Abstract
The use of fibroblast activation protein inhibitors (FAPis) for positron emission tomography (PET) imaging in cancer has garnered significant interest in recent years, yielding promising results in preclinical and clinical settings. FAP is predominantly expressed in pathological conditions such as fibrosis and cancer, making it a compelling target. An optimized approach involves using FAPi homodimers as PET tracers, which enhance tumor uptake and retention, making them more effective candidates for therapy. Here, a UAMC-1110 inhibitor-based homodimer, DOTAGA-Glu(FAPi)2, was synthesized and radiolabeled with gallium-68, and its efficacy was evaluated in vivo for PET imaging in an endogenously FAP-expressing xenografted mouse model, U87MG. Notably, 45 min post-injection, the mean uptake of [68Ga]Ga-DOTAGA-Glu(FAPi)2 was 4.7 ± 0.5% ID/g in the tumor with low off-target accumulation. The ex vivo analysis of the FAP expression in the tumors confirmed the in vivo results. These findings highlight and confirm the tracer’s potential for diagnostic imaging of cancer and as a theranostic companion.
1. Introduction
Despite significant treatment advancement, cancer continues to pose a major global challenge, accounting for nearly 10 million deaths worldwide in 2022. In that year alone, there were an estimated 20 million new cancer cases, emphasizing the increasing disease burden [1]. Early detection and appropriate treatment are crucial for reducing the cancer burden, which can be assisted by advancing diagnostic and therapeutic strategies [2].
Fibroblast activation protein (FAP) is a type II transmembrane serine protease that has emerged as a promising target in various cancer types due to its overexpression in several malignancies, specifically epithelial cancers [3]. FAP cleaves the peptide bond between the proteinogenic amino acid proline and other amino acids, thereby altering various bioactive molecules. Among other proteases with similar activity are dipeptidyl peptidase (DDP) 4, 6, 8, and 9, where FAP is closely related to DPP4 [4]. FAP is predominantly expressed in activated fibroblasts such as cancer-associated fibroblasts (CAFs) present in the tumor stroma, where they actively remodel the extracellular matrix (ECM) and influence tumor behavior. Thus, targeting FAP addresses the tumor microenvironment (TME), compared to other tumor targets that generally target the tumor cells directly [5]. This potentially renders the utility of FAP as a much broader diagnostic and therapeutic target as it can be applied to many cancer types [6,7]. Recent studies have highlighted the role of FAP in the TME, where it not only contributes to ECM remodeling but also influences tumor growth, invasion, and immune evasion [8,9,10,11]. The overexpression of FAP in CAFs is associated with poor prognosis in several cancers, making it a critical target for therapeutic intervention [9]. Moreover, FAP’s enzymatic activities extend beyond its role in cancer, as it is also involved in various pathological conditions such as fibrosis and arthritis [12]. Furthermore, FAP expression in normal adult tissues, where resting fibroblast is present, is minimal, underlying the suitability of FAP as a target for both diagnostic and therapeutic purposes [4,5].
The identification of targeting agents for FAP is of increasing interest driven by the idea of selectively targeting FAP, while DPP4 remains unaffected [4]. The specific targeting of FAP could contribute to targeting the tumor and the TME. FAP inhibitors (FAPis) have been exploited as small-molecule radioligands designed to specifically target FAP with high binding affinity and are actively being investigated for both positron emission tomography (PET) imaging and potential radioligand therapies across various cancer types [5,13].
FAPi dimers represent an innovative approach to enhance tumor uptake, retention, and imaging contrast compared to their monomeric counterparts, optimizing their potential for both imaging and radionuclide therapy [14]. The philosophy of dimerization in PET tracers is grounded in enhancing biological interactions using dual binding sites. This approach has shown considerable promise in improving tumor uptake, retention, and predictive value for therapeutic efficacy [15,16]. Their extended tumor retention makes dimers particularly advantageous for theranostic purposes, especially when paired with radioisotopes like 177-Lutetium (177Lu), which have a prolonged half-life. This combination enhances therapeutic efficacy by leveraging the prolonged retention characteristic of the molecule [15]. Combining diagnostic imaging with therapeutic intervention within a single molecular agent holds immense clinical value, offering personalized treatment strategies and the real-time monitoring of therapeutic response [17].
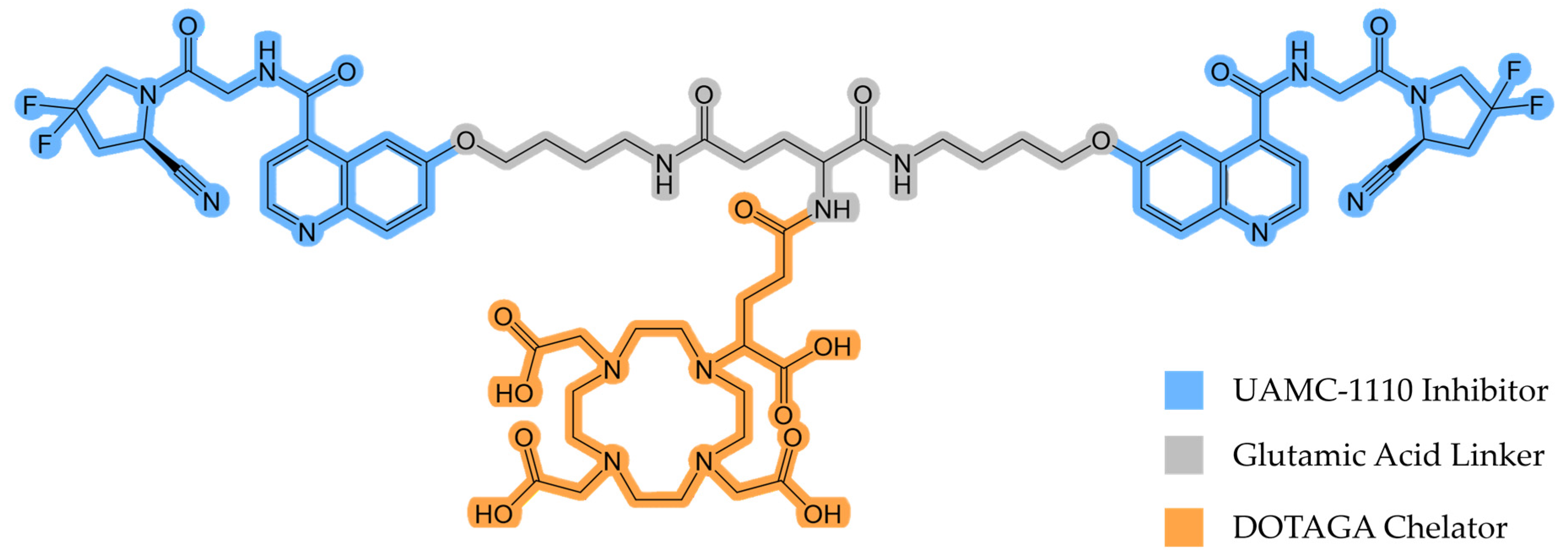
Recently, Martin and colleagues synthesized and evaluated DOTAGA-Glu(FAPi)2 (Figure 1), a promising UAMC-1110 inhibitor-based homodimer [18].
