Photon-Counting Computed Tomography Angiography of Carotid Arteries: A Topical Narrative Review with Case Examples
Abstract
1. Introduction
2. Comparison between Energy-Integrating and Photon-Counting Detectors
3. Strengths of PCDs
3.1. Enhanced Spatial Resolution
3.2. Improved Noise Characteristics
3.3. Improved Contrast
3.4. Enhanced Capabilities of Spectral Imaging and Material Characterization
3.5. Reduced Artifacts
4. Limitations of PCCT
4.1. Technical Challenges
4.2. Clinical Considerations
4.2.1. Alternative Contrast Agents
4.2.2. Clinical Translation and Validation
4.2.3. Data Handling and Analysis
4.3. Economic Considerations
5. Scanning and Reconstruction Protocols
6. PCCT in Carotid Arteries Assessment
6.1. Carotid Lumen Evaluation
6.2. Carotid Stents
6.3. Carotid Plaque Evaluation
6.4. Proliferation of Vasa Vasorum
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Cau, R.; Spinato, G.; Suri, J.S.; Melis, M.; De Rubeis, G.; Antignani, P.; Gupta, A. Carotid stenosis and cryptogenic stroke. J. Vasc. Surg. 2024, 79, 1119–1131. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Bos, D.; van Dam-Nolen, D.H.K.; Gupta, A.; Saba, L.; Saloner, D.; Wasserman, B.A.; van der Lugt, A. Advances in Multimodality Carotid Plaque Imaging: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2021, 217, 16–26. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar] [CrossRef]
- European Carotid Surgery Trialists‘ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Saba, L.; Cau, R.; Murgia, A.; Nicolaides, A.N.; Wintermark, M.; Castillo, M.; Staub, D.; Kakkos, S.K.; Yang, Q.; Paraskevas, K.I.; et al. Carotid Plaque-RADS: A Novel Stroke Risk Classification System. JACC Cardiovasc. Imaging 2024, 17, 62–75. [Google Scholar] [CrossRef]
- Cademartiri, F.; Balestrieri, A.; Cau, R.; Punzo, B.; Cavaliere, C.; Maffei, E.; Saba, L. Insight from imaging on plaque vulnerability: Similarities and differences between coronary and carotid arteries-implications for systemic therapies. Cardiovasc. Diagn. Ther. 2020, 10, 1150–1162. [Google Scholar] [CrossRef]
- Saba, L.; Yuan, C.; Hatsukami, T.S.; Balu, N.; Qiao, Y.; DeMarco, J.K.; Saam, T.; Moody, A.R.; Li, D.; Matouk, C.C.; et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am. J. Neuroradiol. 2018, 39, E9–E31. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Saam, T.; Jager, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, H.; Sartori, S.; Sandholt, B.; Baber, U.; Mehran, R.; Fuster, V. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Griffin, M.B.; Nicolaides, A.N.; Kyriacou, E.; Sabetai, M.M.; Tegos, T.; Makris, G.C.; Thomas, D.J.; Geroulakos, G.; Asymptomatic Carotid, S.; et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J. Vasc. Surg. 2013, 57, 609–618 e601, discussion 617–608. [Google Scholar] [CrossRef]
- Saba, L.; Chen, H.; Cau, R.; Rubeis, G.D.; Zhu, G.; Pisu, F.; Jang, B.; Lanzino, G.; Suri, J.S.; Qi, Y.; et al. Impact Analysis of Different CT Configurations of Carotid Artery Plaque Calcifications on Cerebrovascular Events. AJNR Am. J. Neuroradiol. 2022, 43, 272–279. [Google Scholar] [CrossRef]
- Saba, L.; Moody, A.R.; Saam, T.; Kooi, M.E.; Wasserman, B.A.; Staub, D.; van der Lugt, A.; DeMarco, J.K.; Saloner, D.; Wintermark, M.; et al. Vessel Wall-Imaging Biomarkers of Carotid Plaque Vulnerability in Stroke Prevention Trials: A viewpoint from The Carotid Imaging Consensus Group. JACC Cardiovasc. Imaging 2020, 13, 2445–2456. [Google Scholar] [CrossRef]
