Characterizing Vascular Wall and Lumen Caliber in Eyes with Diabetic Retinopathy Based on Adaptive Optics Scanning Laser Ophthalmoscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Study Procedures
2.3. AOSLO Imaging System
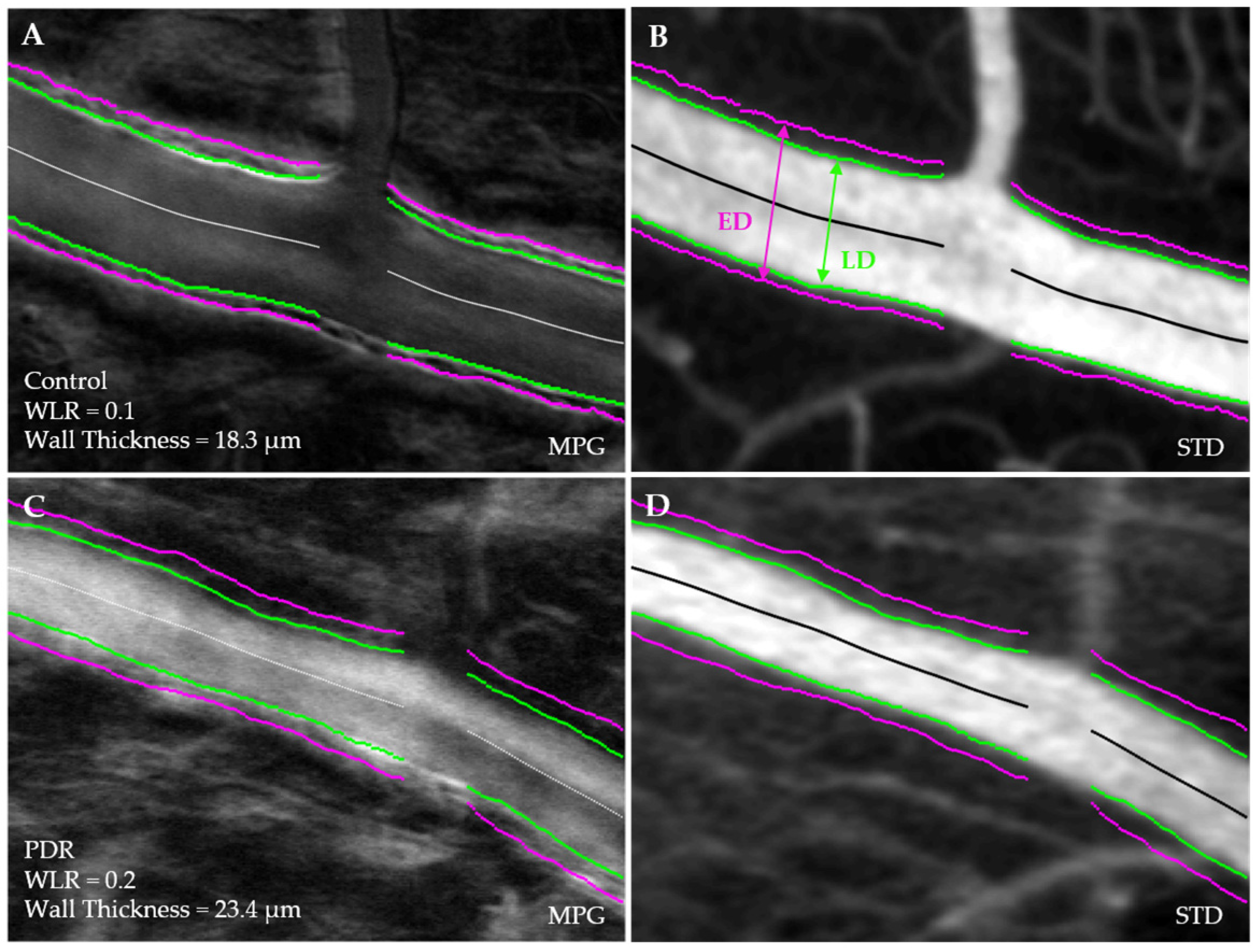
2.4. Vessel Segmentation and Morphological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O'Neal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Aschner, P.; Karuranga, S.; James, S.; Simmons, D.; Basit, A.; Shaw, J.E.; Wild, S.H.; Ogurtsova, K.; Saeedi, P. The International Diabetes Federation’s guide for diabetes epidemiological studies. Diabetes Res. Clin. Pract. 2020, 172, 108630. [Google Scholar] [CrossRef] [PubMed]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.-T.K.S.; de Clerck, E.; Polivka, J.; Potuznik, P.; Stetkarova, I.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Cogan, D.G.; Toussaint, D.; Kuwabara, T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch. Ophthalmol. 1961, 66, 366–378. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F. Pericytes in diabetic retinopathy. Br. J. Ophthalmol. 1966, 50, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N. Vascular basement membrane changes in diabetic retinopathy. Montgomery lecture, 1973. Br. J. Ophthalmol. 1974, 58, 344–366. [Google Scholar] [CrossRef]
- Lorenzi, M.; Feke, G.T.; Pitler, L.; Berisha, F.; Kolodjaschna, J.; McMeel, J.W. Defective myogenic response to posture change in retinal vessels of well-controlled type 1 diabetic patients with no retinopathy. Investig. Opthalmol. Vis. Sci. 2010, 51, 6770–6775. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, L. The cells involved in the pathological process of diabetic retinopathy. Biomed. Pharmacother. 2020, 132, 110818. [Google Scholar] [CrossRef]
- Kollias, A.N.; Ulbig, M.W. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch. Arztebl. Int. 2010, 107, 75–83. [Google Scholar]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Dąbrowska, E.; Harazny, J.M.; Miszkowska-Nagórna, E.; Stefański, A.; Graff, B.; Kunicka, K.; Świerblewska, E.; Rojek, A.; Szyndler, A.; Wolf, J.; et al. Lumen narrowing and increased wall to lumen ratio of retinal microcirculation are valuable biomarkers of hypertension-mediated cardiac damage. Blood Press. 2019, 29, 70–79. [Google Scholar] [CrossRef] [PubMed]
- De Ciuceis, C.; Rosei, C.A.; Malerba, P.; Rossini, C.; Nardin, M.; Chiarini, G.; Famà, F.; Lemoli, M.; Baresi, M.; Petelca, A.; et al. Prognostic significance of the wall to lumen ratio of retinal arterioles evaluated by adaptive optics in humans. Eur. J. Intern. Med. 2023, 122, 86–92. [Google Scholar] [CrossRef]