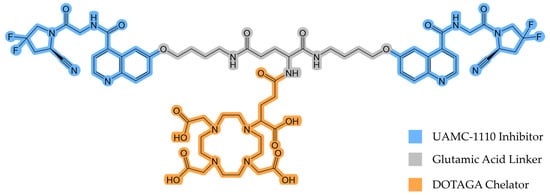
Figure 1.
The chemical structure of DOTAGA-Glu(FAPi)2.
DOTAGA-Glu(FAPi)2 exhibits a very high affinity for FAP (IC50(FAP)/nM 0.26 ± 0.04) [18]. A high affinity for prolyl endopeptidase (PREP) or DPP, ubiquitously expressed in healthy tissue, would reduce tumor selectivity and result in a lower tumor-to-background ratio. Therefore, DOTAGA-Glu(FAPi)2 needs to demonstrate high selectivity for FAP over PREP and DPP. It has been found to have higher selectivity than DOTAGA.(SA.FAPi)2, another promising FAPi derivative [19]. This indicates that it may also have superior properties in vivo compared to other dimeric FAP tracers, making it suitable for further investigations. In this study, we evaluated the potential of gallium-68 (68Ga)-labeled DOTAGA-Glu(FAPi)2 ([68Ga]Ga-DOTAGA-Glu(FAPi)2) for the in vivo PET imaging of mice xenografted with an endogenously FAP-expressing human glioblastoma cell line, aiming to demonstrate enhanced tumor uptake and retention, thereby supporting its use as an effective theranostic agent. By leveraging the unique properties of FAPi dimers, such as their enhanced tumor retention [20], we aimed to explore the potential of the 68Ga-labeled molecule as a theranostic companion.
2. Materials and Methods
2.1. Organic Synthesis
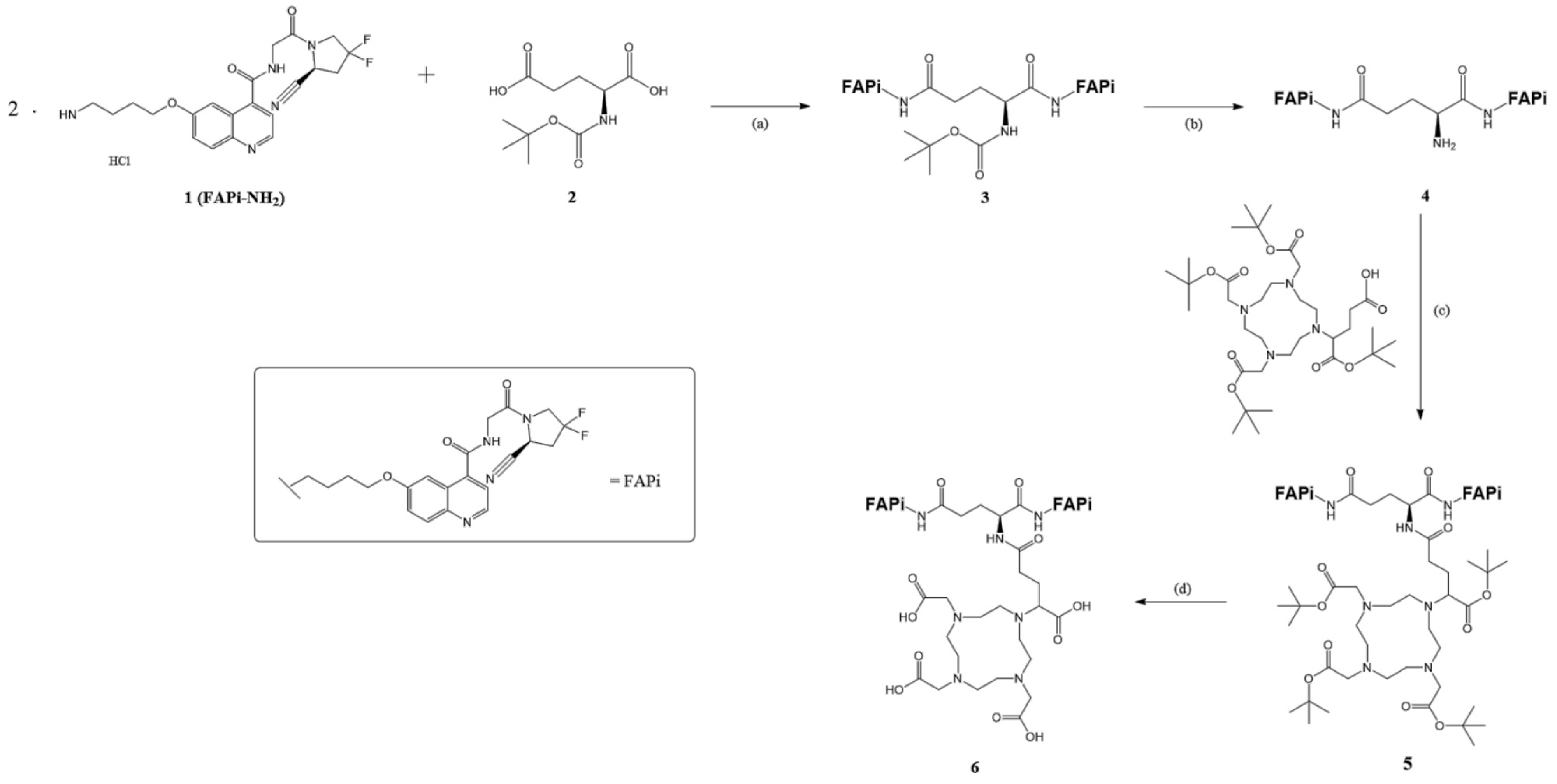
DOTAGA-Glu(FAPi)2 was synthesized according to published procedures [18]. The synthesis process is visualized in Figure 2.
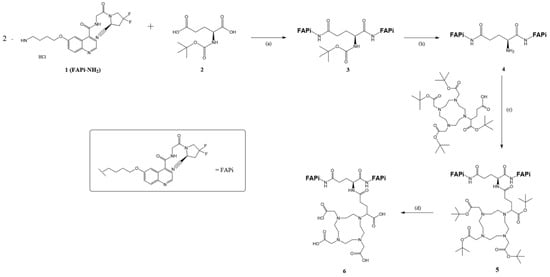
Figure 2.