- Saba, L.; Loewe, C.; Weikert, T.; Williams, M.C.; Galea, N.; Budde, R.P.J.; Vliegenthart, R.; Velthuis, B.K.; Francone, M.; Bremerich, J.; et al. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: Standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2023, 33, 1063–1087. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Loewe, C.; Weikert, T.; Williams, M.C.; Galea, N.; Budde, R.P.J.; Vliegenthart, R.; Velthuis, B.K.; Francone, M.; Bremerich, J.; et al. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: The reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2023, 33, 1088–1101. [Google Scholar] [CrossRef]
- Wildberger, J.E.; Alkadhi, H. New Horizons in Vascular Imaging with Photon-Counting Detector CT. Investig. Radiol. 2023, 58, 499–504. [Google Scholar] [CrossRef]
- Cademartiri, F.; Meloni, A.; Pistoia, L.; Degiorgi, G.; Clemente, A.; De Gori, C.; Positano, V.; Celi, S.; Berti, S.; Emdin, M.; et al. Dual Source Photon-Counting Computed Tomography-Part II: Clinical Overview of Neurovascular Applications. J. Clin. Med. 2023, 12, 3626. [Google Scholar] [CrossRef]
- Cau, R.; Gupta, A.; Kooi, M.E.; Saba, L. Pearls and Pitfalls of Carotid Artery Imaging: Ultrasound, Computed Tomography Angiography, and MR Imaging. Radiol. Clin. N. Am. 2023, 61, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Cademartiri, F.; Positano, V.; Celi, S.; Berti, S.; Clemente, A.; La Grutta, L.; Saba, L.; Bossone, E.; Cavaliere, C.; et al. Cardiovascular Applications of Photon-Counting CT Technology: A Revolutionary New Diagnostic Step. J. Cardiovasc. Dev. Dis. 2023, 10, 363. [Google Scholar] [CrossRef]
- Kreisler, B. Photon counting Detectors: Concept, technical Challenges, and clinical outlook. Eur. J. Radiol. 2022, 149, 110229. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Maffei, E.; Clemente, A.; De Gori, C.; Occhipinti, M.; Positano, V.; Berti, S.; La Grutta, L.; Saba, L.; Cau, R.; et al. Spectral Photon-Counting Computed Tomography: Technical Principles and Applications in the Assessment of Cardiovascular Diseases. J. Clin. Med. 2024, 13, 2359. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology. Radiographics 2019, 39, 729–743. [Google Scholar] [CrossRef]
- Danielsson, M.; Persson, M.; Sjölin, M. Photon-counting X-ray detectors for CT. Phys. Med. Biol. 2021, 66, 03TR01. [Google Scholar] [CrossRef]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- Zheng, Y.; Yveborg, M.; Grönberg, F.; Xu, C.; Su, Q.; Danielsson, M.; Persson, M. Robustness of optimal energy thresholds in photon-counting spectral CT. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 953, 163132. [Google Scholar] [CrossRef]
- Wang, J.; Fleischmann, D. Improving Spatial Resolution at CT: Development, Benefits, and Pitfalls. Radiology 2018, 289, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, M.; Hata, A.; Honda, O.; Kikuchi, N.; Miyata, T.; Uranishi, A.; Tsukagoshi, S.; Tomiyama, N. Subjective and objective comparisons of image quality between ultra-high-resolution CT and conventional area detector CT in phantoms and cadaveric human lungs. Eur. Radiol. 2018, 28, 5060–5068. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Sigovan, M.; Hsu, J.C.; Tatard-Leitman, V.; Chalabreysse, L.; Naha, P.C.; Garrivier, T.; Dessouky, R.; Carnaru, M.; Boussel, L.; et al. In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT. Radiology 2021, 300, 98–107. [Google Scholar] [CrossRef]
