Update on Patient Self-Testing with Portable and Wearable Devices: Advantages and Limitations
Abstract
:1. Introduction
2. Current Wearable Medical Devices and Remote Patient Monitoring
3. Some Paradigmatic Examples of Well-Established Portable Laboratory Testing Devices
3.1. Monitoring of Oral Anticoagulant Therapy
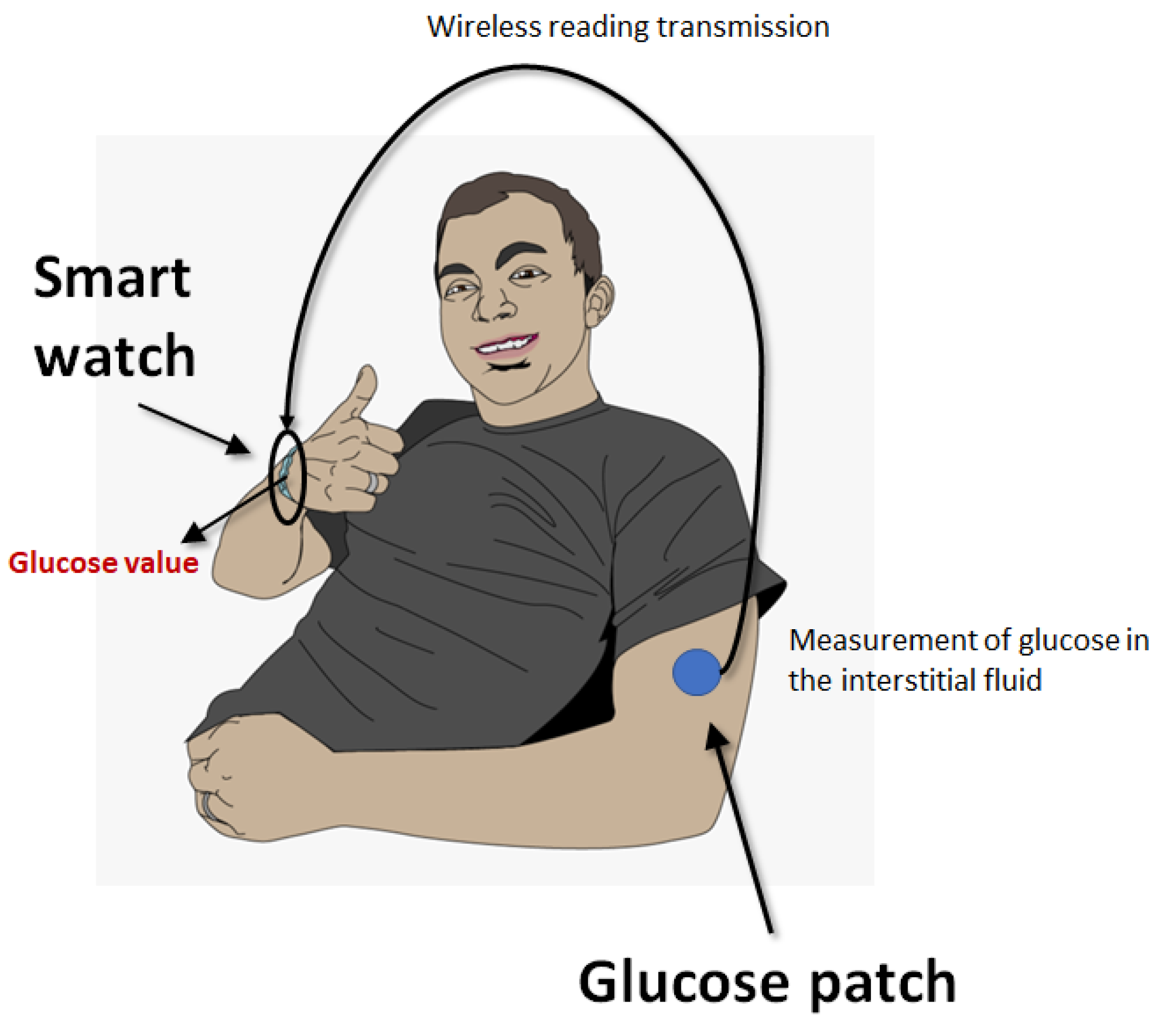
3.2. Blood Glucose Monitoring
4. Innovative Wearable Laboratory Testing Devices
4.1. Cardiac Troponin Testing
4.2. Sespsis Diangosis
5. Potential Problems of Portable or Wearable Lab Testing Devices
5.1. Pre-Analytical Issues
5.2. Analytical Issues
5.3. Post-Analytical Issues
6. All That Glitters Is Not Gold
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lippi, G.; Plebani, M. A modern and pragmatic definition of Laboratory Medicine. Clin. Chem. Lab. Med. 2020, 58, 1171. [Google Scholar] [CrossRef]
- Plebani, M.; Laposata, M.; Lippi, G. A manifesto for the future of laboratory medicine professionals. Clin. Chim. Acta 2019, 489, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Favaloro, E.J. Artificial intelligence in the pre-analytical phase: State-of-the art and future perspectives. J. Med. Biochem. 2024, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Nichols, J.H.; Luppa, P.B.; Greene, D.; Sciacovelli, L.; Shaw, J.; Khan, A.I.; Carraro, P.; Freckmann, G.; Dimech, W.; et al. Point-of-care testing: State-of-the art and perspectives. Clin. Chem. Lab. Med. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.; Sandberg, S.; Balla, J.; Basok, B.I.; Brady, J.J.; Croal, B.; De Vos, N.; Karlsson, M.; Kedars, P.; Ozben, T.; et al. Direct-to-consumer testing as consumer initiated testing: Compromises to the testing process and opportunities for quality improvement. Clin. Chem. Lab. Med. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Bassi, A.; Bovo, C. The future of laboratory medicine in the era of precision medicine. J. Lab. Precis. Med. 2016, 1, 7. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Picard, R.W.; Friend, S.H. Key Issues as Wearable Digital Health Technologies Enter Clinical Care. N. Engl. J. Med. 2024, 390, 1118–1127. [Google Scholar] [CrossRef]
- Farias, F.A.C.; Dagostini, C.M.; Bicca, Y.A.; Falavigna, V.F.; Falavigna, A. Remote Patient Monitoring: A Systematic Review. Telemed. e-Health 2020, 26, 576–583. [Google Scholar] [CrossRef]
- Pronovost, P.J.; Cole, M.D.; Hughes, R.M. Remote Patient Monitoring During COVID-19: An Unexpected Patient Safety Benefit. JAMA 2022, 327, 1125–1126. [Google Scholar] [CrossRef]
- Hilty, D.M.; Armstrong, C.M.; Edwards-Stewart, A.; Gentry, M.T.; Luxton, D.D.; Krupinski, E.A. Sensor, Wearable, and Remote Patient Monitoring Competencies for Clinical Care and Training: Scoping Review. J. Technol. Behav. Sci. 2021, 6, 252–277. [Google Scholar] [CrossRef]
