous Low-Significance Findings
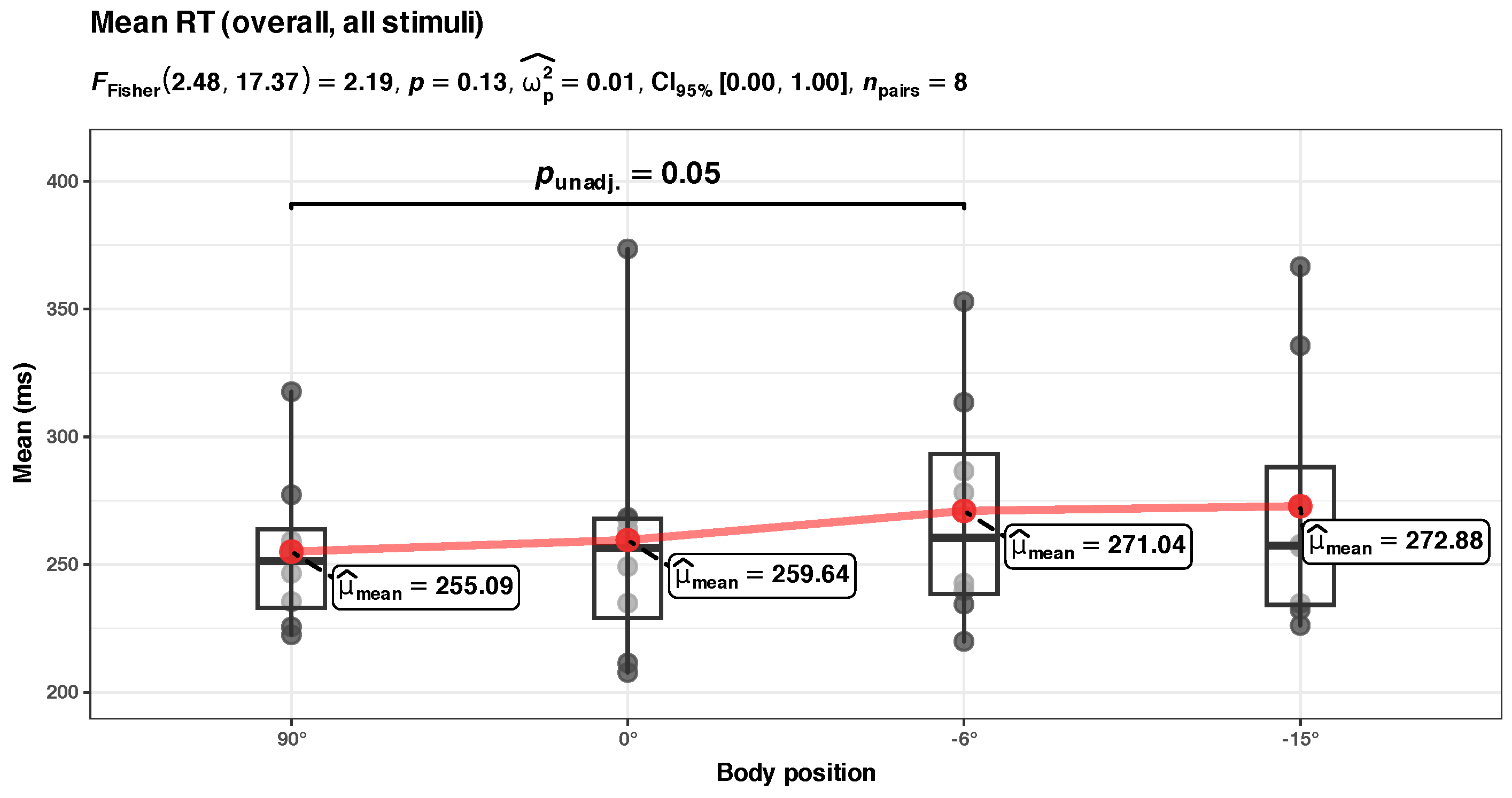
Appendix B.1. Unadjusted Mean RT Analysis (Overall)
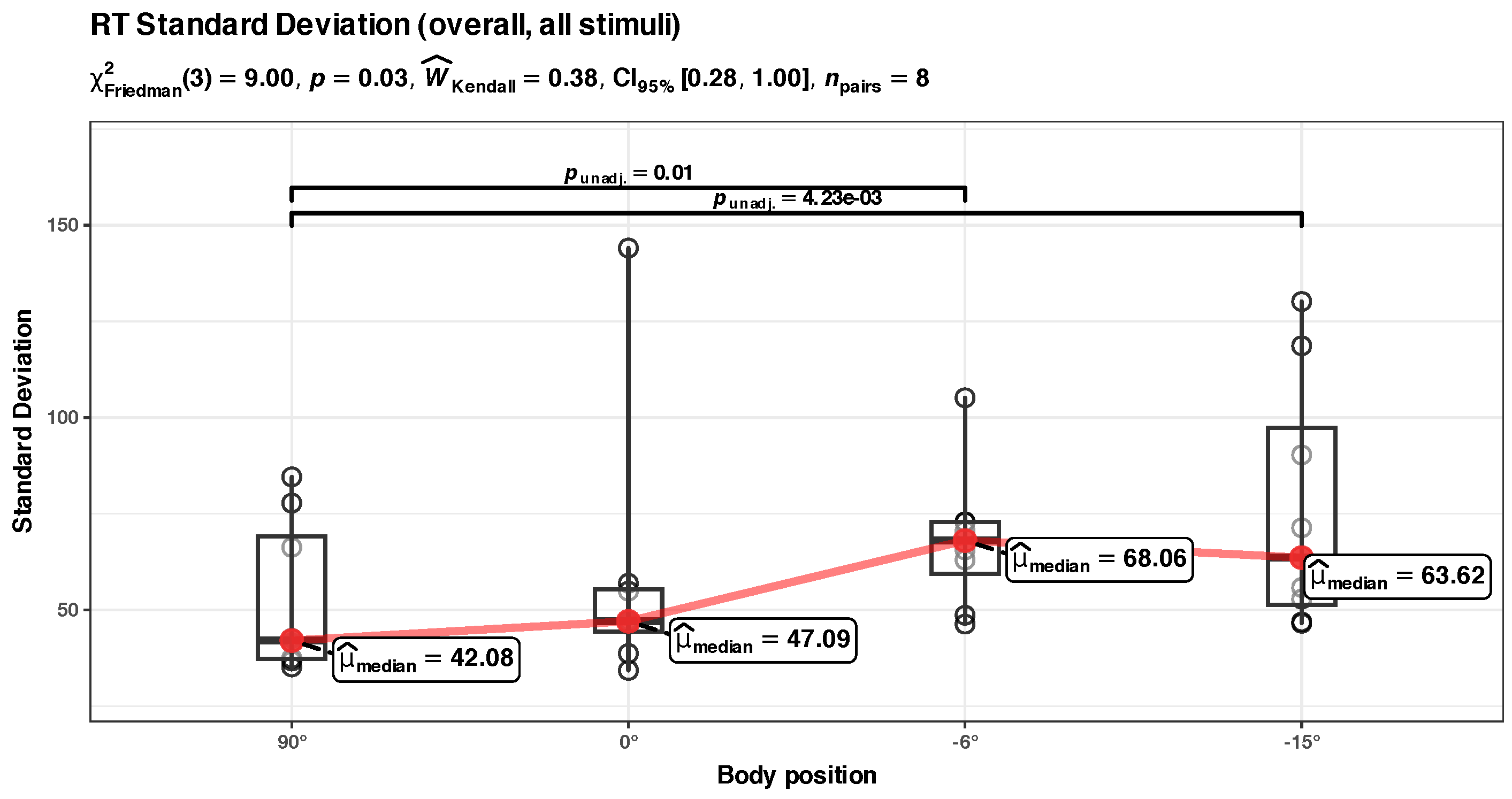
Appendix B.2. Unadjusted Std. Dev. RT Analysis (Overall)
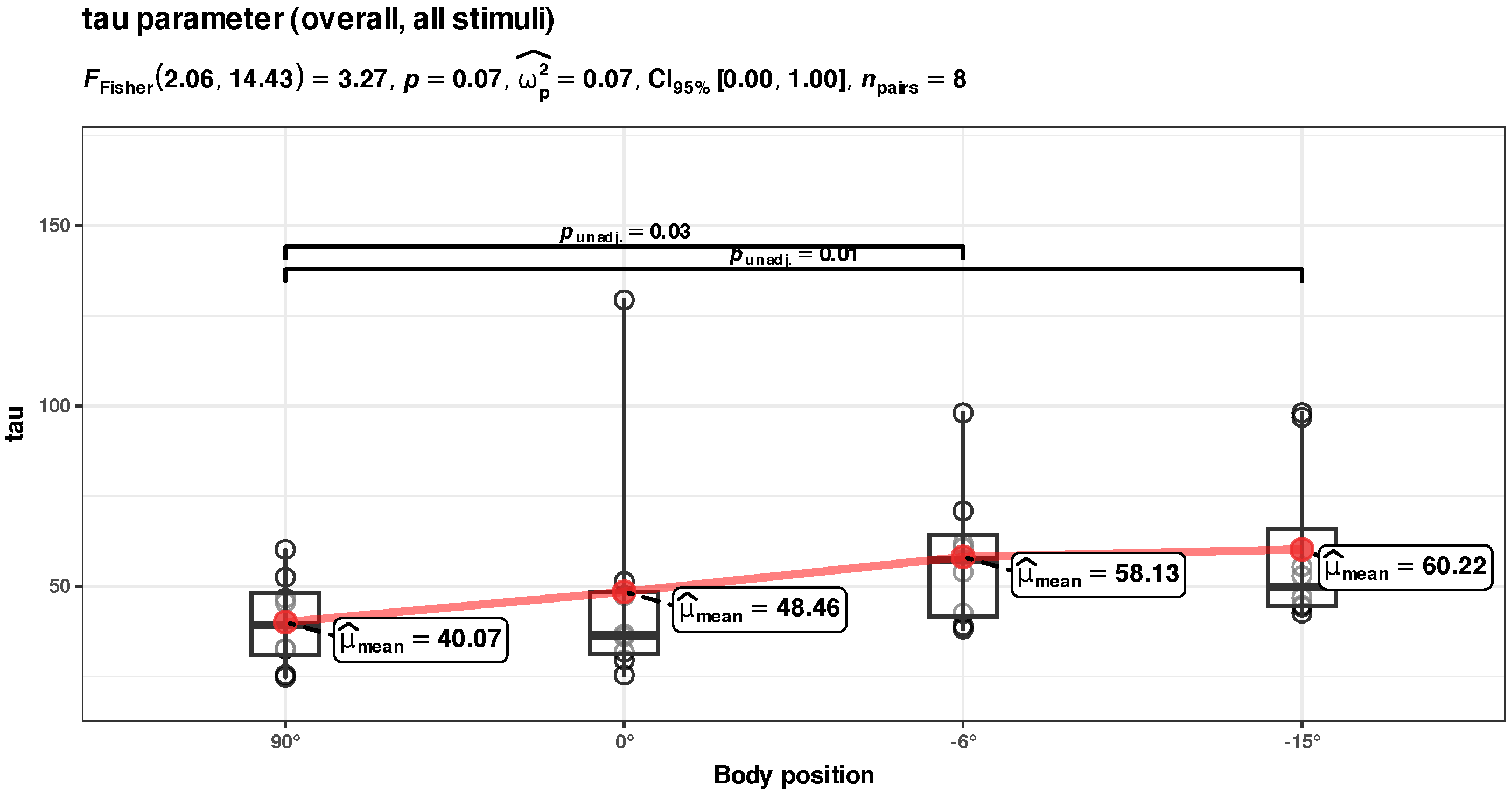
Appendix B.3. Unadjusted Tau Component Analysis
Appendix B.4. Non-Significant Visual Contrast vs. Body Position Model

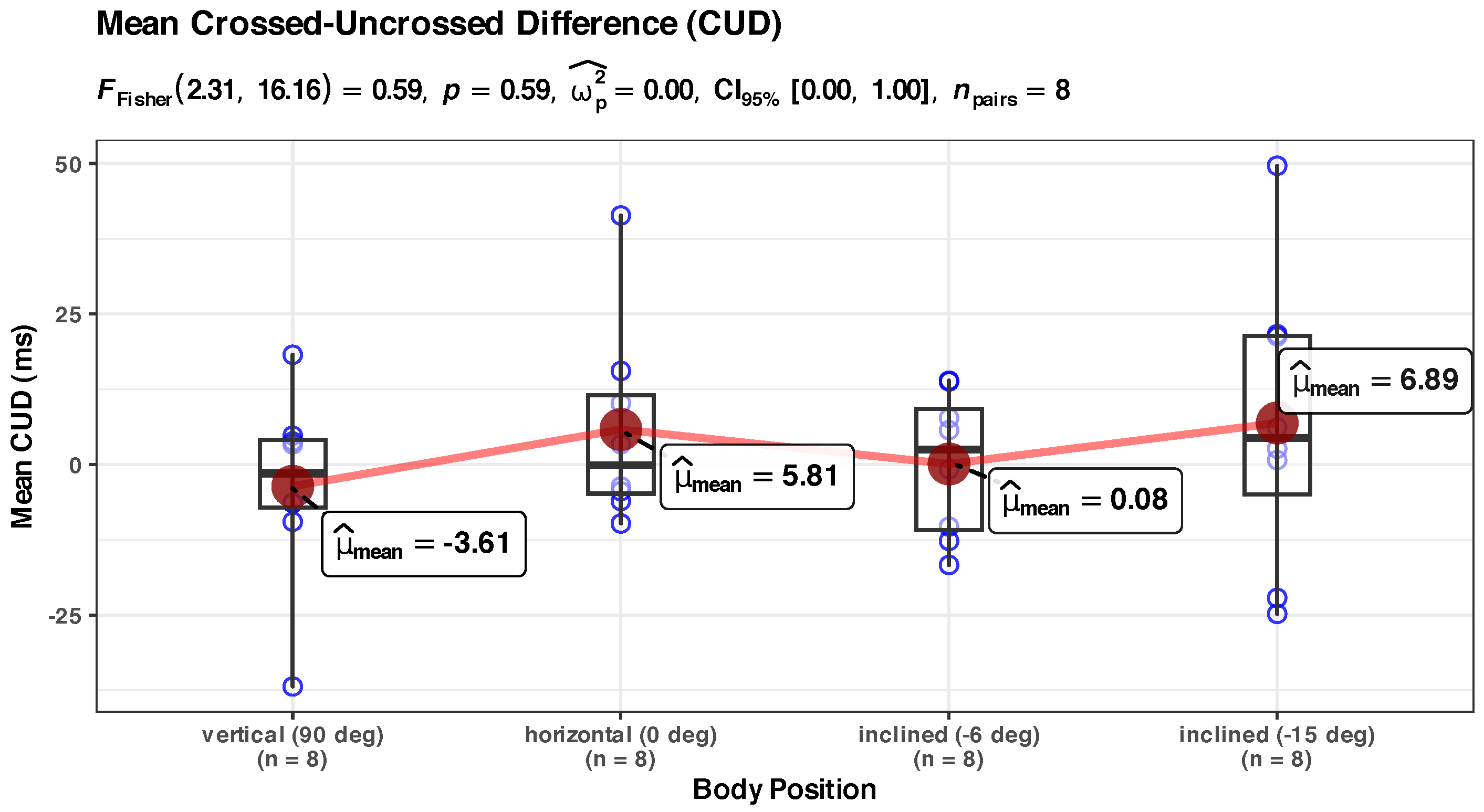
Appendix B.5. Non-Significant CUD
References
- Krittanawong, C.; Singh, N.K.; Scheuring, R.A.; Urquieta, E.; Bershad, E.M.; Macaulay, T.R.; Kaplin, S.; Dunn, C.; Kry, S.F.; Russomano, T.; et al. Human Health during Space Travel: State-of-the-Art Review. Cells 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.H.; Gibson, C.R.; Pass, A.F.; Kramer, L.A.; Lee, A.G.; Fogarty, J.; Tarver, W.J.; Dervay, J.P.; Hamilton, D.R.; Sargsyan, A.; et al. Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight. Ophthalmology 2011, 118, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.; Mulugeta, L.; Myers, J. Microgravity-Induced Fluid Shift and Ophthalmic Changes. Life 2014, 4, 621–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G.; Tarver, W.J.; Mader, T.H.; Gibson, C.R.; Hart, S.F.; Otto, C.A. Neuro-Ophthalmology of Space Flight. J. Neuro-Ophthalmol. 2016, 36, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. npj Microgravity 2020, 6. [Google Scholar] [CrossRef]
- Mader, T.; Gibson, C.; Miller, N.; Subramanian, P.; Patel, N.; Lee, A. An overview of spaceflight-associated neuro-ocular syndrome (SANS). Neurol. India 2019, 67, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Fleischman, D.; Lee, A.G.; Killer, H.E.; Chen, J.J.; Bhatti, M.T. Current concepts of cerebrospinal fluid dynamics and the translaminar cribrosa pressure gradient: A paradigm of optic disk disease. Surv. Ophthalmol. 2020, 65, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; De Winne, F.; Stern, C.; Mader, T.H.; Gibson, C.R.; De Deyn, P.P. Potential Involvement of the Ocular Glymphatic System in Optic Disc Edema in Astronauts. Aerosp. Med. Hum. Perform. 2020, 91, 975–977. [Google Scholar] [CrossRef]
- Wåhlin, A.; Holmlund, P.; Fellows, A.M.; Malm, J.; Buckey, J.C.; Eklund, A. Optic Nerve Length before and after Spaceflight. Ophthalmology 2021, 128, 309–316. [Google Scholar] [CrossRef]
- Zwart, S.R.; Gibson, C.R.; Mader, T.H.; Ericson, K.; Ploutz-Snyder, R.; Heer, M.; Smith, S.M. Vision Changes after Spaceflight Are Related to Alterations in Folate- and Vitamin B-12-Dependent One-Carbon Metabolism. J. Nutr. 2012, 142, 427–431. [Google Scholar] [CrossRef]
- Marshall-Goebel, K.; Mulder, E.; Donoviel, D.; Strangman, G.; Suarez, J.I.; Venkatasubba Rao, C.; Frings-Meuthen, P.; Limper, U.; Rittweger, J.; Bershad, E.M. An international collaboration studying the physiological and anatomical cerebral effects of carbon dioxide during head-down tilt bed rest: The SPACECOT study. J. Appl. Physiol. 2017, 122, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Nasrini, J.; Hermosillo, E.; McGuire, S.; Dinges, D.F.; Moore, T.M.; Gur, R.C.; Rittweger, J.; Mulder, E.; Wittkowski, M.; et al. Effects of -12° head-down tilt with and without elevated levels of CO2 on cognitive performance: The SPACECOT study. J. Appl. Physiol. 