Organic synthesis of DOTA.GA.Glu.(FAPi)2 6: (a) HOBt, EDC*HCl, DIPEA, DMF, RT, overnight, 69%; (b) 4 M HCl in 1,4-dioxane, 1,4-dioxane, 0 °C-RT, 3 h, 95%; (c) HATU, DIPEA, DMF, and RT, overnight, 98%; (d) TFA:MeCN:TIPS:H2O (85:10:5:2.5), RT, 5 h, 28%.
2.1.1. Synthesis of Glu.(FAPi)2
In our synthesis, we prepared tert-Butyl ((S)-1,5-bis((4-((4-((2-((S)-2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)caRbamoyl)-quinolin-6-yl)oxy)butyl)amino)-1,5-dioxopentan-2-yl)carbamate (3,Boc-Glu.(FAPi)2).
N-tert-Butoxycarbonyl-L-glutamic acid 2 (Boc-Glu-OH, 118 mg, 0.476 mmol, 1.00 eq), 1-hydroxybenzotriazole (HOBt, 167 mg, 1.233 mmol, 2.59 eq), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC*HCl, 238 mg, 1.243 mmol, 2.61 eq) were dissolved in dry N,N-dimethylformamide (DMF, 3 mL). N,N-diisopropylethylamine (DIPEA, 41 μL, 0.238 mmol, 0.50 eq) was then added under argon condition. The solution was stirred at room temperature for one hour, during which it turned yellow. Subsequently, a solution of FAPi-NH2 1 (446 mg, 0.952 mmol, 2.00 eq) and DIPEA (412 μL, 2.380 mmol, 5.00 eq) in DMF (3 mL) was added. The reaction mixture was stirred at room temperature overnight, and the solvent was removed in vacuo. The solution was then diluted with water (5 times the volume of the organic solvent), and the aqueous phase was extracted with EtOAc (3 × 30 mL). The organic phase was dried over Na2SO4, and the solvent was removed under reduced pressure. After column chromatography (CH2Cl2/MeOH, 95:0.5–10) rf: 0.375 3 was obtained as a colorless oil (350 mg, 0.326 mmol, 69%). 1H-NMR (400 MHz, MeOD-d4): δ [ppm] 8.71 (t, J = 4.6 Hz, 2H), 7.93 (d, J = 9.2 Hz, 2H), 7.89 (d, J = 2.7 Hz, 2H), 7.53 (dd, J = 4.5, 2.8 Hz, 2H), 7.42 (dd, J = 9.2, 2.7 Hz, 2H), 5.15 (dt, J = 9.5, 2.8 Hz, 2H), 4.40–3.87 (m, 13H), 3.30–3.20 (m, 3H), 3.02–2.74 (m, 4H), 2.24 (t, J = 7.5 Hz, 2H), 1.87 (dq, J = 12.5, 6.6 Hz, 5H), 1.72 (q, J = 7.0 Hz, 4H), 1.38 (s, 9H). MS (ESI+): m/z (%) = 537.8 (95, [M+H]2+), 1074.4 (72, [M+H]+), 1075.4 (50, [M+H]+), 1076.4 (20, [M+H]+) calculated for C52H59F4N11O10: 1073.44 [M].
(S)-2-Amino-N1,N5-bis(4-((4-((2-((S)-2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)-quinolin-6-yl)oxy)butyl)pentanediamide (4, Glu.(FAPi)2) was then prepared from Boc-Glu.(FAPi)2 3 (350 mg, 0.326 mmol, 1.00 eq), which was dissolved in dry 1,4-dioxane (5 mL) under an argon atmosphere. At 0 °C, 4 M hydrochloric acid (HCl) in 1,4-dioxane (1 mL, 4.24 mmol, 13.00 eq) was added, resulting in the formation of a white precipitate. The reaction was allowed to warm to room temperature, and after 3 h, the solvent was completely removed in vacuo, yielding Glu.(FAPi)2 as a yellowish solid 4 (311 mg, 0.308 mmol, 95%). 1H NMR (600 MHz, MeOD) δ 9.02 (dd, J = 8.3, 5.4 Hz, 2H), 8.21 (dd, J = 8.4, 2.7 Hz, 2H), 8.14 (dd, J = 9.3, 2.7 Hz, 2H), 7.99 (dt, J = 10.5, 4.1 Hz, 2H), 7.77 (ddd, J = 8.9, 5.8, 2.6 Hz, 2H), 5.16 (td, J = 10.0, 3.1 Hz, 2H), 4.48–4.03 (m, 12H), 3.92 (t, J = 6.3 Hz, 1H), 3.07–2.72 (m, 6H), 2.43 (p, J = 7.4 Hz, 2H), 2.11 (hept, J = 7.5 Hz, 2H), 1.95 (dq, J = 21.1, 6.9 Hz, 4H), 1.78 (dp, J = 30.0, 7.3 Hz, 4H). MS (ESI+): m/z (%) = 325.6 (100, [M-Boc+H]3+), 487.8 (85, [M+H]2+), 974.3 (45, [M+H]+), calculated for C47H51F4N11O8: 973.39 [M].
2.1.2. Synthesis of DOTAGA.Glu.(FAPi)2
We prepared tri-tert-Butyl 2,2′,2″-(10-(5-(((S)-1,5-bis((4-((4-((2-((S)-2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxo-ethyl)carbamoyl)quinolin-6-yl)oxy)butyl)amino)-1,5-dioxopentan-2-yl)amino)-1-(tert-butoxy)-1,5-dioxopentan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate (6, DOTAGA(tBu)4.Glu.(FAPi)2).
DOTAGA(tBu)4 5 (40 mg, 0.057 mmol, 1.15 eq), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo [4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU, 21.6 mg, 0.057 mmol, 1.15 eq) and DIPEA (10.7 μL, 0.062 mmol, 1.25 eq) were dissolved in dry DMF (1 mL) under argon atmosphere and stirred at room temperature for one hour. The solution changed color from clear pink to yellow. Then, the solution of Glu.(FAPi)2 (50 mg, 0.05 mmol, 1.00 eq) and DIPEA (21.4 μL, 0.124 mmol, 2.50 eq) in dry DMF (2 mL) was added. The reaction mixture reacted for 2 h, and the solvent was removed in vacuo. The crude product was purified by reverse phase (gradient from 0 to 100% MeCN, tR = 12.4 min) and a yellow solid was obtained. MS (ESI+): m/z (%) = 415.23 (75, [M+H]4+), 553.46 (100, [M+H]3+), 829.28 (80, [M+H]2+), 830.17 (20, [M+H]2+), 1656.85 (87, [M+H]+), 1657.85 (85, [M+H]+), 1658.85 (43, [M+H]+), 1659.86 (15, [M+H]+), calculated for C82H113F4N15O17: 1655.84 [M].