- Bernasconi, S.; Angelucci, A.; De Cesari, A.; Masotti, A.; Pandocchi, M.; Vacca, F.; Zhao, X.; Paganelli, C.; Aliverti, A. Recent Technologies for Transcutaneous Oxygen and Carbon Dioxide Monitoring. Diagnostics 2024, 14, 785. [Google Scholar] [CrossRef]
- Santosh, K.C.; Rasmussen, N.; Mamun, M.; Aryal, S. A systematic review on cough sound analysis for COVID-19 diagnosis and screening: Is my cough sound COVID-19? PeerJ Comput. Sci. 2022, 8, e958. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Liumbruno, G.M.; Bonfanti, C.; Lippi, G. The evolution of anticoagulant therapy. Blood Transfus. 2016, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Pasalic, L.; Lippi, G. Oral anticoagulation therapy: An update on usage, costs and associated risks. Pathology 2020, 52, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, Y.; Huang, L.; Han, Z. The Value of Anticoagulation Management Combining Telemedicine and Self-Testing in Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. Ther. Clin. Risk Manag. 2023, 19, 279–290. [Google Scholar] [CrossRef]
- Chan, J.; Michaelsen, K.; Estergreen, J.K.; Sabath, D.E.; Gollakota, S. Micro-mechanical blood clot testing using smartphones. Nat. Commun. 2022, 13, 831. [Google Scholar] [CrossRef]
- Xu, W.; Althumayri, M.; Mohammad, A.; Ceylan Koydemir, H. Foldable low-cost point-of-care device for testing blood coagulation using smartphones. Biosens. Bioelectron. 2023, 242, 115755. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S126–S144. [Google Scholar] [CrossRef]
- Hughes, M.S.; Addala, A.; Buckingham, B. Digital Technology for Diabetes. N. Engl. J. Med. 2023, 389, 2076–2086. [Google Scholar] [CrossRef]
- Reddy, M.; Oliver, N. The role of real-time continuous glucose monitoring in diabetes management and how it should link to integrated personalized diabetes management. Diabetes Obes. Metab. 2024, 26 (Suppl. 1), 46–56. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.; Hassan, N.; Tam, W.; Koh, S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: A systematic review of randomised controlled trials and meta-analysis. Diabetologia 2022, 65, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Jancev, M.; Vissers, T.A.C.M.; Visseren, F.L.J.; van Bon, A.C.; Serné, E.H.; DeVries, J.H.; de Valk, H.W.; van Sloten, T.T. Continuous glucose monitoring in adults with type 2 diabetes: A systematic review and meta-analysis. Diabetologia 2024, 67, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Li, H.; Zhang, N.; Jiang, X.; Yu, X.; Yang, Q.; Jin, Z.; Meng, H.; Chang, L. Highly integrated watch for noninvasive continual glucose monitoring. Microsyst. Nanoeng. 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, B.M. Flux-Type versus Concentration-Type Sensors in Transdermal Measurements. Biosensors 2023, 13, 845. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Shi, H.; Xu, C. Current Technological Trends in Transdermal Biosensing. Adv. NanoBiomed Res. 2022, 2, 2200040. [Google Scholar] [CrossRef]