- Leng, S.; Rajendran, K.; Gong, H.; Zhou, W.; Halaweish, A.F.; Henning, A.; Kappler, S.; Baer, M.; Fletcher, J.G.; McCollough, C.H. 150-μm Spatial Resolution Using Photon-Counting Detector Computed Tomography Technology: Technical Performance and First Patient Images. Investig. Radiol. 2018, 53, 655–662. [Google Scholar] [CrossRef]
- Ferda, J.; Vendiš, T.; Flohr, T.; Schmidt, B.; Henning, A.; Ulzheimer, S.; Pecen, L.; Ferdová, E.; Baxa, J.; Mírka, H. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur. J. Radiol. 2021, 137, 109614. [Google Scholar] [CrossRef]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Leng, S.; Kappler, S.; Hahn, K.; Li, Z.; Halaweish, A.F.; Henning, A.; McCollough, C.H. Noise performance of low-dose CT: Comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner. J. Med. Imaging 2016, 3, 043503. [Google Scholar] [CrossRef]
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; Bluemke, D.A.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2017, 62, 202–213. [Google Scholar] [CrossRef]
- Sandfort, V.; Persson, M.; Pourmorteza, A.; Noël, P.B.; Fleischmann, D.; Willemink, M.J. Spectral photon-counting CT in cardiovascular imaging. J. Cardiovasc. Comput. Tomogr. 2021, 15, 218–225. [Google Scholar] [CrossRef]
- Swank, R.K. Absorption and noise in X-ray phosphors. J. Appl. Phys. 1973, 44, 4199–4203. [Google Scholar] [CrossRef]
- Iwanczyk, J.S.; Nygård, E.; Meirav, O.; Arenson, J.; Barber, W.C.; Hartsough, N.E.; Malakhov, N.; Wessel, J.C. Photon Counting Energy Dispersive Detector Arrays for X-ray Imaging. IEEE Trans. Nucl. Sci. 2009, 56, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Silkwood, J.D.; Matthews, K.L.; Shikhaliev, P.M. Photon counting spectral breast CT: Effect of adaptive filtration on CT numbers, noise, and contrast to noise ratio. Med. Phys. 2013, 40, 051905. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Energy-resolved computed tomography: First experimental results. Phys. Med. Biol. 2008, 53, 5595–5613. [Google Scholar] [CrossRef]
- Shikhaliev, P.M.; Fritz, S.G. Photon counting spectral CT versus conventional CT: Comparative evaluation for breast imaging application. Phys. Med. Biol. 2011, 56, 1905–1930. [Google Scholar] [CrossRef]
- Giersch, J.; Niederlöhner, D.; Anton, G. The influence of energy weighting on X-ray imaging quality. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2004, 531, 68–74. [Google Scholar] [CrossRef]
- Schmidt, T.G. Optimal “image-based” weighting for energy-resolved CT. Med. Phys. 2009, 36, 3018–3027. [Google Scholar] [CrossRef]
- Adam, S.Z.; Rabinowich, A.; Kessner, R.; Blachar, A. Spectral CT of the abdomen: Where are we now? Insights Imaging 2021, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Abbara, S.; Halliburton, S.S. Spectral detector CT for cardiovascular applications. Diagn. Interv. Radiol. 2017, 23, 187–193. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 2009, 36, 1602–1609. [Google Scholar] [CrossRef]
