Fibrosis-4 Score Is Associated with Mortality in Hemodialysis Patients with Chronic Viral Hepatitis: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
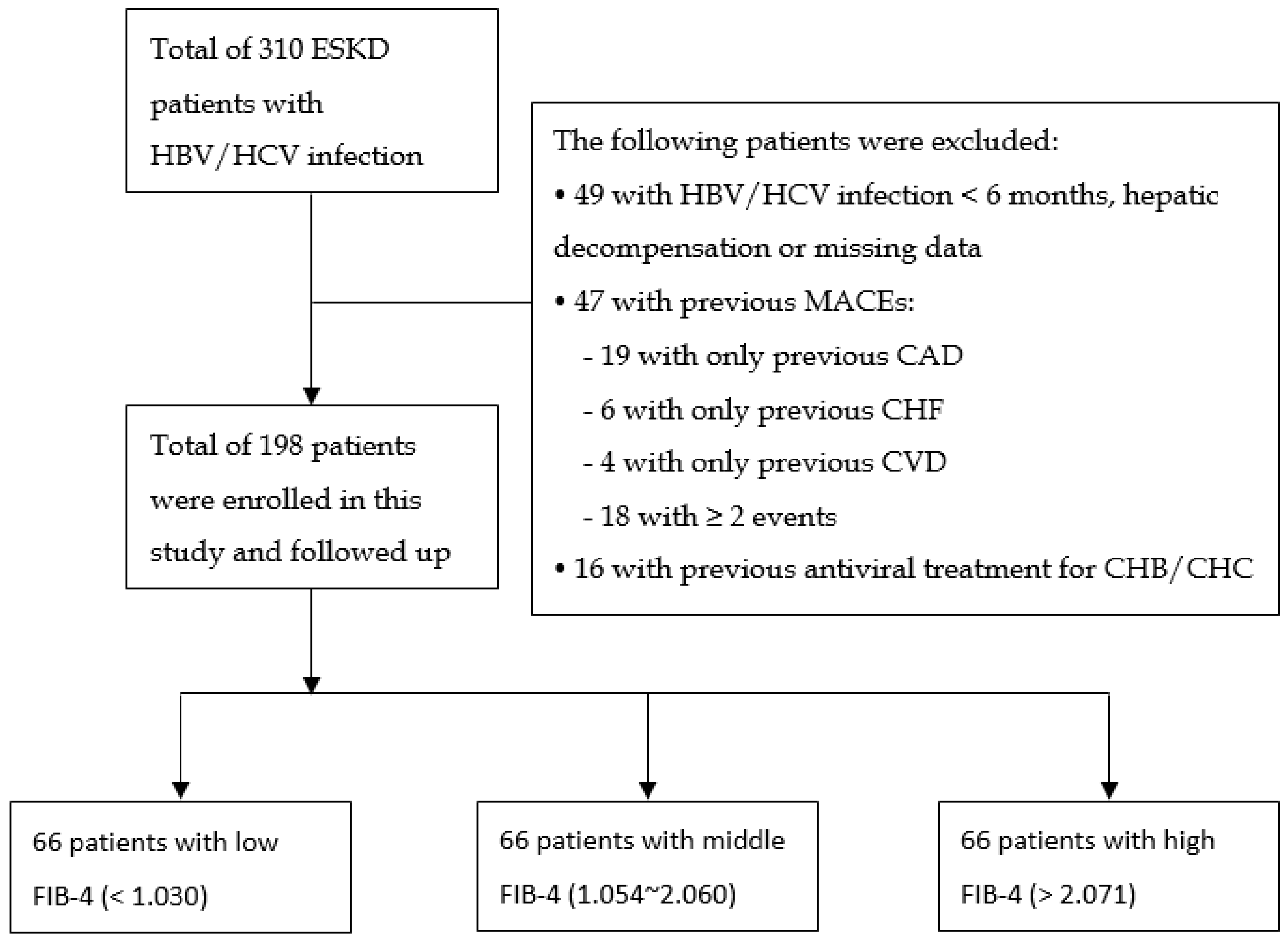
2.1. Patient Recruitment
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Predictors for 5-Year MACEs in HD Patients with Chronic Viral Hepatitis
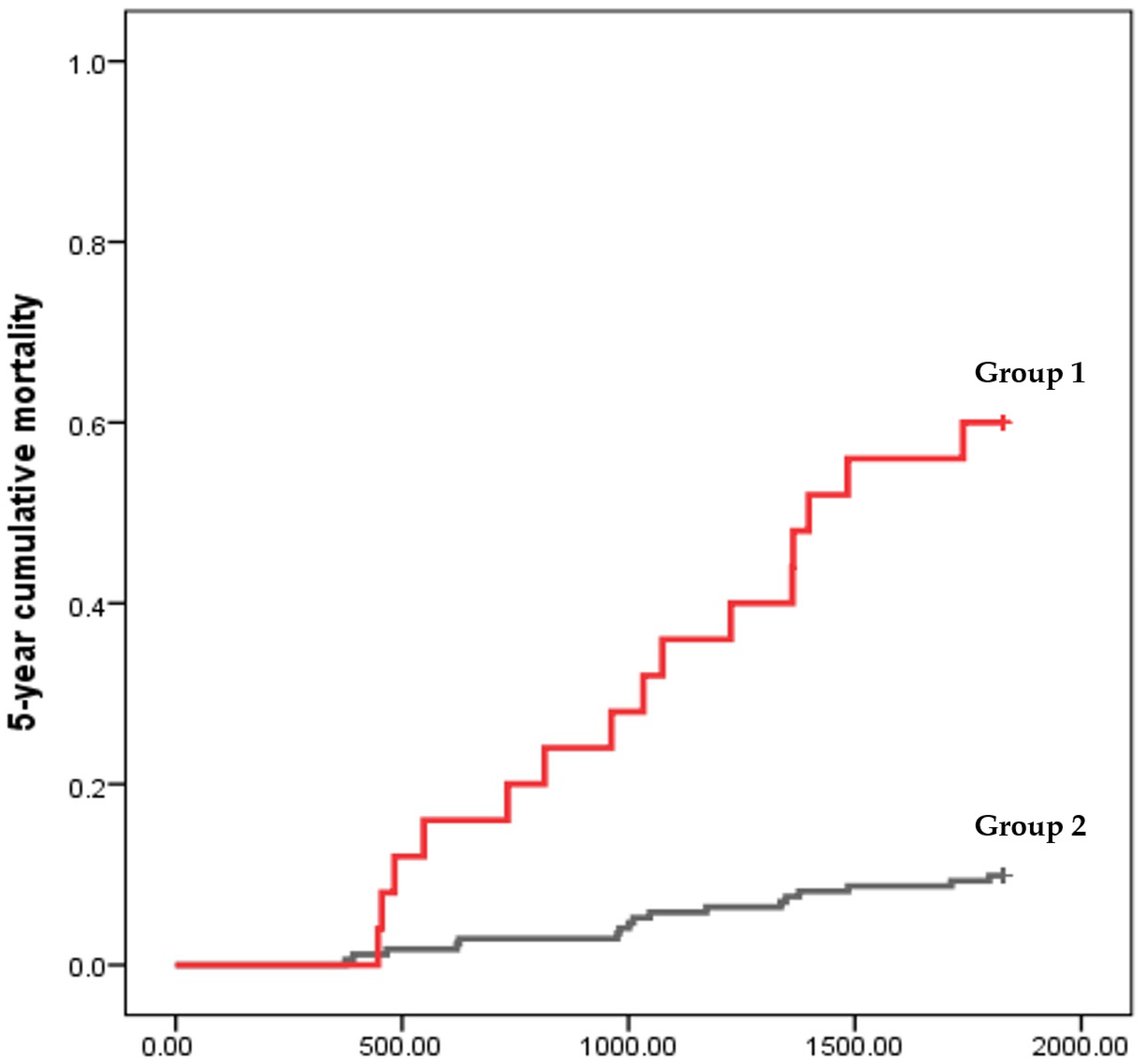
3.3. Predictors for 5-Year Mortality in HD Patients with Chronic Viral Hepatitis
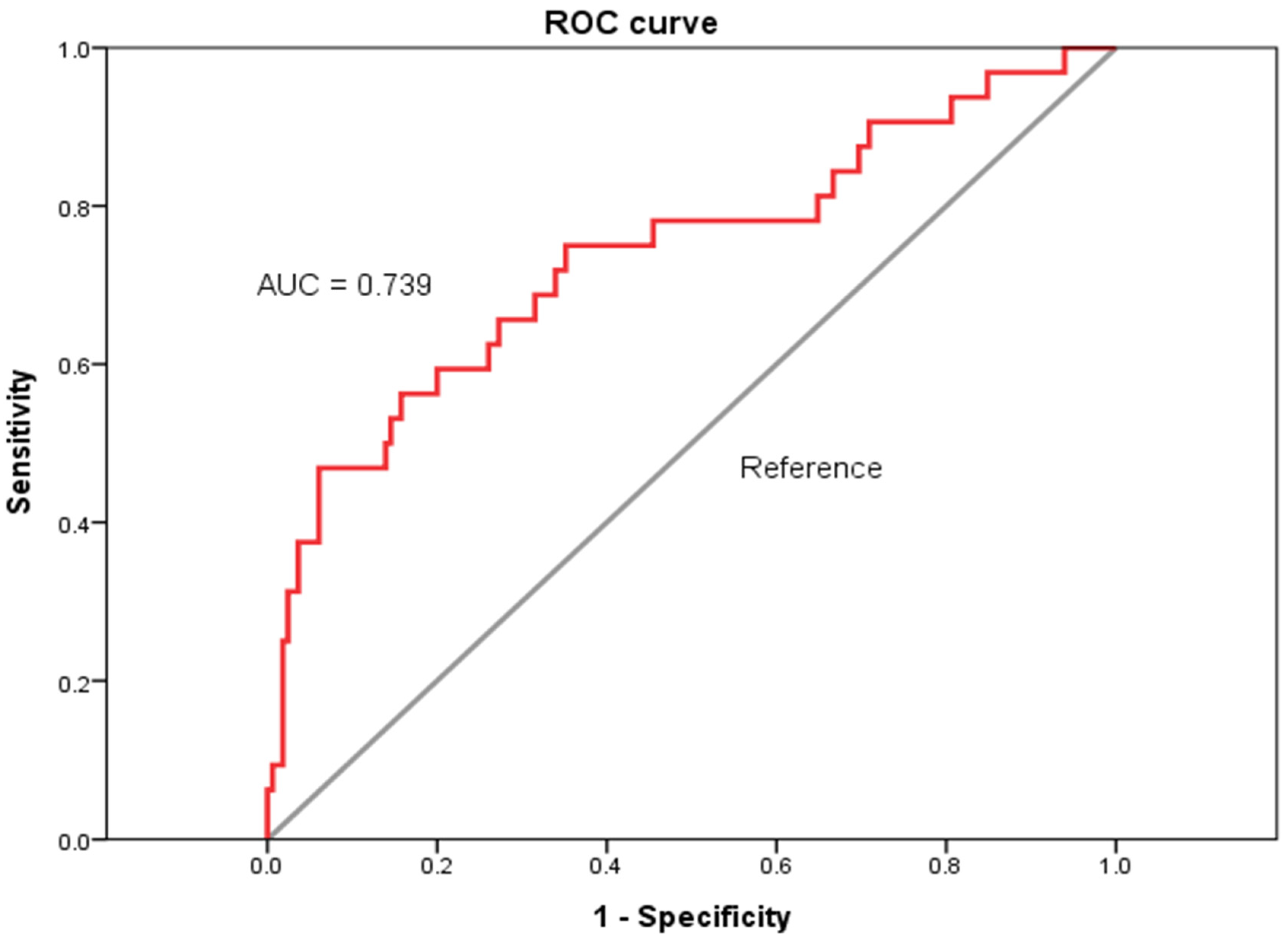
3.4. Diagnostic Performances of FIB-4 Score for Predicting All-Cause 5-Year Mortality in HD Patients with Chronic Viral Hepatitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- de Avila, R.E.; Carmo, R.A.; de Paula Farah, K.; Teixeira, A.L.; Coimbra, L.V.; de Figueiredo Antunes, C.M.; Lambertucci, J.R. Hyaluronic acid in the evaluation of liver fibrosis in patients with hepatitis C on haemodialysis. Braz. J. Infect. Dis. 2010, 14, 335–341. [Google Scholar] [CrossRef][Green Version]