- Tiwari, D.; Aw, T.C. Optimizing the Clinical Use of High-Sensitivity Troponin Assays: A Review. Diagnostics 2023, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Gokhan, I.; Dong, W.; Grubman, D.; Mezue, K.; Yang, D.; Wang, Y.; Gandhi, P.U.; Kwan, J.M.; Hu, J.R. Clinical Biochemistry of Serum Troponin. Diagnostics 2024, 14, 378. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: Every minute of delay counts. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef]
- Polonschii, C.; Potara, M.; Iancu, M.; David, S.; Banciu, R.M.; Vasilescu, A.; Astilean, S. Progress in the Optical Sensing of Cardiac Biomarkers. Biosensors 2023, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Titus, J.; Wu, A.H.B.; Biswal, S.; Burman, A.; Sengupta, S.P.; Sengupta, P.P. Development and preliminary validation of infrared spectroscopic device for transdermal assessment of elevated cardiac troponin. Commun. Med. 2022, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Biswal, S.; Titus, J.; Burman, A.; Reddy, K.; Fulwani, M.C.; Khan, A.; Deshpande, N.; Shrivastava, S.; Yanamala, N.; et al. A novel breakthrough in wrist-worn transdermal troponin-I-sensor assessment for acute myocardial infarction. Eur. Heart J. Digit. Health 2023, 4, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.X.; Bhimarao, M.; Greene, J.D.; Manickam, R.N.; Martinez, A.; Schuler, A.; Barreda, F.; Escobar, G.J. The Presentation, Pace, and Profile of Infection and Sepsis Patients Hospitalized Through the Emergency Department: An Exploratory Analysis. Crit. Care Explor. 2021, 3, e0344. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G. Sepsis biomarkers: Past, present and future. Clin. Chem. Lab. Med. 2019, 57, 1281–1283. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Yang, Y.; Zhou, J.; Yao, K.; Li, J.; Zhou, Y.; Li, M.; Wong, T.H.; Yu, X. Wearable and battery-free wound dressing system for wireless and early sepsis diagnosis. Bioeng. Transl. Med. 2023, 8, e10445. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Plebani, M. Can biomarkers help us to better diagnose and manage sepsis? Diagnosis 2015, 2, 81–87. [Google Scholar] [CrossRef]
- Banfi, G.; Bozic, B.; Cihan, M.; Paaalic, D.; Pennestrì, F.; Plebani, M. Point-of-care testing, near-patient testing and patient self-testing: Warning points. Clin. Chem. Lab. Med. 2024. ahead of print. [Google Scholar] [CrossRef]
- Plebani, M.; Laposata, M.; Lundberg, G.D. The brain-to-brain loop concept for laboratory testing 40 years after its introduction. Am. J. Clin. Pathol. 2011, 136, 829–833. [Google Scholar] [CrossRef]