2,2′,2″-(10-(4-(((S)-1,5-bis((4-((4-((2-((S)-2-Cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)quinolin-6-yl)oxy)butyl)amino)-1,5-dioxopentan-2-yl)amino)-1-carboxy-4-oxobutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (7, DOTAGA.Glu.(FAPi)2) was also obtained.
DOTAGA(tBu)4.Glu.(FAPi)2 6 was dissolved in TFA (601 μL), MeCN (70 μL), triisopropylsilane (TIPS, 36 μL) and H20 (18 μL) and stirred at room temperature for 4 h.
The crude product was purified by semipreparative RP-HPLC (35% MeCN in 30 min, tR = 31 min), and 7 was obtained as a yellow solid (20.0 mg, 0.014 mmol, 28%). MS (ESI+): m/z (%) = 359.1 (55, [M+H]4+), 478.4 (100, [M+H]3+), 716.9 (40, [M+H]2+), 1432.40 (20, [M+H]+), calculated for C66H81F4N15O17: 1431.59 [M].
2.2. Labeling
Gallium-68 chloride was obtained by eluting the 68Ge/68Ga generator (Galliapharm, Eckert & Ziegler, Berlin, Germany) with 0.1 M HCl. Ammonium acetate buffer (0.1 M, pH 5.5), and a DOTAGA-Glu(FAPi)2 stock solution (2 mg/mL) for labeling was prepared using water for ultratrace analysis (Merck, Darmstadt, Germany). [68Ga]GaCl3 solution in 0.1 M HCl (500 µL, ca. 200 MBq 68Ga) was mixed with ammonium acetate buffer (80 µL) and DOTAGA-Glu-(FAPi)2 stock solution (4 µL). The resulting mixture was placed on an agitating mixer and shaken at 600 rpm for 5 min at 60 °C. Afterward, the reaction mixture was diluted with 5 mL water and passed through a Strata-X 33 µm polymeric reversed-phase cartridge (Phenomenex, Torrance, CA, USA). The cartridge was rinsed with more water (0.3 mL) and eluted with absolute ethanol (0.5 mL). Ethanol was evaporated under nitrogen flow and gentle heating to 40 °C. The residue was resolubilized in an aliquot of fresh ethanol (20 µL) and further diluted with phosphate buffer (0.1 M, pH 7) to a total volume of 2 mL.
Radiochemical conversion (RCC) for the 68Ga-labeling process and radiochemical purity (RCP) of the formulated [68Ga]Ga-DOTAGA-Glu(FAPi)2 were assessed by radio-HPLC.
2.3. In Vivo Evaluation
Six-week-old female NMRI nude mice were purchased (Janvier, Le Genest-Saint-Isle, France) and housed in groups of 4–8 mice in individually ventilated cages under regular lighting conditions. They were fed a standard pathogen-free pellet diet and provided water ad libitum. All animal experiments were approved by The Animal Experiments Inspectorate in Denmark (2021-15-0201-01041). The animals were acclimatized for two weeks before study initiation.
To establish a tumor model, the mice were subcutaneously inoculated on the right flank with a human xenograft glioblastoma U87MG (HTB-14, ATCC, Manassas, VA, USA). These cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (FBS) and penicillin–streptomycin (Pen/Strep). Tumor growth was monitored two times weekly using calipers.
After adequate tumor establishment, the animals were injected intravenously in the tail vein with 4.49 ± 0.04 MBq [68Ga]Ga-DOTAGA-Glu(FAPi)2 in a 100 μL phosphate buffer (0.1 M, pH 7) with 1% ethanol (v/v). PET/CT imaging (Inveon, Siemens Healthineers, Knoxville, TN, USA) was performed immediately after injection, and PET data were acquired for 3000 s. The imaging protocol included a dynamic framing setup, with five consecutive frames of 600 s each, resulting in average time intervals of 0–10, 10–20, 20–30, 30–40, and 40–50 min post-injection. During imaging, body temperature was maintained using a heated platform.
PET images were reconstructed using the 3D-OSEM/SP-MAP algorithm, and attenuation correction was performed using the co-registered CT images. The activity concentration was decay-corrected to the time of injection and quantified using Inveon Research Workplace (IRW). Regions of interest were manually drawn on CT images to delineate organs and derive volume and activity [21]. Subsequently, the percentage of injected dose per gram (% ID/g) was calculated for tumor, blood, liver, kidney, and muscle tissues for each frame.
All graphical representations of the data were generated using Prism version 10.1.0 (1994–2023, GraphPad Software, LLC, Boston, MA, USA). The results were expressed as the mean ± standard error of the mean (SEM).
2.4. Ex Vivo Evaluation
All tumor xenografts were resected and fixed in 4% paraformaldehyde. Following fixation, the tissues were embedded in paraffin and sectioned into slices, which were then deparaffinized and rehydrated to prepare them for immunohistochemical (IHC) analysis. The tissue sections were incubated for 24 h at 4 °C with the recombinant anti-FAP ɑ antibody (ab218164 Abcam, Cambridge, UK) at a dilution of 1:50. The secondary antibody was then applied, followed by staining with DAB+ chromogen, which produced a brown precipitate, indicating positive immunoreactivity. The sections were counterstained with hematoxylin to visualize cell nuclei and then dehydrated and mounted for microscopy. The specific binding of the antibody was ensured through the inclusion of negative controls.
High-quality virtual slides of the IHC results were obtained using the ZEISS Axio Scan Z1 whole slide scanner (ZEISS, Oberkochen, Germany), which allowed for the capture of detailed, high-resolution images of the entire tissue sections. Image analysis and quantification were performed using ZEISS ZEN 3.8 software, enabling the assessment of the FAP expression.
3. Results
3.1. Organic Synthesis
DOTAGA-Glu-(FAPi)2 was obtained with a purity of 99% as initially reported by Martin et al. [18]. The identity of the compound was confirmed by LC-MS, and the purity was defined by HPLC-MS (Figure S1).
3.2. Radiosynthesis
[68Ga]Ga-DOTAGA-Glu-(FAPi)2 was successfully obtained in 61% isolated radiochemical yield (RCY) [22]. Labeling and formulation took about 20 min. The RCC of 68Ga chelation was 96%, and the RCP of the final formulated tracer was 88% (Figure S2). RCP was stable for at least 1 h post-formulation.