- Soliman, A.; Fathy, A.; Khashab, S.; Shaheen, N. The burden of anti-HCV genotye-4 positivity in renal transplant recipients: 8 years follow-up. Int. Urol. Nephrol. 2013, 45, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.L.; Alhamad, T.; Abendroth, C.S.; Farag, H.A.; Verma, N. An unexpected presentation: Minimal change disease in an adult with treatment-naïve hepatitis C. Int. Urol. Nephrol. 2013, 45, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, W.; Tan, Z.; Li, D. Comparison of prednisolone and lamivudine combined therapy with prednisolone monotherapy on carriers of hepatitis B virus with IgA nephropathy: A prospective cohort study. Int. Urol. Nephrol. 2014, 46, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Keyvani, H.; Agah, S.; Kabir, A.; Alavian, S.-M. Prevalence and risk factors of isolated anti-HBc antibody and occult hepatitis B infection in hemodialysis patients: A nationwide study. Ann. Hepatol. 2015, 12, 213–219. [Google Scholar] [CrossRef]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. Liver biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef]
- Sterling, R.K.; Sanyal, A.J.; Luketic, V.A.; Stravitz, R.T.; King, A.L.; Post, A.B.; Mills, A.S.; Contos, M.J.; Shiffman, M.L. Chronic hepatitis C infection in patients with end stage renal disease: Characterization of liver histology and viral load in patients awaiting renal transplantation. Am. J. Gastroenterol. 1999, 94, 3576–3582. [Google Scholar] [CrossRef]