- Triantafyllidis, A.; Kondylakis, H.; Katehakis, D.; Kouroubali, A.; Alexiadis, A.; Segkouli, S.; Votis, K.; Tzovaras, D. Smartwatch interventions in healthcare: A systematic review of the literature. Int. J. Med. Inform. 2024, 190, 105560. [Google Scholar] [CrossRef]
- Thapa, S.; Bello, A.; Maurushat, A.; Farid, F. Security Risks and User Perception towards Adopting Wearable Internet of Medical Things. Int. J. Environ. Res. Public Health 2023, 20, 5519. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.M. Using Continuous Glucose Monitoring in Clinical Practice. Clin. Diabetes 2020, 38, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bellido, V.; Freckman, G.; Pérez, A.; Galindo, R.J. Accuracy and Potential Interferences of Continuous Glucose Monitoring Sensors in the Hospital. Endocr. Pract. 2023, 29, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Erbach, M.; Freckmann, G.; Hinzmann, R.; Kulzer, B.; Ziegler, R.; Heinemann, L.; Schnell, O. Interferences and Limitations in Blood Glucose Self-Testing: An Overview of the Current Knowledge. J. Diabetes Sci. Technol. 2016, 10, 1161–1168. [Google Scholar] [CrossRef]
- Asarani, N.A.M.; Reynolds, A.N.; Boucher, S.E.; de Bock, M.; Wheeler, B.J. Cutaneous Complications with Continuous or Flash Glucose Monitoring Use: Systematic Review of Trials and Observational Studies. J. Diabetes Sci. Technol. 2020, 14, 328–337. [Google Scholar] [CrossRef]
- Freckmann, G.; Pleus, S.; Grady, M.; Setford, S.; Levy, B. Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. J. Diabetes Sci. Technol. 2019, 13, 575–583. [Google Scholar] [CrossRef]
- Heinemann, L. Interferences with CGM Systems: Practical Relevance? J. Diabetes Sci. Technol. 2022, 16, 271–274. [Google Scholar] [CrossRef]
- Khan, M.; Wahid, N.; Musser, T.; Bergenstal, R.M.; Ebekozien, O.; Snow, K.; Thomas, K.; Aprigliano, C. Advancing Diabetes Quality Measurement in the Era of Continuous Glucose Monitoring. Sci. Diabetes Self Manag. Care 2023, 49, 112–125. [Google Scholar] [CrossRef]
- Plebani, M. Harmonizing the post-analytical phase: Focus on the laboratory report. Clin. Chem. Lab. Med. 2024, 62, 1053–1062. [Google Scholar] [CrossRef]
- Larkin, H.D. Cybersecurity Risk for Medtronic Insulin Pump. JAMA 2022, 328, 1679. [Google Scholar] [CrossRef]
- Carraro, P.; Plebani, M. Post-analytical errors with portable glucose meters in the hospital setting. Clin. Chim. Acta 2009, 404, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and recommendations for wearable devices in digital health: Data quality, interoperability, health equity, fairness. PLoS Digit. Health 2022, 1, e0000104. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Shah, P.; Raymond, J. Integrating Continuous Glucose Monitor Data Directly into the Electronic Health Record: Proof of Concept. Diabetes Technol. Ther. 2020, 22, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Kidzinski, L.; Runge, R.; Witt, D.; Hicks, J.L.; Schüssler-Fiorenza Rose, S.M.; Li, X.; Bahmani, A.; Delp, S.L.; Hastie, T.; et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat. Med. 2021, 27, 1105–1112. [Google Scholar] [CrossRef]