- Yveborg, M.; Danielsson, M.; Bornefalk, H. Theoretical comparison of a dual energy system and photon counting silicon detector used for material quantification in spectral CT. IEEE Trans. Med. Imaging 2015, 34, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Michoux, N.; Coche, E.; Dragean, C.A. Virtual unenhanced phase with spectral dual-energy CT: Is it an alternative to conventional true unenhanced phase for abdominal tissues? Diagn. Interv. Imaging 2019, 100, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Radiol. 2017, 42, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.M.; Kang, C.K.; Yoon, J.; Chae, K.J.; Goo, J.M. Effect of CT Acquisition Parameters on Iodine Density Measurement at Dual-Layer Spectral CT. AJR Am. J. Roentgenol. 2018, 211, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Racine, D.; Jungblut, L.; Sartoretti, T.; Bickel, S.; Monnin, P.; Higashigaito, K.; Martini, K.; Alkadhi, H.; Euler, A. Virtual Noncontrast Abdominal Imaging with Photon-counting Detector CT. Radiology 2022, 305, 107–115. [Google Scholar] [CrossRef]
- Leng, S.; Zhou, W.; Yu, Z.; Halaweish, A.; Krauss, B.; Schmidt, B.; Yu, L.; Kappler, S.; McCollough, C. Spectral performance of a whole-body research photon counting detector CT: Quantitative accuracy in derived image sets. Phys. Med. Biol. 2017, 62, 7216–7232. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Lennartz, S.; Neuhaus, V.F.; Große Hokamp, N.; Rau, R.; Le Blanc, M.; Abdullayev, N.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. CT metal artifacts in patients with total hip replacements: For artifact reduction monoenergetic reconstructions and post-processing algorithms are both efficient but not similar. Eur. Radiol. 2018, 28, 4524–4533. [Google Scholar] [CrossRef]
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First In Vivo Human Results. Investig. Radiol. 2018, 53, 135–142. [Google Scholar] [CrossRef]
- Rassouli, N.; Chalian, H.; Rajiah, P.; Dhanantwari, A.; Landeras, L. Assessment of 70-keV virtual monoenergetic spectral images in abdominal CT imaging: A comparison study to conventional polychromatic 120-kVp images. Abdom. Radiol. 2017, 42, 2579–2586. [Google Scholar] [CrossRef]
- Kappler, S.; Henning, A.; Kreisler, B.; Schoeck, F.; Stierstorfer, K.; Flohr, T. Photon Counting CT at Elevated X-ray Tube Currents: Contrast Stability, Image Noise and Multi-Energy Performance; SPIE: Bellingham, WA, USA, 2014; Volume 9033. [Google Scholar]
- Faby, S.; Kuchenbecker, S.; Sawall, S.; Simons, D.; Schlemmer, H.P.; Lell, M.; Kachelrieß, M. Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: A simulation study. Med. Phys. 2015, 42, 4349–4366. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Kondo, S.; Kawashita, I.; Takahashi, I.; Awai, K. An introduction to photon-counting detector CT (PCD CT) for radiologists. Jpn. J. Radiol. 2022, 41, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Schirra, C.O.; Brendel, B.; Anastasio, M.A.; Roessl, E. Spectral CT: A technology primer for contrast agent development. Contrast Media Mol. Imaging 2014, 9, 62–70. [Google Scholar] [CrossRef]
- Pan, D.; Schirra, C.O.; Senpan, A.; Schmieder, A.H.; Stacy, A.J.; Roessl, E.; Thran, A.; Wickline, S.A.; Proska, R.; Lanza, G.M. An early investigation of ytterbium nanocolloids for selective and quantitative “multicolor” spectral CT imaging. ACS Nano 2012, 6, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M.; Schlattl, H.; Hoeschen, C.; Dietrich, O. Feasibility of spectral CT imaging for the detection of liver lesions with gold-based contrast agents—A simulation study. Phys. Med. 2015, 31, 875–881. [Google Scholar] [CrossRef]