2018, 124, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.S.; Macias, B.R.; Dunn, J.T.; Young, M.; Stern, C.; Lee, S.M.; Stenger, M.B. Optic Disc Edema after 30 Days of Strict Head-down Tilt Bed Rest. Ophthalmology 2019, 126, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Diaz-Artiles, A.; Zaman, S.Y.; Ridolfi, L.; Scarsoglio, S. Linking cerebral hemodynamics and ocular microgravity-induced alterations through an in silico-in vivo head-down tilt framework. npj Microgravity 2024, 10, 22. [Google Scholar] [CrossRef]
- Mader, T.H.; Gibson, C.R.; Otto, C.A.; Sargsyan, A.E.; Miller, N.R.; Subramanian, P.S.; Hart, S.F.; Lipsky, W.; Patel, N.B.; Lee, A.G. Persistent Asymmetric Optic Disc Swelling After Long-Duration Space Flight: Implications for Pathogenesis. J. Neuro-Ophthalmol. 2017, 37, 133–139. [Google Scholar] [CrossRef]
- Patel, N.; Pass, A.; Mason, S.; Gibson, C.R.; Otto, C. Optical Coherence Tomography Analysis of the Optic Nerve Head and Surrounding Structures in Long-Duration International Space Station Astronauts. JAMA Ophthalmol. 2018, 136, 193. [Google Scholar] [CrossRef]
- Stern, C.; Yücel, Y.H.; zu Eulenburg, P.; Pavy-Le Traon, A.; Petersen, L.G. Eye-brain axis in microgravity and its implications for Spaceflight Associated Neuro-ocular Syndrome. npj Microgravity 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Watenpaugh, D.E. Analogs of microgravity: Head-down tilt and water immersion. J. Appl. Physiol. 2016, 120, 904–914. [Google Scholar] [CrossRef]
- Pandiarajan, M.; Hargens, A.R. Ground-Based Analogs for Human Spaceflight. Front. Physiol. 2020, 11, 716. [Google Scholar] [CrossRef]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef]
- Smith, J.D.; Cromwell, R.L.; Kundrot, C.E.; Charles, J.B. Six-degree head-down tilt bed rest: Forty years of development as a physiological analog for weightlessness. In Proceedings of the American Society for Gravitational and Space Biology Conference, San Jose, CA, USA, 3 November 2011. number ARC-E-DAA-TN4096. [Google Scholar]
- Nicogossian, A.E.; Huntoon, C.L.; Polk, J.D.; Williams, R.S.; Schneider, C.R.D.V.S. (Eds.) Simulations and Analogs (Test-Beds). In Space Physiology and Medicine; Springer: New York, NY, USA, 2017; pp. 441–445. [Google Scholar]
- Galanis, D.S.; Naka, K.K.; Veziraki, P.; Simos, Y.V.; Kalfakakou, V.; Evangelou, A.M. Cardiovascular and pulmonary adaptations during short-term 15° and 30° head-down posture in healthy male volunteers. Hell. J.Cardiol. HJC Hell. Kardiol. Ep. 2013, 54, 273–280. [Google Scholar]
- Mader, T.H.; Taylor, G.R.; Hunter, N.; Caputo, M.; Meehan, R.T. Intraocular pressure, retinal vascular, and visual acuity changes during 48 h of 10 degrees head-down tilt. Aviation Space Environ. Med. 1990, 61, 810–813. [Google Scholar]
- Kermorgant, M.; Chedmail, T.; Varenne, F.; Bareille, M.P.; Beck, A.; Billette de Villemeur, R.; Fournié, P.; Grondin, L.; Hélissen, O.; Membrives, C.; et al. Neuro-ophthalmological changes in healthy females exposed to a 5-day dry immersion: A pilot study. npj Microgravity 2024, 10, 4. [Google Scholar] [CrossRef]