3.3. In Vivo Evaluation
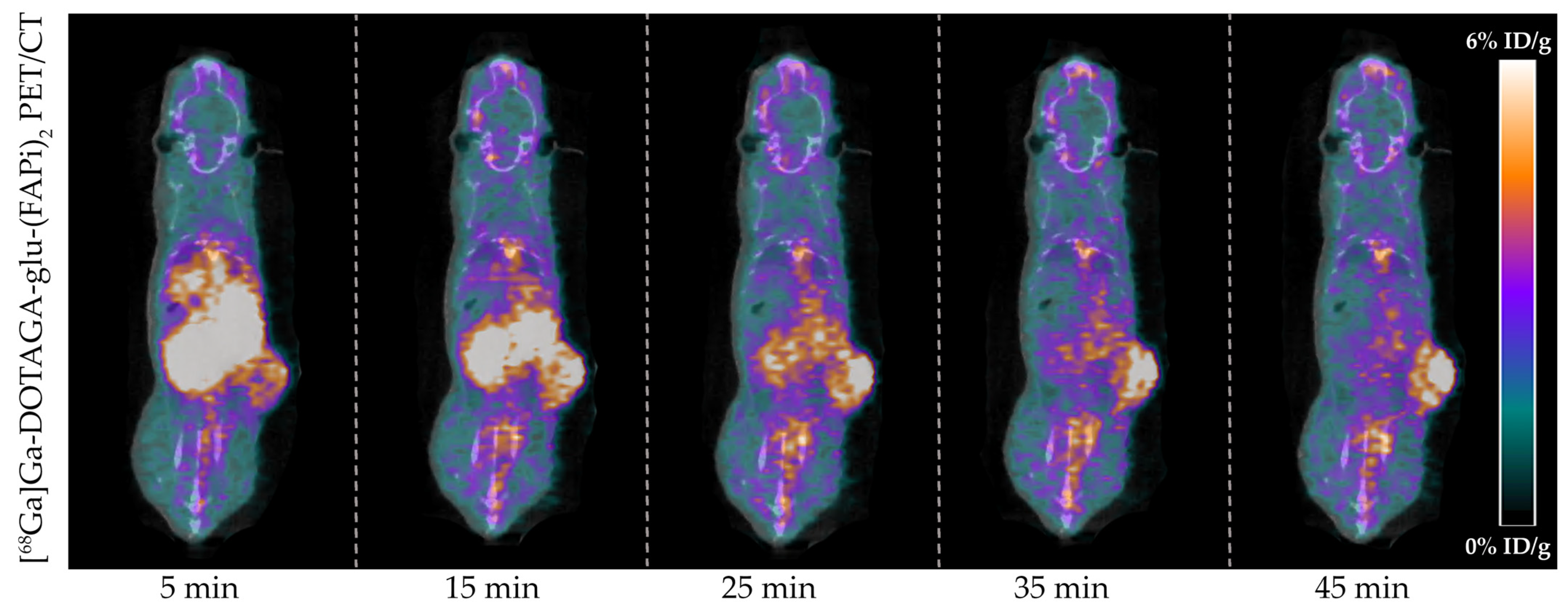
Tumor-bearing mice (n = 4) were successfully imaged with [68Ga]Ga-DOTAGA-Glu-(FAPi)2 PET/CT. A representative dynamic PET/CT image series of the in vivo cancer model is presented in Figure 3 showing the radioactive uptake at 5, 15, 25, 35, and 45 min post-injection.
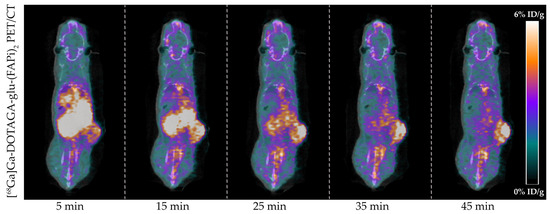
Figure 3.
In vivo [68Ga]Ga-DOTAGA-Glu-(FAPi)2 PET/CT imaging of U87MG cancer model. Representative images of the radioactive uptake are visualized from 0 to 6%ID/g at 5, 15, 25, 35, and 45 min post-injection.
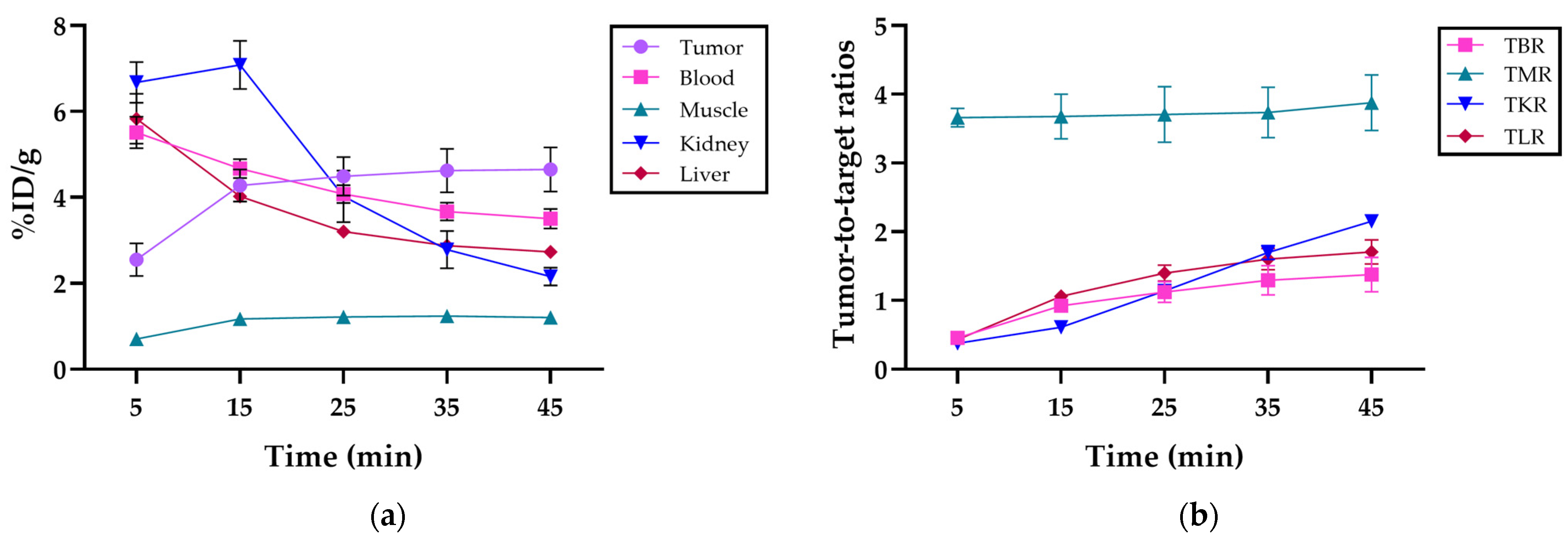
The mean uptake at 45 min post-injection was 4.7 ± 0.5% ID/g in the tumor, 3.5 ± 0.2% ID/g in blood, 1.2 ± 0.1% ID/g in muscle, 2.2 ± 0.2% ID/g in kidneys, and 2.7 ± 0.1% ID/g in the liver and was cleared mainly by the kidneys (Figure 4a). Detailed uptake data at the various time points post-injection are presented in Table S1.