- Kim, J.; Bar-Ness, D.; Si-Mohamed, S.; Coulon, P.; Blevis, I.; Douek, P.; Cormode, D.P. Assessment of candidate elements for development of spectral photon-counting CT specific contrast agents. Sci. Rep. 2018, 8, 12119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Wang, Y.-X.; Lin, Y.; Zhang, J.-S.; Yang, F.; Zhou, Q.-L.; Liao, Y.-Y. Advance of Molecular Imaging Technology and Targeted Imaging Agent in Imaging and Therapy. BioMed Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef]
- Balegamire, J.; Vandamme, M.; Chereul, E.; Si-Mohamed, S.; Azzouz Maache, S.; Almouazen, E.; Ettouati, L.; Fessi, H.; Boussel, L.; Douek, P.; et al. Iodinated polymer nanoparticles as contrast agent for spectral photon counting computed tomography. Biomater. Sci. 2020, 8, 5715–5728. [Google Scholar] [CrossRef]
- Dong, Y.C.; Kumar, A.; Rosario-Berríos, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noël, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274–39284. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.; Cormode, D.P.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Langlois, J.-B.; Chalabreysse, L.; Coulon, P.; Blevis, I.; Roessl, E.; et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale 2017, 9, 18246–18257. [Google Scholar] [CrossRef]
- Cormode, D.P.; Roessl, E.; Thran, A.; Skajaa, T.; Gordon, R.E.; Schlomka, J.P.; Fuster, V.; Fisher, E.A.; Mulder, W.J.; Proksa, R.; et al. Atherosclerotic plaque composition: Analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010, 256, 774–782. [Google Scholar] [CrossRef]
- Brooks, R.A.; Chiro, G.D. Beam hardening in X-ray reconstructive tomography. Phys. Med. Biol. 1976, 21, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Shikhaliev, P.M. Beam hardening artefacts in computed tomography with photon counting, charge integrating and energy weighting detectors: A simulation study. Phys. Med. Biol. 2005, 50, 5813–5827. [Google Scholar] [CrossRef]
- Lee, C.-L.; Park, J.; Nam, S.; Choi, J.; Choi, Y.; Lee, S.; Lee, K.-Y.; Cho, M. Metal artifact reduction and tumor detection using photon-counting multi-energy computed tomography. PLoS ONE 2021, 16, e0247355. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, R.; Halaweish, A.F.; Yu, Z.; Leng, S.; Yu, L.; Li, Z.; Jorgensen, S.M.; Ritman, E.L.; Kappler, S.; McCollough, C.H. Human Imaging with Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Investig. Radiol. 2016, 51, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Pack, J.D.; Xu, M.; Wang, G.; Baskaran, L.; Min, J.; De Man, B. Cardiac CT blooming artifacts: Clinical significance, root causes and potential solutions. Vis. Comput. Ind. Biomed. Art. 2022, 5, 29. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef]
- Flohr, T.; Schmidt, B. Technical Basics and Clinical Benefits of Photon-Counting CT. Investig. Radiol. 2023, 58, 441–450. [Google Scholar] [CrossRef]
- Wang, A.S.; Pelc, N.J. Spectral Photon Counting CT: Imaging Algorithms and Performance Assessment. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 453–464. [Google Scholar] [CrossRef]