- Laurie, S.S.; Greenwald, S.H.; Marshall-Goebel, K.; Pardon, L.P.; Gupta, A.; Lee, S.M.C.; Stern, C.; Sangi-Haghpeykar, H.; Macias, B.R.; Bershad, E.M. Optic disc edema and chorioretinal folds develop during strict 6° head-down tilt bed rest with or without artificial gravity. Physiol. Rep. 2021, 9, e14977. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Trick, G.L.; Wolf, M.L. Altering body position affects intraocular pressure and visual function. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1492–1497. [Google Scholar]
- Schroeder, J.E.; Tuttle, M.L. Investigation of Possible Causes for Human-Performance Degradation during Microgravity Flight; Resreport (SwRI Project No. 12-4075, NASA Grant No. NAG 9-487); NASA: Washington, DC, USA, 1991. [Google Scholar]
- Oneal, M.R.; Task, H.L.; Genco, L.V. Effect of Microgravity on Several Visual Functions During STS Shuttle Missions: Visual Function Tester-model 1 (VFT-1). In Proceedings of the Flight Experiments Technical Interchange Meeting Proceedings, Monterey, CA, USA, 5–9 October 1992; NASA: Washington, DC, USA, 1992. [Google Scholar]
- Wollseiffen, P.; Vogt, T.; Abeln, V.; Strüder, H.K.; Askew, C.D.; Schneider, S. Neuro-cognitive performance is enhanced during short periods of microgravity. Physiol. Behav. 2016, 155, 9–16. [Google Scholar] [CrossRef]
- Strangman, G.E.; Sipes, W.; Beven, G. Human Cognitive Performance in Spaceflight and Analogue Environments. Aviat. Space Environ. Med. 2014, 85, 1033–1048. [Google Scholar] [CrossRef]
- Karpinskaia, V.Y.; Pechenkova, E.V.; Zelenskaya, I.S.; Lyakhovetskii, V.A. Vision for Perception and Vision for Action in Space Travelers. Front. Physiol. 2022, 13, 806578. [Google Scholar] [CrossRef]
- Kosinski, R.J. A Literature Review on Reaction Time; Clemson University: Clemson, SC, USA, 2012. [Google Scholar]
- Luce, R.D. Response Times. Their Role in Inferring Elementary Mental Organization; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Whelan, R. Effective Analysis of Reaction Time Data. Psychol. Rec. 2008, 58, 475–482. [Google Scholar] [CrossRef]
- Van Zandt, T. How to fit a response time distribution. Psychon. Bull. Rev. 2000, 7, 424–465. [Google Scholar] [CrossRef]
- Matzke, D.; Wagenmakers, E.J. Psychological interpretation of the ex-Gaussian and shifted Wald parameters: A diffusion model analysis. Psychon. Bull. Rev. 2009, 16, 798–817. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.M.; Horowitz, T.S.; Torralba, A.; Wolfe, J.M. What are the shapes of response time distributions in visual search? J. Exp. Psychol. Hum. Percept. Perform. 2011, 37, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Rouder, J.N.; Lu, J.; Speckman, P.; Sun, D.; Jiang, Y. A hierarchical model for estimating response time distributions. Psychon. Bull. Rev. 2005, 12, 195–223. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, A. RTSYS: A DOS application for the analysis of reaction time data. Behav. Res. Methods Instrum. Comput. 1996, 28, 427–445. [Google Scholar] [CrossRef]