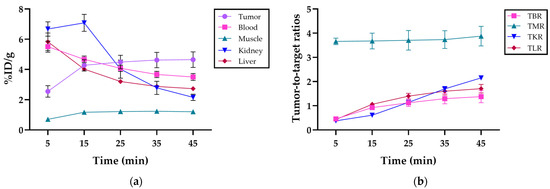
Figure 4.
(a) Organ distribution in %ID/g of [68Ga]Ga-DOTAGA-Glu-(FAPi)2 in the tumor, blood, muscle, kidney, and liver, respectively, at 5, 15, 25, 35, and 45 min post-injection; (b) the tumor-to-blood ratio (TBR), tumor-to-muscle ratio (TMR), tumor-to-kidney ratio (TKR), and tumor-to-liver ratio (TLR) of [68Ga]Ga-DOTAGA-Glu-(FAPi)2 at 5, 15, 25, 35, and 45 min post-injection.
The mean tumor-to-background ratios at 45 min post-injection were 1.4 ± 0.3 for the tumor-to-blood ratio (TBR), 3.9 ± 0.4 for the tumor-to-muscle ratio (TMR), 2.6 ± 0.1 for the tumor-to-kidney ratio (TKR), and 1.7 ± 0.2 for the tumor-to-liver ratio (TLR). The tumor-to-organ ratios at different time intervals can be seen in Figure 4b. The detailed ratios over the imaging period are provided in Table S2.
3.4. Ex Vivo Evaluation
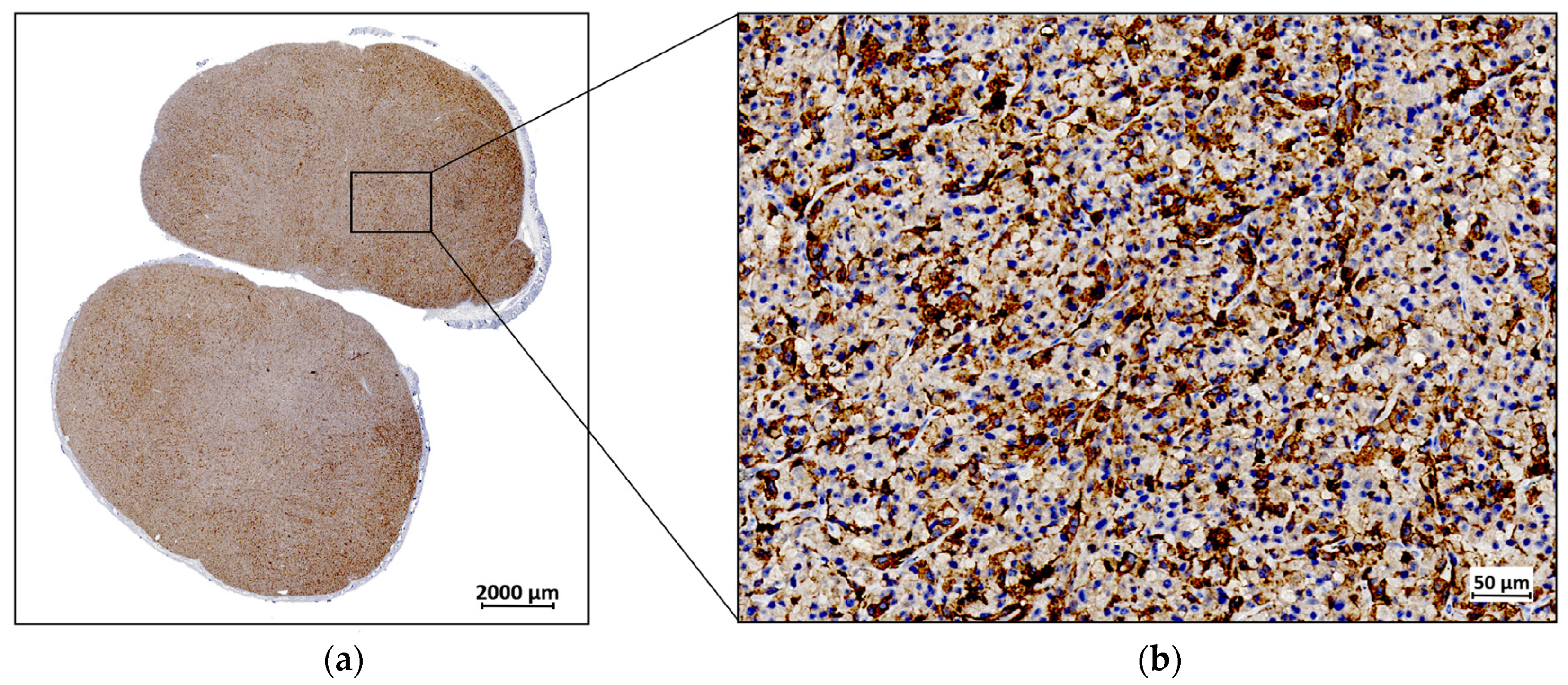
The ex vivo IHC analysis of the resected tumor tissue revealed distinctive FAP expression. The observed immunopositivity in the tumor cells was consistent with the expected endogenous expression of FAP. The brown DAB staining, which marks FAP-positive areas, was visible across the tumor cells, with additional staining detected in the stromal regions. Figure 5 illustrates these findings, with FAP immunopositivity depicted in brown, while the cell nuclei are counterstained in blue with hematoxylin.
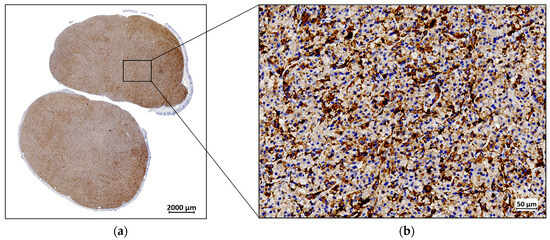
Figure 5.
A representative example of fibroblast activation protein (FAP) expression by immunohistochemistry (IHC) in a U87MG resected tumor: (a) IHC staining for FAP at 1× magnification; (b) IHC staining for FAP at 40× magnification.
4. Discussion
Here, we demonstrate the suitability of [68Ga]Ga-DOTAGA-Glu(FAPi)2 for in vivo PET imaging. Previous studies have evaluated the therapeutic potential of the molecule labeled with 177Lu [18,20,23]. To the authors’ knowledge, this is the first in vivo evaluation of [68Ga]Ga-DOTAGA-Glu(FAPi)2.