- Cammin, J.; Xu, J.; Barber, W.C.; Iwanczyk, J.S.; Hartsough, N.E.; Taguchi, K. A cascaded model of spectral distortions due to spectral response effects and pulse pileup effects in a photon-counting X-ray detector for CT. Med. Phys. 2014, 41, 041905. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Harrison, D.; Lobastov, V.; Tkaczyk, J.E. Pulse pileup statistics for energy discriminating photon counting x-ray detectors. Med. Phys. 2011, 38, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Stierstorfer, K.; Polster, C.; Lee, O.; Kappler, S. Spatio-energetic cross-talk in photon counting detectors: Numerical detector model (PcTK) and workflow for CT image quality assessment. Med. Phys. 2018, 45, 1985–1998. [Google Scholar] [CrossRef]
- Pourmorteza, A. Photon-counting CT: Scouting for Quantitative Imaging Biomarkers. Radiology 2021, 298, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Leng, S.; Halaweish, A.F.; Yu, Z.; Yu, L.; Ritman, E.L.; McCollough, C.H. Overcoming calcium blooming and improving the quantification accuracy of percent area luminal stenosis by material decomposition of multi-energy computed tomography datasets. J. Med. Imaging 2020, 7, 053501. [Google Scholar] [CrossRef]
- Sartoretti, T.; Eberhard, M.; Rüschoff, J.H.; Pietsch, H.; Jost, G.; Nowak, T.; Schmidt, B.; Flohr, T.; Euler, A.; Alkadhi, H. Photon-counting CT with tungsten as contrast medium: Experimental evidence of vessel lumen and plaque visualization. Atherosclerosis 2020, 310, 11–16. [Google Scholar] [CrossRef]
- Michael, A.E.; Boriesosdick, J.; Schoenbeck, D.; Lopez-Schmidt, I.; Kroeger, J.R.; Moenninghoff, C.; Horstmeier, S.; Pennig, L.; Borggrefe, J.; Niehoff, J.H. Photon Counting CT Angiography of the Head and Neck: Image Quality Assessment of Polyenergetic and Virtual Monoenergetic Reconstructions. Diagnostics 2022, 12, 1306. [Google Scholar] [CrossRef]
- Clark, D.J.; Lessio, S.; O’Donoghue, M.; Schainfeld, R.; Rosenfield, K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter. Cardiovasc. Interv. 2004, 63, 355–362. [Google Scholar] [CrossRef]
- Yadav, J.S.; Wholey, M.H.; Kuntz, R.E.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Whitlow, P.; Strickman, N.E.; Jaff, M.R.; et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2004, 351, 1493–1501. [Google Scholar] [CrossRef]
- Lettau, M.; Kotter, E.; Bendszus, M.; Hahnel, S. Carotid artery stents on CT angiography: In vitro comparison of different stent designs and sizes using 16-, 64- and 320-row CT scanners. J. Neuroradiol. 2014, 41, 259–268. [Google Scholar] [CrossRef]
- Halliburton, S.S.; Tanabe, Y.; Partovi, S.; Rajiah, P. The role of advanced reconstruction algorithms in cardiac CT. Cardiovasc. Diagn. Ther. 2017, 7, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Verelst, E.; Buls, N.; De Mey, J.; Nieboer, K.H.; Vandenbergh, F.; Crotty, D.; Deak, P.; Sundvall, A.; Holmin, S.; De Smet, A.; et al. Stent appearance in a novel silicon-based photon-counting CT prototype: Ex vivo phantom study in head-to-head comparison with conventional energy-integrating CT. Eur. Radiol. Exp. 2023, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, H.; Crotty, D.; Nyren, S.; Yu, J.; Arnberg-Sandor, F.; Brismar, T.; Tovatt, C.; Linder, H.; Dagotto, J.; Fredenberg, E.; et al. Initial Clinical Images from a Second-Generation Prototype Silicon-Based Photon-Counting Computed Tomography System. Acad. Radiol. 2024, 31, 572–581. [Google Scholar] [CrossRef]