- Luce, R.D. Chapter 2. Simple Reaction Times: Basic Data. In Response Times. Their Role in Inferring Elementary Mental Organization; Oxford Science Publications; Oxford University Press: Oxford, UK, 1991; pp. 49–95. [Google Scholar]
- Kuldavletova, O.; Navarro Morales, D.C.; Quarck, G.; Denise, P.; Clément, G. Spaceflight alters reaction time and duration judgment of astronauts. Front. Physiol. 2023, 14, 1141078. [Google Scholar] [CrossRef]
- Li, J.; Lam, C.S.Y.; Yu, M.; Hess, R.F.; Chan, L.Y.L.; Maehara, G.; Woo, G.C.; Thompson, B. Quantifying Sensory Eye Dominance in the Normal Visual System: A New Technique and Insights into Variation across Traditional Tests. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6875. [Google Scholar] [CrossRef]
- Buteikienė, D.; Kybartaitė-Žilienė, A.; Kriaučiūnienė, L.; Barzdžiukas, V.; Janulevičienė, I.; Paunksnis, A. Morphometric parameters of the optic disc in normal and glaucomatous eyes based on time-domain optical coherence tomography image analysis. Medicina 2017, 53, 242–252. [Google Scholar] [CrossRef]
- Waisberg, E.; Micieli, J.A. Neuro-Ophthalmological Optic Nerve Cupping: An Overview. Eye Brain 2021, 13, 255–268. [Google Scholar] [CrossRef]
- Golnik, K.C. Congenital and Acquired Abnormalities of the Optic Nerve. UpToDate, Wolters Kluwer (Ed. Paysse E.A. and Tehrani N.). 2024. Available online: https://www.uptodate.com/contents/congenital-and-acquired-abnormalities-of-the-optic-nerve (accessed on 21 August 2024).
- Kingma, I.; Toussaint, H.M.; Commissaris, D.A.C.M.; Savelsbergh, G.J.P. Adaptation of center of mass control under microgravity in a whole-body lifting task. Exp. Brain Res. 1999, 125, 35–42. [Google Scholar] [CrossRef]
- van de Langenberg, R.; Kingma, I.; Beek, P.J. Perception of limb orientation in the vertical plane depends on center of mass rather than inertial eigenvectors. Exp. Brain Res. 2007, 180, 595–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, J.J.; Hughes, C.V.; Ptacin, M.J.; Barney, J.A.; Tristani, F.E.; Ebert, T.J. The Effect of Age on Hemodynamic Response to Graded Postural Stress in Normal Men. J. Gerontol. 1987, 42, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Critchley, L.A.H.; Conway, F.; Anderson, P.J.; Tomlinson, B.; Critchley, J.A.J.H. Non-invasive continuous arterial pressure, heart rate and stroke volume measurements during graded head-up tilt in normal man. Clin. Auton. Res. 1997, 7, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Whittle, R.S.; Keller, N.; Hall, E.A.; Vellore, H.S.; Stapleton, L.M.; Findlay, K.H.; Dunbar, B.J.; Diaz-Artiles, A. Gravitational Dose-Response Curves for Acute Cardiovascular Hemodynamics and Autonomic Responses in a Tilt Paradigm. J. Am. Heart Assoc. 2022, 11, e024175. [Google Scholar] [CrossRef] [PubMed]
- Pewsey, A. Chapter 8.4.1 Basic Cosine Regression Model. In Circular Statistics in R; Oxford University Press: Oxford, UK, 2013; pp. 160–162. [Google Scholar]
- Cremers, J.; Klugkist, I. One Direction? A Tutorial for Circular Data Analysis Using R With Examples in Cognitive Psychology. Front. Psychol. 2018, 9, 2040. [Google Scholar] [CrossRef]