The tumor uptake of [68Ga]Ga-DOTAGA-Glu-(FAPi)2 showed increased accumulation with high tumor retention at 45 min post-injection, whereas the blood, kidney, and liver uptake decreased over the same period, depicting rapid clearance as the compound cleared renally. The kinetic profile of [68Ga]Ga-DOTAGA-Glu-(FAPi)2 is explained considering its low lipophilicity, estimated by measuring the logD7.4 value using the shake flask method with n-octanol and phosphate-buffered saline (pH 7.4). The logD7.4 of −2.5 ± 0.05 indicates good hydrophilicity, leading to renal excretion over biliary excretion [9]. The hydrophilicity is comparable to the monomer [68Ga]Ga-DOTA.SA.FAPi (−2.7 ± 0.1) ([24]) but with the advantage of high tumor uptake due to its dimeric nature. Moreover, the compound is more hydrophilic than the first-generation dimer [68Ga]Ga-DOTAGA.(SA.FAPi)2 (−2.0 ± 0.1) ([25]), resulting in lower background uptake and higher tumor-to-background image contrast.
During the initial 15 min post-injection, a small accumulation of [68Ga]Ga-DOTAGA-Glu(FAPi)2 was observed in the muscle tissue, most likely due to blood perfusion; however, uptake remained low.
An elevated tumor-to-muscle ratio was consistently observed throughout the imaging period, indicating specific tumor tissue accumulation. Additionally, the continuous increase in tumor-to-blood, tumor-to-kidney, and tumor-to-liver ratios further validates the compound’s sensitivity. These findings emphasize the efficacy of the tracer for accurate diagnostics and imaging. A comparison to monomeric counterparts suggests that dimerization enhances contrast and uptake, potentially improving diagnostic precision and therapeutic outcomes [14].
The ex vivo analysis of the FAP expression in the tumors corroborates the in vivo findings, confirming the expected endogenous expression of FAP within these tumor cells. The distinct FAP immunopositivity observed in both the tumor cells and the stromal component underscores the effectiveness of [68Ga]Ga-DOTAGA-Glu(FAPi)2 in specifically targeting FAP-positive tumors. This specificity is crucial for accurate imaging and subsequent therapeutic targeting, as it ensures that the tracer is selectively accumulating in FAP-expressing tumor cells rather than in non-target tissues. Importantly, the selective uptake of [68Ga]Ga-DOTAGA-Glu(FAPi)2 in FAP-expressing tissues, rather than non-target tissues, highlights its precision in targeting and imaging FAP-expressing tumors, making it a promising tool for the management of FAP-positive cancers.
Radiolabeling with 68Ga is advantageous due to its physical properties, including a short half-life of 68 min, which matches the kinetics and rapid tumor accumulation of [68Ga]Ga-DOTAGA-Glu(FAPi)2. Exploiting the compatibility of 68Ga decay kinetics with the circulation and tumor uptake kinetics of the FAPi molecule resulted in excellent image quality within 45 min. Furthermore, the relatively short half-life of 68Ga facilitates timely imaging procedures while minimizing radiation exposure to patients, thus making it an ideal choice for routine clinical PET imaging [26,27]. For this reason, [68Ga]Ga-DOTAGA-Glu(FAPi)2 could represent a better diagnostic compared to [68Ga]Ga-FAPI-04, a commonly used tracer that targets FAP. The latter has demonstrated a strong uptake in various types of cancer with improved biodistribution, tumor-to-background contrast, and faster kinetics compared to [18F]FDG. However, its relatively short tumor retention time makes it unsuitable for theranostic applications [28].
Long tumor retention is advantageous for therapeutic purposes, for instance, when labeling with long-lived radioisotopes like 177Lu [29]. Yadav et al. [23] demonstrated the efficacy and safety of [177Lu]Lu-DOTAGA-FAPi dimer treatment, yielding promising outcomes. This study compared the dimer and monomer forms, revealing significantly higher absorbed doses within the tumor with the dimer formulation.
The development of FAPi dimers represents a significant advancement in molecular imaging and targeted cancer therapy [30]. By leveraging the increased tumor uptake, retention, and imaging contrast achieved through dimerization, these agents not only improve diagnostic accuracy but also present a viable approach for developing radiotherapeutic analogs of FAPi-based molecules [31].
5. Conclusions
We successfully demonstrated the applicability of [68Ga]Ga-DOTAGA-Glu(FAPi)2 PET in vivo, highlighting its potential as a diagnostic tracer. Compared to existing tracers such as [68Ga]Ga-FAPI-04, [68Ga]Ga-DOTAGA-Glu(FAPi)2 offers the advantage of longer tumor retention and improved tumor-to-background contrast, making it a promising candidate for both diagnostic and therapeutic applications.
Given the dimers’ extended tumor retention time, this tracer also has significant potential as a theranostic agent when labeled with, e.g., 177Lu. The dimeric form of FAPi may provide a better predictive value for therapeutic efficacy than monomeric forms, underscoring its promise achieved through dimerization; thus, [68Ga]Ga-DOTAGA-Glu(FAPi)2 shows potential as an effective theranostic companion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14182024/s1, Figure S1: LC-MS and HPLC-MS analyses of the final compound; Figure S2: Radio-HPLC chromatograms for the preparation of [68Ga]Ga-DOTAGA-Glu-(FAPi)2; Table S1: Organ distribution in the percentage of injected dose per gram (%ID/g) of [68Ga]Ga-DOTAGA-Glu-(FAPi)2 in the tumor, blood, muscle, kidney, and liver, respectively, at 5, 15, 25, 35, and 45 min post-injection obtained from PET/CT data; Table S2: The tumor-to-blood ratio (TBR), tumor-to-muscle ratio (TMR), tumor-to-kidney ratio (TKR), and tumor-to-liver ratio (TLR) of [68Ga]Ga-DOTAGA-Glu-(FAPi)2 at 5, 15, 25, 35, and 45 min post-injection obtained from PET/CT data.
Author Contributions
Conceptualization, J.v.K.M., L.H., U.M.B., M.M.H. and A.K.; data curation, J.v.K.M. and E.G.L.; formal analysis, J.v.K.M., S.M., L.H. and E.G.L.; funding acquisition, M.M.H. and A.K.; investigation, J.v.K.M., S.M., E.G.L. and V.S.; methodology, J.v.K.M., S.M., L.H. and V.S.; project administration, J.v.K.M.; resources, M.M.H. and A.K.; supervision, L.H., M.M.H. and A.K.; writing—original draft preparation, J.v.K.M.; writing—review and editing, S.M., L.H., V.S., F.R., U.M.B., M.M.H. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 670261 (ERC Advanced Grant) and 668532 (Click-It), the Lundbeck Foundation, the Novo Nordisk Foundation, the Innovation Fund Denmark, the Neuroendocrine Tumor Research Foundation, the Danish Cancer Society, Arvid Nilsson Foundation, the Neye. Foundation, the Sygeforsikringen danmark, the Research Foundation of Rigshospitalet, the Danish National Research Foundation (grant 126)—PERSIMUNE, the Research Council of the Capital Region of Denmark, the Danish Health Authority, the John and Birthe Meyer Foundation, and Research Council for Independent Research. Andreas Kjaer is a Lundbeck Foundation Professor. OncoProTools has received funding from the European Union’s Horizon Europe Research and Innovation programme under the Marie Skłodowska-Curie Action No 101073231.