- Meixner, E.; Michelson, G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research. Graefe's Arch. Clin. Exp. Ophthalmol. 2015, 253, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Paini, A.; Muiesan, M.L.; Agabiti-Rosei, C.; Aggiusti, C.; De Ciuceis, C.; Bertacchini, F.; Duse, S.; Semeraro, F.; Rizzoni, D.; Agabiti-Rosei, E.; et al. Carotid stiffness is significantly correlated with wall-to-lumen ratio of retinal arterioles. J. Hypertens. 2018, 36, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Stefański, A.; Wolf, J.; Harazny, J.M.; Miszkowska-Nagórna, E.; Wolnik, B.; Murawska, J.; Narkiewicz, K.; Schmieder, R.E. Impact of type 1 diabetes and its duration on wall-to-lumen ratio and blood flow in retinal arterioles. Microvasc. Res. 2023, 147, 104499. [Google Scholar] [CrossRef] [PubMed]
- Damian, I.; Nicoară, S.D. Correlations between Retinal Arterial Morphometric Parameters and Neurodegeneration in Patients with Type 2 Diabetes Mellitus with No or Mild Diabetic Retinopathy. Medicina 2021, 57, 244. [Google Scholar] [CrossRef]
- Ueno, Y.; Iwase, T.; Goto, K.; Tomita, R.; Ra, E.; Yamamoto, K.; Terasaki, H. Association of changes of retinal vessels diameter with ocular blood flow in eyes with diabetic retinopathy. Sci. Rep. 2021, 11, 4653. [Google Scholar] [CrossRef]
- Merino, D.; Loza-Alvarez, P. Adaptive optics scanning laser ophthalmoscope imaging: Technology update. Clin. Ophthalmol. 2016, 10, 743–755. [Google Scholar] [CrossRef]
- Zhang, B.; Li, N.; Kang, J.; He, Y.; Chen, X.-M. Adaptive optics scanning laser ophthalmoscopy in fundus imaging, a review and update. Int. J. Ophthalmol. 2017, 10, 1751–1758. [Google Scholar]
- Mujat, M.; Sampani, K.; Patel, A.H.; Sun, J.K.; Iftimia, N. Cellular-Level Analysis of Retinal Blood Vessel Walls Based on Phase Gradient Images. Diagnostics 2023, 13, 3399. [Google Scholar] [CrossRef]
- Mujat, M.; Ferguson, R.D.; Hammer, D.X.; Patel, A.H.; Iftimia, N. High-Resolution Retinal Imaging: Technology Overview and Applications. Photonics 2024, 11, 522. [Google Scholar] [CrossRef]
- Chui, T.Y.; Vannasdale, D.A.; Burns, S.A. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed. Opt. Express 2012, 3, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Mujat, M.; Akula, J.D.; Fulton, A.B.; Ferguson, R.D.; Iftimia, N. Non-Rigid Registration for High-Resolution Retinal Imaging. Diagnostics 2023, 13, 2285. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, C.N.; Mcgeown, J.G.; Curtis, T.M. Cellular physiology of retinal and choroidal arteriolar smooth muscle cells. Microcirculation 2007, 14, 11–24. [Google Scholar] [CrossRef]
- Das, A.; Byrd, J. Retinal Vasculature: Structure and Pathologies. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 2137–2161. [Google Scholar]
- Gardiner, T.A.; Stitt, A.W.; Anderson, H.R.; Archer, D.B. Selective loss of vascular smooth muscle cells in the retinal microcirculation of diabetic dogs. Br. J. Ophthalmol. 1994, 78, 54–60. [Google Scholar] [CrossRef]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef]