- Wodarg, F.; Turner, E.L.; Dobson, J.; Ringleb, P.A.; Mali, W.P.; Fraedrich, G.; Chatellier, G.; Bequemin, J.P.; Brown, M.M.; Algra, A.; et al. Influence of stent design and use of protection devices on outcome of carotid artery stenting: A pooled analysis of individual patient data. J. Neurointerv. Surg. 2018, 10, 1149–1154. [Google Scholar] [CrossRef]
- Govsa, F.; Yagdi, T.; Ozer, M.A.; Eraslan, C.; Alagoz, A.K. Building 3D anatomical model of coiling of the internal carotid artery derived from CT angiographic data. Eur. Arch. Otorhinolaryngol. 2017, 274, 1097–1102. [Google Scholar] [CrossRef]
- Spiliopoulos, S.; Blanc, R.; Gandini, R.; Müller-Hülsbeck, S.; Reith, W.; Moschovaki-Zeiger, O. CIRSE Standards of Practice on Carotid Artery Stenting. CardioVascular Interv. Radiol. 2024, 47, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Roubin, G.S.; Iyer, S.; Halkin, A.; Vitek, J.; Brennan, C. Realizing the potential of carotid artery stenting: Proposed paradigms for patient selection and procedural technique. Circulation 2006, 113, 2021–2030. [Google Scholar] [CrossRef]
- Choi, H.M.; Hobson, R.W.; Goldstein, J.; Chakhtoura, E.; Lal, B.K.; Haser, P.B.; Cuadra, S.A.; Padberg, F.T.; Jamil, Z. Technical challenges in a program of carotid artery stenting. J. Vasc. Surg. 2004, 40, 746–751, discussion 751. [Google Scholar] [CrossRef]
- Dahal, S.; Raja, A.Y.; Searle, E.; Colgan, F.E.; Crighton, J.S.; Roake, J.; Saba, L.; Gieseg, S.; Butler, A.P.H. Components of carotid atherosclerotic plaque in spectral photon-counting CT with histopathologic comparison. Eur. Radiol. 2023, 33, 1612–1619. [Google Scholar] [CrossRef]
- Shami, A.; Sun, J.; Gialeli, C.; Markstad, H.; Edsfeldt, A.; Aurumskjold, M.L.; Goncalves, I. Atherosclerotic plaque features relevant to rupture-risk detected by clinical photon-counting CT ex vivo: A proof-of-concept study. Eur. Radiol. Exp. 2024, 8, 14. [Google Scholar] [CrossRef]
- Healy, J.; Searle, E.; Panta, R.K.; Chernoglazov, A.; Roake, J.; Butler, P.; Butler, A.; Gieseg, S.P.; Adebileje, S.A.; Alexander, S.D.; et al. Ex-vivo atherosclerotic plaque characterization using spectral photon-counting CT: Comparing material quantification to histology. Atherosclerosis 2023, 378, 117160. [Google Scholar] [CrossRef] [PubMed]
- Keser, Z.; Diehn, F.E.; Lanzino, G. Photon-Counting Detector CT Angiography in Cervical Artery Dissection. Stroke 2024, 55, e48–e49. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Underhill, H.R.; Hippe, D.S.; Xue, Y.; Yuan, C.; Hatsukami, T.S. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: A long-term time course study. JACC Cardiovasc. Imaging 2012, 5, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Pletsch-Borba, L.; Selwaness, M.; van der Lugt, A.; Hofman, A.; Franco, O.H.; Vernooij, M.W. Change in Carotid Plaque Components: A 4-Year Follow-Up Study with Serial MR Imaging. JACC Cardiovasc. Imaging 2018, 11, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Fayad, Z.A.; Fuster, V.; Worthley, S.G.; Helft, G.; Chesebro, J.; Mercuri, M.; Badimon, J.J. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: A longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation 2001, 104, 249–252. [Google Scholar] [CrossRef]
- Saba, L.; Brinjikji, W.; Spence, J.D.; Wintermark, M.; Castillo, M.; de Borst, G.J.; Yang, Q.; Yuan, C.; Buckler, A.; Edjlali, M.; et al. Roadmap Consensus on Carotid Artery Plaque Imaging and Impact on Therapy Strategies and Guidelines: An International, Multispecialty, Expert Review and Position Statement. Am. J. Neuroradiol. 2021, 42, 1566–1575. [Google Scholar] [CrossRef]