Institutional Review Board Statement
All animal experiments were approved by The Animal Experiments Inspectorate in Denmark (2021-15-0201-01041).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
Author Frank Roesch holds a patent related to the technology discussed in this manuscript (WO2022258637).
References
- World Health Organization. Global Cancer Burden Growing, amidst Mounting Need for Services; World Health Organization: Geneva, Switzerland, 2024.
- Velikyan, I. Chapter 17—Radionuclides for Imaging and Therapy in Oncology. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Oxford, UK, 2014; pp. 285–325. [Google Scholar]
- Chandekar, K.R.; Prashanth, A.; Vinjamuri, S.; Kumar, R. FAPI PET/CT Imaging—An Updated Review. Diagnostics 2023, 13, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Huang, Y.; Simms, A.E.; Mazur, A. Fibroblast activation protein-alpha: A key modulator of the microenvironment in multiple pathologies. Int. Rev. Cell Mol. Biol. 2012, 297, 83–116. [Google Scholar] [PubMed]
- Gilardi, L.; Airò Farulla, L.S.; Demirci, E.; Clerici, I.; Omodeo Salè, E.; Ceci, F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines 2022, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef]
- Mousavi, M.J.; Karami, J.; Alimohammadi, M.; Solaymani-Mohammadi, F.; Rezaei, N. Fibroblast Activation Protein (FAP): A Key Modulator of the Cancer Microenvironment. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–23. [Google Scholar]
- Shahvali, S.; Rahiman, N.; Jaafari, M.R.; Arabi, L. Targeting fibroblast activation protein (FAP): Advances in CAR-T cell, antibody, and vaccine in cancer immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 2041–2056. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Dziadek, S.; Kraxner, A.; Cheng, W.Y.; Ou Yang, T.H.; Flores, M.; Theiss, N.; Tsao, T.-S.; Andersson, E.; Harring, S.V.; Bröske, A.-M.E.; et al. Comprehensive analysis of fibroblast activation protein expression across 23 tumor indications: Insights for biomarker development in cancer immunotherapies. Front. Immunol. 2024, 15, 1352615. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid 2021, 32, 65–77. [Google Scholar] [CrossRef]
- Tan, Y.; Li, J.; Zhao, T.; Zhou, M.; Liu, K.; Xiang, S.; Tang, Y.; Jakobsson, V.; Xu, P.; Chen, X.; et al. Clinical translation of a novel FAPI dimer [68Ga]Ga-LNC1013. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2761–2773. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.H.; Lan, X.; Cai, W. PET with a 68Ga-Labeled FAPI Dimer: Moving Toward Theranostics. J. Nucl. Med. 2022, 63, 860. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Bauder-Wüst, U.; Leotta, K.; Zoller, F.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Eder, M. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoğlu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef]
- Martin, M.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; De Loose, J.; Verhulst, E.; De Meester, I.; Van Der Veken, P.; Roesch, F. Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers 2023, 15, 1889. [Google Scholar] [CrossRef]
- Läppchen, T.; Bilinska, A.; Pilatis, E.; Menéndez, E.; Imlimthan, S.; Moon, E.S.; Afshar-Oromieh, A.; Rösch, F.; Rösch, A.; Gourni, E. Tailoring Fibroblast-Activation Protein Targeting for Theranostics: A Comparative Preclinical Evaluation of the 68Ga- and 177Lu-Labeled Monomeric and Dimeric Fibroblast-Activation Protein Inhibitors DOTA.SA.FAPi and DOTAGA.(SA.FAPi)2. Molecules 2024, 29, 3093. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Raju, S.; Roesch, F.; Martin, M.; Tripathi, M.; Bal, C. [(177)Lu]Lu-DOTAGA.Glu.(FAPi)(2) Radionuclide Therapy: A New Treatment Option for Patients with Glioblastoma Multiforme. Nucl. Med. Mol. Imaging 2024, 58, 32–34. [Google Scholar] [CrossRef]
- Jensen, M.M.; Jørgensen, J.T.; Binderup, T.; Kjær, A. Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med. Imaging 2008, 8, 16. [Google Scholar] [CrossRef]
- Herth, M.M.; Ametamey, S.; Antuganov, D.; Bauman, A.; Berndt, M.; Brooks, A.F.; Bormans, G.; Choe, Y.S.; Gillings, N.; Häfeli, U.O.; et al. On the consensus nomenclature rules for radiopharmaceutical chemistry—Reconsideration of radiochemical conversion. Nucl. Med. Biol. 2021, 93, 19–21. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Martin, M.; Roesch, F.; Satapathy, S.; Moon, E.S.; Tripathi, M.; Gogia, A.; Bal, C. Therapeutic potential of [(177)Lu]Lu-DOTAGA-FAPi dimers in metastatic breast cancer patients with limited treatment options: Efficacy and safety assessment. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 805–819. [Google Scholar] [CrossRef]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; Stephan, S.; Bracke, A.; Van der Veken, P.; De Meester, I.; Roesch, F. Fibroblast Activation Protein (FAP) targeting homodimeric FAP inhibitor radiotheranostics: A step to improve tumor uptake and retention time. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 476–491. [Google Scholar] [PubMed]
- Michael, M.; Yury, S.; Elisabeth, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In Medical Isotopes; Syed Ali Raza, N., Muhammad Babar, I., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 2. [Google Scholar]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F.; Spreckelmeyer, S. Good practices for 68Ga radiopharmaceutical production. EJNMMI Radiopharm. Chem. 2022, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Verena, A.; Kuo, H.-T.; Merkens, H.; Zeisler, J.; Bendre, S.; Wong, A.A.W.L.; Bénard, F.; Lin, K.-S. Novel 68Ga-Labeled Pyridine-Based Fibroblast Activation Protein-Targeted Tracers with High Tumor-to-Background Contrast. Pharmaceuticals 2023, 16, 449. [Google Scholar] [CrossRef]
- Dhoundiyal, S.; Srivastava, S.; Kumar, S.; Singh, G.; Ashique, S.; Pal, R.; Mishra, N.; Taghizadeh-Hesary, F. Radiopharmaceuticals: Navigating the frontier of precision medicine and therapeutic innovation. Eur. J. Med. Res. 2024, 29, 26. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-art of FAPI-PET imaging: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Pang, Y.; Fang, J.; Fu, K.; Meng, L.; Zhang, X.; Guo, Z.; Wu, H.; Sun, L.; et al. Development of Fibroblast Activation Protein Inhibitor-Based Dimeric Radiotracers with Improved Tumor Retention and Antitumor Efficacy. Mol. Pharm. 2022, 19, 3640–3651. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).