- Alva, S.; Brazg, R.; Castorino, K.; Kipnes, M.; Liljenquist, D.R.; Liu, H. Accuracy of the Third Generation of a 14-Day Continuous Glucose Monitoring System. Diabetes Ther. 2023, 14, 767–776. [Google Scholar] [CrossRef]
- Alva, S.; Bailey, T.; Brazg, R.; Budiman, E.S.; Castorino, K.; Christiansen, M.P.; Forlenza, G.; Kipnes, M.; Liljenquist, D.R.; Liu, H. Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System with Advanced Algorithm in Pediatric and Adult Population with Diabetes. J. Diabetes Sci. Technol. 2022, 16, 70–77. [Google Scholar] [CrossRef]
- Wadwa, R.P.; Laffel, L.M.; Shah, V.N.; Garg, S.K. Accuracy of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System During 10 Days of Use in Youth and Adults with Diabetes. Diabetes Technol. Ther. 2018, 20, 395–402. [Google Scholar] [CrossRef]
- Park, J. Smartphone based lateral flow immunoassay quantifications. J. Immunol. Methods 2024, 533, 113745. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Islam, T.; Washington, P. Non-Invasive Biosensing for Healthcare Using Artificial Intelligence: A Semi-Systematic Review. Biosensors 2024, 14, 183. [Google Scholar] [CrossRef]
| Phase of the Testing Process | Problem |
|---|---|
| Pre-analytical | Regulatory challenges |
| Cost | |
| Inappropriate purchasing | |
| Patient variables | |
| Appropriateness | |
| Sensor placement | |
| Maintenance and replacement | |
| Long-term injuries | |
| Analytical | Calibration |
| Chemical interreference | |
| Environmental conditions | |
| Lot-to-lot variation | |
| Cyberterrorism | |
| Lack of connectivity | |
| Transcription errors | |
| Post-analytical | Cyberterrorism |
| Lack of connectivity | |
| Transcription errors | |
| Misinterpretation of test results | |
| Information overload | |
| Integration within the medical record | |
| Variability in reporting formats and reference ranges |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, G.; Pighi, L.; Mattiuzzi, C. Update on Patient Self-Testing with Portable and Wearable Devices: Advantages and Limitations. Diagnostics 2024, 14, 2037. https://doi.org/10.3390/diagnostics14182037
Lippi G, Pighi L, Mattiuzzi C. Update on Patient Self-Testing with Portable and Wearable Devices: Advantages and Limitations. Diagnostics. 2024; 14(18):2037. https://doi.org/10.3390/diagnostics14182037
Chicago/Turabian StyleLippi, Giuseppe, Laura Pighi, and Camilla Mattiuzzi. 2024. "Update on Patient Self-Testing with Portable and Wearable Devices: Advantages and Limitations" Diagnostics 14, no. 18: 2037. https://doi.org/10.3390/diagnostics14182037