- Fisher, N.I. Chapter 6.2 Linear-Circular Association and Circular-Linear Association. In Statistical Analysis of Circular Data; Cambridge University Press: Cambridge, MA, USA, 2000; pp. 139–145. [Google Scholar]
- Fisher, N.I. Chapter 2.4 Modifications for Axial Data. In Statistical Analysis of Circular Data; Cambridge University Press: Cambridge, MA, USA, 2000; p. 37. [Google Scholar]
- Ong, J.; Lee, A.G.; Moss, H.E. Head-Down Tilt Bed Rest Studies as a Terrestrial Analog for Spaceflight Associated Neuro-Ocular Syndrome. Front. Neurol. 2021, 12, 648958. [Google Scholar] [CrossRef]
- O’Carroll, D.C.; Wiederman, S.D. Contrast sensitivity and the detection of moving patterns and features. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130043. [Google Scholar] [CrossRef] [PubMed]
- Elze, T. Achieving precise display timing in visual neuroscience experiments. J. Neurosci. Methods 2010, 191, 171–179. [Google Scholar] [CrossRef]
- Woods, D.L.; Wyma, J.M.; Yund, E.W.; Herron, T.J.; Reed, B. Factors influencing the latency of simple reaction time. Front. Hum. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2011, 44, 314–324. [Google Scholar] [CrossRef]
- Garaizar, P.; Vadillo, M.A.; López-de Ipiña, D.; Matute, H. Measuring Software Timing Errors in the Presentation of Visual Stimuli in Cognitive Neuroscience Experiments. PLoS ONE 2014, 9, e85108. [Google Scholar] [CrossRef]
- Bridges, D.; Pitiot, A.; MacAskill, M.R.; Peirce, J.W. The timing mega-study: Comparing a range of experiment generators, both lab-based and online. PeerJ 2020, 8, e9414. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.W. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Budiawan, W.; Sakakibara, H.; Tsuzuki, K. Brain Response and Reaction Time in Natural and Comfort Conditions, with Energy-Saving Potential in an Office Environment. Energies 2021, 14, 7598. [Google Scholar] [CrossRef]
- Ando, S.; Kida, N.; Oda, S. Practice Effects on Reaction Time for Peripheral and Central Visual Fields. Percept. Motor Skills 2002, 95, 747–751. [Google Scholar] [CrossRef]
- Kanai, R.; Dalmaijer, E.S.; Sherman, M.T.; Kawakita, G.; Paffen, C.L.E. Larger Stimuli Require Longer Processing Time for Perception. Perception 2017, 46, 605–623. [Google Scholar] [CrossRef]
- Quinn, N.; Csincsik, L.; Flynn, E.; Curcio, C.A.; Kiss, S.; Sadda, S.R.; Hogg, R.; Peto, T.; Lengyel, I. The clinical relevance of visualising the peripheral retina. Prog. Retin. Eye Res. 2019, 68, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Armaly, M.F. The Size and Location of the Normal Blind Spot. Arch. Ophthalmol. 1969, 81, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Sommer, A. Asymmetry and Variation in the Normal Hill of Vision. Arch. Ophthalmol. 1986, 104, 65–68. [Google Scholar] [CrossRef]
- Christoforidis, J. Volume of visual field assessed with kinetic perimetry and its application to static perimetry. Clin. Ophthalmol. 2011, 5, 535. [Google Scholar] [CrossRef][Green Version]
- Wong, A.L.; Goldsmith, J.; Forrence, A.D.; Haith, A.M.; Krakauer, J.W. Reaction times can reflect habits rather than computations. eLife 2017, 6, 28075. [Google Scholar] [CrossRef]