- Fu, J.; Yu, M.G.; Li, Q.; Park, K.; King, G.L. Insulin’s actions on vascular tissues: Physiological effects and pathophysiological contributions to vascular complications of diabetes. Mol. Metab. 2021, 52, 101236. [Google Scholar] [CrossRef]
- Roy, S.; Ha, J.; Trudeau, K.; Beglova, E. Vascular basement membrane thickening in diabetic retinopathy. Curr. Eye Res. 2010, 35, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trudeau, K.; Behl, Y.; Dhar, S.; Chronopoulos, A. New insights into hyperglycemia-induced molecular changes in microvascular cells. J. Dent. Res. 2009, 89, 116–127. [Google Scholar] [CrossRef]
- Huang, B.B.; Fukuyama, H.; Burns, S.A.; Fawzi, A.A. Imaging the Retinal Vascular Mural Cells In Vivo: Elucidating the Timeline of Their Loss in Diabetic Retinopathy. Arter. Thromb. Vasc. Biol. 2024, 44, 465–476. [Google Scholar] [CrossRef]
- Matuszewski, W.; Gontarz-Nowak, K.; Harazny, J.M.; Bandurska-Stankiewicz, E. Evaluation of Morphological Changes in Retinal Vessels in Type 1 Diabetes Mellitus Patients with the Use of Adaptive Optics. Biomedicines 2022, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Sapoznik, K.A.; Gast, T.J.; Carmichael-Martins, A.; Walker, B.R.; Warner, R.L.; Burns, S.A. Retinal Arteriolar Wall Remodeling in Diabetes Captured With AOSLO. Transl. Vis. Sci. Technol. 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, I.E.; Zagrean, L.; Balta, F.; Branisteanu, D.C. Retinal Microcirculation Investigation in Type I and Ii Diabetic Patients without Retinopathy Using an Adaptive Optics Retinal Camera. Acta. Endocrinol. 2019, 15, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, e2655–e2664. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Garvin, M.K.; Sonka, M.; Lee, K.; DeVries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef]
- McAnany, J.J.; Park, J.C.; Liu, K.; Liu, M.; Chen, Y.; Chau, F.Y.; Lim, J.I. Contrast sensitivity is associated with outer-retina thickness in early-stage diabetic retinopathy. Acta Ophthalmol. 2019, 98, e224–e231. [Google Scholar] [CrossRef]
- Clermont, A.C.; Aiello, L.P.; Mori, F.; Bursell, S.-E. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: A potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Arch. Ophthalmol. 1997, 124, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Muir, E.R.; Rentería, R.C.; Duong, T.Q. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Investig. Opthalmol. Vis. Sci. 2012, 53, 6488–6494. [Google Scholar] [CrossRef]
- Hanaguri, J.; Yokota, H.; Watanabe, M.; Yamagami, S.; Kushiyama, A.; Kuo, L.; Nagaoka, T. Retinal blood flow dysregulation precedes neural retinal dysfunction in type 2 diabetic mice. Sci. Rep. 2021, 11, 18401. [Google Scholar] [CrossRef]