- Fleiner, M.; Kummer, M.; Mirlacher, M.; Sauter, G.; Cathomas, G.; Krapf, R.; Biedermann, B.C. Arterial neovascularization and inflammation in vulnerable patients: Early and late signs of symptomatic atherosclerosis. Circulation 2004, 110, 2843–2850. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Ritman, E.L.; Lerman, A. The dynamic vasa vasorum. Cardiovasc. Res. 2007, 75, 649–658. [Google Scholar] [CrossRef]
- Marsh, J., Jr.; Rajendran, K.; Tao, S.; Vercnocke, A.; Anderson, J.; Leng, S.; Ritman, E.; McCollough, C. A Blooming correction technique for improved vasa vasorum detection using an ultra-high-resolution photon-counting detector CT. Proc. SPIE Int. Soc. Opt. Eng. 2020, 11312, 1169–1176. [Google Scholar] [CrossRef]
- Marsh, J.F., Jr.; Vercnocke, A.J.; Rajendran, K.; Tao, S.; Anderson, J.L.; Ritman, E.L.; Leng, S.; McCollough, C.H. Measurement of enhanced vasa vasorum density in a porcine carotid model using photon counting detector CT. J. Med. Imaging 2023, 10, 016001. [Google Scholar] [CrossRef] [PubMed]

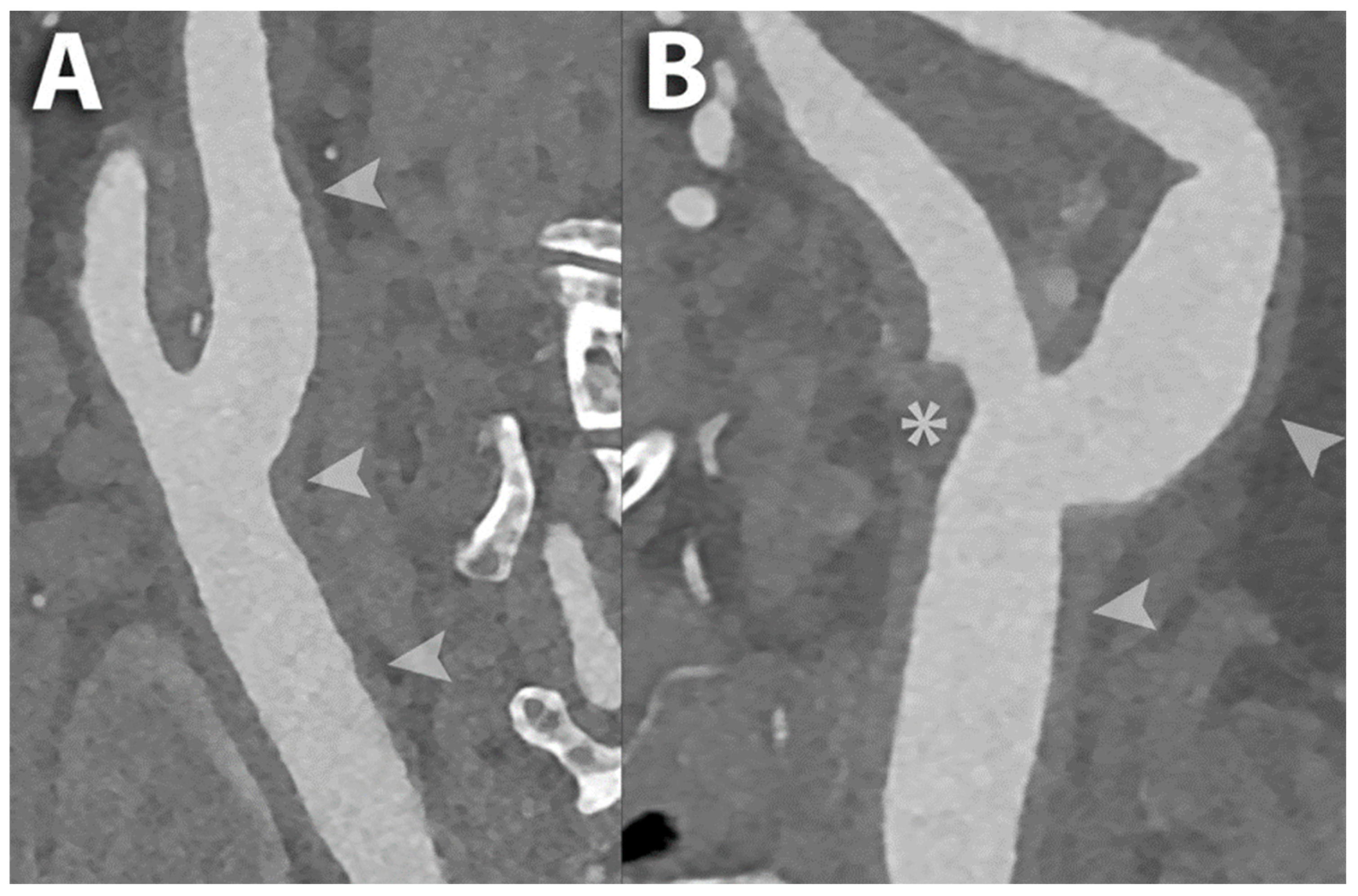

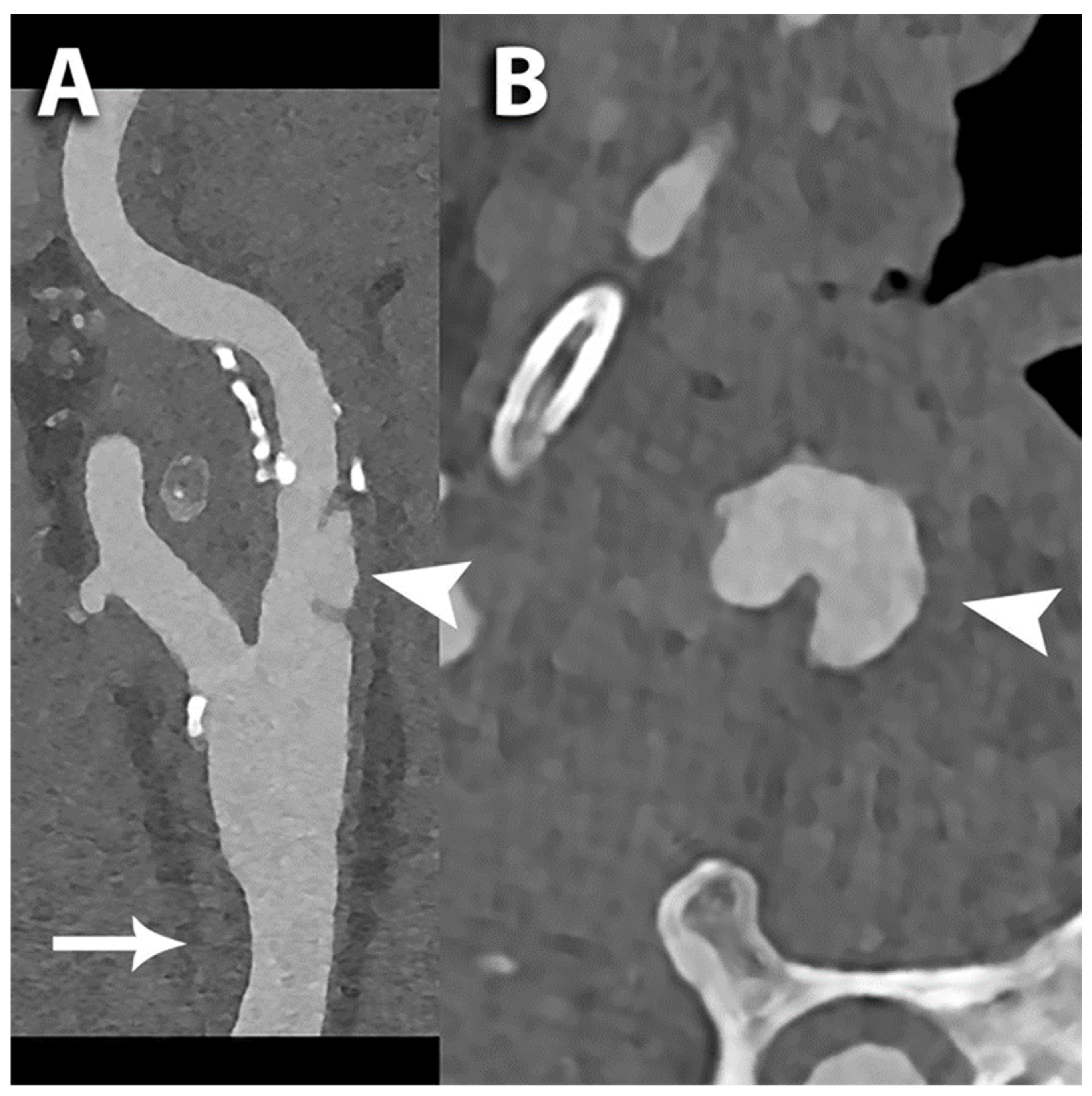


| Benefits of Photon-Counting Detectors | Impact in Carotid Arteries Assessment |
|---|---|
| Enhanced spatial resolution | Improved assessment of the carotid vessel lumen Improved stent imaging Improved atherosclerotic plaque characterization |
| Improved contrast and noise | Improved assessment of the carotid vessel lumen Improved stent imaging Dose reduction |
| Enhanced spectral capabilities | Improved assessment of the carotid vessel lumen Improved atherosclerotic plaque characterization Dose reduction |
| Reduced artifacts | Improved assessment of the carotid vessel lumen Improved stent imaging Improved atherosclerotic plaque characterization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Cau, R.; Saba, L.; Positano, V.; De Gori, C.; Occhipinti, M.; Celi, S.; Bossone, E.; Bertacchi, J.; Punzo, B.; et al. Photon-Counting Computed Tomography Angiography of Carotid Arteries: A Topical Narrative Review with Case Examples. Diagnostics 2024, 14, 2012. https://doi.org/10.3390/diagnostics14182012
Meloni A, Cau R, Saba L, Positano V, De Gori C, Occhipinti M, Celi S, Bossone E, Bertacchi J, Punzo B, et al. Photon-Counting Computed Tomography Angiography of Carotid Arteries: A Topical Narrative Review with Case Examples. Diagnostics. 2024; 14(18):2012. https://doi.org/10.3390/diagnostics14182012
Chicago/Turabian StyleMeloni, Antonella, Riccardo Cau, Luca Saba, Vincenzo Positano, Carmelo De Gori, Mariaelena Occhipinti, Simona Celi, Eduardo Bossone, Jacopo Bertacchi, Bruna Punzo, and et al. 2024. "Photon-Counting Computed Tomography Angiography of Carotid Arteries: A Topical Narrative Review with Case Examples" Diagnostics 14, no. 18: 2012. https://doi.org/10.3390/diagnostics14182012
APA StyleMeloni, A., Cau, R., Saba, L., Positano, V., De Gori, C., Occhipinti, M., Celi, S., Bossone, E., Bertacchi, J., Punzo, B., Mantini, C., Cavaliere, C., Maffei, E., & Cademartiri, F. (2024). Photon-Counting Computed Tomography Angiography of Carotid Arteries: A Topical Narrative Review with Case Examples. Diagnostics, 14(18), 2012. https://doi.org/10.3390/diagnostics14182012