- Pins, D.; Bonnet, C. On the relation between stimulus intensity and processing time: Piéron’s law and choice reaction time. Percept. Psychophys. 1996, 58, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, R.; Van Dongen, H.P.A. Diffusion model for one-choice reaction-time tasks and the cognitive effects of sleep deprivation. Proc. Natl. Acad. Sci. USA 2011, 108, 11285–11290. [Google Scholar] [CrossRef] [PubMed]
- Parker, S. Were the Victorians cleverer than us? Maybe, maybe not. Intelligence 2014, 47, 1–2. [Google Scholar] [CrossRef]
- Miller, J. How Many Participants? How Many Trials? Maximizing the Power of Reaction Time Studies. Behav. Res. Methods 2023, 56, 2398–2421. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, R. Group reaction time distributions and an analysis of distribution statistics. Psychol. Bull. 1979, 86, 446–461. [Google Scholar] [CrossRef]
- Guy, N.; Lancry-Dayan, O.C.; Pertzov, Y. Not all fixations are created equal: The benefits of using ex-Gaussian modeling of fixation durations. J. Vision 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Massidda, D. Retimes: Reaction Time Analysis; 2013. R Package Version 0.1-2. Available online: https://CRAN.R-project.org/package=retimes (accessed on 30 November 2023).
- Rouder, J.N.; Speckman, P.L. An evaluation of the Vincentizing method of forming group-level response time distributions. Psychon. Bull. Rev. 2004, 11, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A. An Introduction to Linear Mixed-Effects Modeling in R. Adv. Methods Pract. Psychol. Sci. 2021, 4, 251524592096035. [Google Scholar] [CrossRef]
- Meteyard, L.; Davies, R.A. Best practice guidance for linear mixed-effects models in psychological science. J. Memory Lang. 2020, 112, 104092. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2012, 4, 133–142. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Ellis, P.D. (Ed.) Part I, Effect sizes and the interpretation of results. In The Essential Guide to Effect Sizes, 6th ed.; Cambridge Univ. Press: Cambridge, UK, 2013. [Google Scholar]
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. Effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Aickin, M.; Gensler, H. Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R. Correction for Multiple Testing. Chest 2011, 140, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means; 2024. R package version 1.10.1. Available online: https://doi.org/10.32614/CRAN.package.emmeans (accessed on 6 April 2024).
- Daróczi, G.; Tsegelskyi, R. Pander: An R ’Pandoc’ Writer; 2022. R package version 0.6.5. Available online: https://doi.org/10.32614/CRAN.package.pander (accessed on 30 November 2023).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.S.; Wiernik, B.M. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption; CRAN: Vienna, Austria, 2023. [Google Scholar]
- Coombes, K.R.; Brock, G.; Abrams, Z.B.; Abruzzo, L.V. Polychrome: Creating and Assessing Qualitative Palettes with Many Colors. J. Stat. Softw. Code Snippets 2019, 90, 1–23. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests; 2023. R package version 0.7.2. Available online: https://doi.org/10.32614/CRAN.package.rstatix (accessed on 30 November 2023).