- Rizzoni, D.; Rosei, E.A. Small artery remodeling in hypertension and diabetes. Curr. Hypertens. Rep. 2006, 8, 90–95. [Google Scholar] [CrossRef]
- Heagerty, A.M.; Heerkens, E.H.; Izzard, A.S. Small artery structure and function in hypertension. J. Cell. Mol. Med. 2010, 14, 1037–1043. [Google Scholar] [CrossRef] [PubMed]


| Participants Total N = 19 | N (%) Mean ± SD |
|---|---|
| Age (years) | 37.7 ± 8.1 |
| Gender (Female) | 10 (52.6) |
| Type 1 diabetes | 14 (73.7) |
| T1D duration (years) | 24.0 ± 8.2 |
| Hemoglobin A1c (%) | 7.4 ± 1.9 |
| DR Distribution | Eyes N (%) | Arterioles N (%) |
|---|---|---|
| Control | 5 (20.8) | 30 (27.3) |
| No DR | 4 (16.7) | 22 (20.0) |
| Mild–moderate NPDR | 9 (37.5) | 40 (36.4) |
| Severe NPDR–PDR | 6 (25.0) | 18 (16.4) |
| Total | 24 | 110 |
| Arteriolar Parameter N = 110 | Controls IQR (Q1, Q3) | T1D IQR (Q1, Q3) | p Value |
|---|---|---|---|
| Wall-to-lumen ratio | 0.02 (0.10, 0.12) | 0.05 (0.11, 0.16) | 0.002 * |
| Mean wall thickness (μm) | 4.3 (13.7, 18.0) | 6.0 (15.7, 21.7) | 0.0006 * |
| Luminal diameter (μm) | 28.0 (131.6, 159.6) | 91.4 (91.8, 183.3) | 0.907 |
| External diameter (μm) | 38.6 (159.0, 197.5) | 99.6 (123.2, 222.7) | 0.548 |
| Arteriolar Parameter N = 110 | Controls IQR (Q1, Q3) | No DR IQR (Q1, Q3) | Mild-Mod. NPDR IQR (Q1, Q3) | Severe—PDR IQR (Q1, Q3) |
|---|---|---|---|---|
| Wall-to-lumen ratio | 0.02 (0.10, 0.12) | 0.12 (0.11, 0.14) | 0.05 (0.12, 0.16) | 0.10 (0.10, 0.20) |
| Mean wall thickness (μm) | 4.3 (13.7, 18.0) | 10.07 (13.16, 23.23) | 4.63 (14.91, 19.53) | 3.3817.96, 21.34) |
| Luminal diameter (μm) | 28.0 (131.6, 159.6) | 127.20 (91.38, 218.58)) | 62.07 (91.40, 153.47) | 79.63 (102.64, 182.27) |
| External diameter (μm) | 38.6 (159.0, 197.5) | 145.15 (115.24, 260.39) | 63.92 (121.11, 185.02) | 82.31 (140.31, 222.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampani, K.; Mujat, M.; Patel, A.H.; Kang, C.; Iftimia, N.; Chatziralli, I.; Sun, J.K. Characterizing Vascular Wall and Lumen Caliber in Eyes with Diabetic Retinopathy Based on Adaptive Optics Scanning Laser Ophthalmoscopy. Diagnostics 2024, 14, 2020. https://doi.org/10.3390/diagnostics14182020
Sampani K, Mujat M, Patel AH, Kang C, Iftimia N, Chatziralli I, Sun JK. Characterizing Vascular Wall and Lumen Caliber in Eyes with Diabetic Retinopathy Based on Adaptive Optics Scanning Laser Ophthalmoscopy. Diagnostics. 2024; 14(18):2020. https://doi.org/10.3390/diagnostics14182020
Chicago/Turabian StyleSampani, Konstantina, Mircea Mujat, Ankit H. Patel, Chaerim Kang, Nicusor Iftimia, Irini Chatziralli, and Jennifer K. Sun. 2024. "Characterizing Vascular Wall and Lumen Caliber in Eyes with Diabetic Retinopathy Based on Adaptive Optics Scanning Laser Ophthalmoscopy" Diagnostics 14, no. 18: 2020. https://doi.org/10.3390/diagnostics14182020