- Hlavac, M. Stargazer: Well-Formatted Regression and Summary Statistics Tables; Social Policy Institute: Bratislava, Slovakia, 2022; R Package Version 5.2.3. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Heitz, R.P. The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Front. Neurosci. 2014, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, E.J.; Brown, S. On the linear relation between the mean and the standard deviation of a response time distribution. Psychol. Rev. 2007, 114, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Mekari, S.; Murphy, R.J.L.; MacKinnon, A.R.S.; Hollohan, Q.; Macdougall, S.C.; Courish, M.K.; Kimmerly, D.S.; Neyedli, H.F. The impact of a short-period head-down tilt on executive function in younger adults. Sci. Rep. 2022, 12, 20888. [Google Scholar] [CrossRef] [PubMed]
- Dayal, D.; Jesudasen, S.; Scott, R.; Stevens, B.; Hazel, R.; Nasrini, J.; Donoviel, D.; Basner, M. Effects of short-term-12° head-down tilt on cognitive performance. Acta Astronaut. 2020, 175, 582–590. [Google Scholar] [CrossRef]
- Komada, Y.; Mizuno, K.; Mishima, K.; Sato, H.; Inoue, Y.; Tanaka, H.; Shirakawa, S. Effects of Acute Simulated Microgravity on Nocturnal Sleep, Daytime Vigilance, and Psychomotor Performance: Comparison of Horizontal and 6° Head-Down Bed Rest. Percept. Mot. Ski 2006, 103, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Ulrich, R. Mental chronometry and individual differences: Modeling reliabilities and correlations of reaction time means and effect sizes. Psychon. Bull. Rev. 2013, 20, 819–858. [Google Scholar] [CrossRef]
- Spaide, R.F. CHOROIDAL BLOOD FLOW: Review and Potential Explanation for the Choroidal Venous Anatomy Including the Vortex Vein System. Retina 2020, 40, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Roos, L.E.; Knight, E.L.; Beauchamp, K.G.; Berkman, E.T.; Faraday, K.; Hyslop, K.; Fisher, P.A. Acute stress impairs inhibitory control based on individual differences in parasympathetic nervous system activity. Biol. Psychol. 2017, 125, 58–63. [Google Scholar] [CrossRef]
- Goriely, A.; Budday, S.; Kuhl, E. Neuromechanics. In Advances in Applied Mechanics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 79–139. [Google Scholar] [CrossRef]
- Taibbi, G.; Cromwell, R.L.; Zanello, S.B.; Yarbough, P.O.; Ploutz-Snyder, R.J.; Godley, B.F.; Vizzeri, G. Ocular Outcomes Comparison Between 14- and 70-Day Head-Down-Tilt Bed Rest. Investig. Ophthalmol. Vis. Sci. 2016, 57, 495. [Google Scholar] [CrossRef]
- Ong, J.; Tarver, W.; Brunstetter, T.; Mader, T.H.; Gibson, C.R.; Mason, S.S.; Lee, A. Spaceflight associated neuro-ocular syndrome: Proposed pathogenesis, terrestrial analogues, and emerging countermeasures. Br. J. Ophthalmol. 2023, 107, 895–900. [Google Scholar] [CrossRef]
- Shinojima, A.; Iwasaki, K.i.; Aoki, K.; Ogawa, Y.; Yanagida, R.; Yuzawa, M. Subfoveal Choroidal Thickness and Foveal Retinal Thickness During Head-Down Tilt. Aviat. Space Environ. Med. 2012, 83, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, H.; Lovasik, J.V. Seven-degree head-down tilt reduces choroidal pulsatile ocular blood flow. Aviat. Space Environ. Med. 2005, 76, 930–934. [Google Scholar] [PubMed]
- Carvil, P.; Baptista, R.; Russomano, T. The human body in a microgravity environment: Long term adaptations and countermeasures. Aviat. Focus 2013, 4, 10–22. [Google Scholar]
- Li, C.; Fitzgerald, M.E.; Del Mar, N.; Reiner, A. Disinhibition of neurons of the nucleus of solitary tract that project to the superior salivatory nucleus causes choroidal vasodilation: Implications for mechanisms underlying choroidal baroregulation. Neurosci. Lett. 2016, 633, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Heron, W.; Hyman, R.; Rizzolatti, G.; Umiltà, C. Simple Reaction Times of Ipsilateral and Contralateral Hand to Lateralized Visual Stimuli. Brain 1971, 94, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Poggel, D.; Strasburger, H. Visual perception in space and time—Mapping the visual field of temporal resolution. Acta Neurobiol. Exp. 2004, 64, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Nistorescu, A.; Busnatu, S.S.; Dinculescu, A.; Olteanu, G.; Marin, M.; Jercalau, C.E.; Vizitiu, C.; Papacocea, I.R. Striated Muscle Evaluation Based on Velocity and Amortization Ratio of Mechanical Impulse Propagation in Simulated Microgravity Environment. Biology 2022, 11, 1677. [Google Scholar] [CrossRef]
- Iskovitz, I.; Kassemi, M.; Thomas, J. Impact of Microgravity and Partial Gravity on Cardiac Shape. In Proceedings of the 42nd International Conference on Environmental Systems, San Diego, CA, USA, 15–19 July 2012; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2012. [Google Scholar] [CrossRef]
- McCarthy, I.D. Fluid Shifts Due to Microgravity and Their Effects on Bone: A Review of Current Knowledge. Ann. Biomed. Eng. 2005, 33, 95–103. [Google Scholar] [CrossRef]
- Wei, F.; Flowerdew, K.; Kinzel, M.; Perotti, L.E.; Asiatico, J.; Omer, M.; Hovell, C.; Reumers, V.; Coathup, M.J. Changes in interstitial fluid flow, mass transport and the bone cell response in microgravity and normogravity. Bone Res. 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Vernice, N.A.; Meydan, C.; Afshinnekoo, E.; Mason, C.E. Long-term spaceflight and the cardiovascular system. Precis. Clin. Med. 2020, 3, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.R.; Suh, K.I.; Moret, F.; Messer, R.K.; Manuel, F.K. Measurement of Choroidal blood flow in zero gravity. In Ophthalmic Technologies XIII; Manns, F., Söderberg, Eds.; SPIE: San Jose, CA, USA, 2003. [Google Scholar] [CrossRef]
- Boote, C.; Sigal, I.A.; Grytz, R.; Hua, Y.; Nguyen, T.D.; Girard, M.J. Scleral structure and biomechanics. Prog. Retin. Eye Res. 2020, 74, 100773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Li, Z.; Hao, S.; Wang, B. A novel perspective on neuron study: Damaging and promoting effects in different neurons induced by mechanical stress. Biomech. Model. Mechanobiol. 2015, 15, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- LaPlaca, M.C.; Prado, G.R. Neural mechanobiology and neuronal vulnerability to traumatic loading. J. Biomech. 2010, 43, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lu, S.; Madhukar, A. Real-Time Dynamics of Ca2+, Caspase-3/7, and Morphological Changes in Retinal Ganglion Cell Apoptosis under Elevated Pressure. PLoS ONE 2010, 5, e13437. [Google Scholar] [CrossRef] [PubMed]
- Feghali, J.G.; Jin, J.C.; Odom, J.V. Effect of short-term intraocular pressure elevation on the rabbit electroretinogram. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2184–2189. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2160453 (accessed on 30 April 2024).
- Arbeille, P.; Fomina, G.; Roumy, J.; Alferova, I.; Tobal, N.; Herault, S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur. J. Appl. Physiol. 2001, 86, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Deng, S.; Zhao, X.; Xia, G.; Zhao, R.; Chan, H.F. Deciphering and engineering tissue folding: A mechanical perspective. Acta Biomater. 2021, 134, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Tallinen, T.; Biggins, J.S. Mechanics of invagination and folding: Hybridized instabilities when one soft tissue grows on another. Phys. Rev. E 2015, 92, 022720. [Google Scholar] [CrossRef]
- Ferguson, C.R.; Pardon, L.P.; Laurie, S.S.; Young, M.H.; Gibson, C.R.; Brunstetter, T.J.; Tarver, W.J.; Mason, S.S.; Sibony, P.A.; Macias, B.R. Incidence and Progression of Chorioretinal Folds During Long-Duration Spaceflight. JAMA Ophthalmol. 2023, 141, 168. [Google Scholar] [CrossRef]
- Liu, C.S.J.; Bryan, R.N.; Miki, A.; Woo, J.H.; Liu, G.T.; Elliott, M.A. Magnocellular and parvocellular visual pathways have different blood oxygen level-dependent signal time courses in human primary visual cortex. Am. J. Neuroradiol. 2006, 27, 1628–1634. [Google Scholar]
- Corballis, M. Hemispheric interactions in simple reaction time. Neuropsychologia 2002, 40, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M.; Zaidel, E. Crossed–uncrossed difference in simple reaction times to lateralized flashes: Between- and within-subjects variability. Neuropsychologia 2000, 38, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M.; Zaidel, E. Interhemispheric visuo-motor integration in humans: The role of the superior parietal cortex. Neuropsychologia 2004, 42, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Womack, K.B.; Paliotta, C.; Strain, J.F.; Ho, J.S.; Skolnick, Y.; Lytton, W.W.; Turtzo, L.C.; McColl, R.; Diaz-Arrastia, R.; Bergold, P.J. Measurement of Peripheral Vision Reaction Time Identifies White Matter Disruption in Patients with Mild Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1539–1545. [Google Scholar] [CrossRef]
- Wolski, P.; Asanowicz, D. Does CUD measure interhemispheric transfer time? The allocation of attention influences the Poffenberger effect. Neuropsychologia 2023, 185, 108581. [Google Scholar] [CrossRef] [PubMed]
- Seiple, W.; Overbury, O.; Rosenthal, B.; Arango, T.; Odom, J.V.; Morse, A.R. Effects of Lighting on Reading Speed as a Function of Letter Size. Am. J. Occup. Ther. 2018, 72, 7202345020p1–7202345020p7. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, E.; Ong, J.; Zaman, N.; Paladugu, P.; Kamran, S.A.; Tavakkoli, A.; Lee, A.G. The spaceflight contrast sensitivity hypothesis and its role to investigate the pathophysiology of spaceflight-associated neuro-ocular syndrome. Front. Ophthalmol. 2023, 3, 1229748. [Google Scholar] [CrossRef]
- Waisberg, E.; Ong, J.; Masalkhi, M.; Zaman, N.; Kamran, S.A.; Sarker, P.; Tavakkoli, A.; Lee, A.G. The Case for Expanding Visual Assessments During Spaceflight. Prehospital Disaster Med. 2023, 38, 518–521. [Google Scholar] [CrossRef]
- Masood, K.; Ahmed, B.; Choi, J.; Gutierrez-Osuna, R. Consistency and Validity of Self-reporting Scores in Stress Measurement Surveys. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012. [Google Scholar] [CrossRef]
- Dunning, D.; Heath, C.; Suls, J.M. Flawed Self-Assessment: Implications for Health, Education, and the Workplace. Psychol. Sci. Public Interest 2004, 5, 69–106. [Google Scholar] [CrossRef]
- Althouse, A.D. Adjust for Multiple Comparisons? It’s Not That Simple. Ann. Thorac. Surg. 2016, 101, 1644–1645. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftime, A.; Tofolean, I.T.; Pintilie, V.; Călinescu, O.; Busnatu, S.; Papacocea, I.R. Differential Functional Changes in Visual Performance during Acute Exposure to Microgravity Analogue and Their Potential Links with Spaceflight-Associated Neuro-Ocular Syndrome. Diagnostics 2024, 14, 1918. https://doi.org/10.3390/diagnostics14171918
Iftime A, Tofolean IT, Pintilie V, Călinescu O, Busnatu S, Papacocea IR. Differential Functional Changes in Visual Performance during Acute Exposure to Microgravity Analogue and Their Potential Links with Spaceflight-Associated Neuro-Ocular Syndrome. Diagnostics. 2024; 14(17):1918. https://doi.org/10.3390/diagnostics14171918
Chicago/Turabian StyleIftime, Adrian, Ioana Teodora Tofolean, Victor Pintilie, Octavian Călinescu, Stefan Busnatu, and Ioana Raluca Papacocea. 2024. "Differential Functional Changes in Visual Performance during Acute Exposure to Microgravity Analogue and Their Potential Links with Spaceflight-Associated Neuro-Ocular Syndrome" Diagnostics 14, no. 17: 1918. https://doi.org/10.3390/diagnostics14171918
APA StyleIftime, A., Tofolean, I. T., Pintilie, V., Călinescu, O., Busnatu, S., & Papacocea, I. R. (2024). Differential Functional Changes in Visual Performance during Acute Exposure to Microgravity Analogue and Their Potential Links with Spaceflight-Associated Neuro-Ocular Syndrome. Diagnostics, 14(17), 1918. https://doi.org/10.3390/diagnostics14171918