- Cotler, S.J.; Diaz, G.; Gundlapalli, S.; Jakate, S.; Chawla, A.; Mital, D.; Jensik, S.; Jensen, D.M. Characteristics of hepatitis C in renal transplant candidates. J. Clin. Gastroenterol. 2002, 35, 191–195. [Google Scholar] [CrossRef]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.-Z.; Reddy, K.R.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef]
- Ichikawa, S.; Motosugi, U.; Ichikawa, T.; Sano, K.; Morisaka, H.; Enomoto, N.; Matsuda, M.; Fujii, H.; Araki, T. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magn. Reson. Med. Sci. 2012, 11, 291–297. [Google Scholar] [CrossRef]
- Stebbing, J.; Farouk, L.; Panos, G.; Anderson, M.; Jiao, L.R.; Mandalia, S.; Bower, M.; Gazzard, B.; Nelson, M. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J. Clin. Gastroenterol. 2010, 44, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Kaplan, M.M. The SGOT/SGPT ratio—An indicator of alcoholic liver disease. Dig. Dis. Sci. 1979, 24, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sakaguchi, K.; Fujiwara, A.; Fujioka, S.-i.; Iwasaki, Y.; Makino, Y.; Araki, Y.; Shiratori, Y. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med. Okayama 2006, 60, 77–84. [Google Scholar]
- Caragea, D.C.; Ungureanu, B.S.; Florescu, D.; Popa, P.; Sacerdotianu, M.V.; Gheonea, D.; Vere, C.C. Noninvasive fibrosis assessment in chronic viral hepatitis C associated with end stage renal disease. Curr. Health Sci. J. 2018, 44, 206. [Google Scholar] [PubMed]
- Mikolasevic, I.; Orlic, L.; Zaputovic, L.; Racki, S.; Cubranic, Z.; Anic, K.; Devcic, B.; Stimac, D. Usefulness of liver test and controlled attenuation parameter in detection of nonalcoholic fatty liver disease in patients with chronic renal failure and coronary heart disease. Wien. Klin. Wochenschr. 2015, 127, 451–458. [Google Scholar] [CrossRef]
- Wadhva, R.K.; Haque, M.M.; Luck, N.H.; Tasneem, A.A.; Abbas, Z.; Mubarak, M. Diagnostic Accuracy of Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis 4 Scores in Predicting Advanced Liver Fibrosis in Patients with End-stage Renal Disease and Chronic Viral Hepatitis: Experience from Pakistan. J. Transl. Int. Med. 2018, 6, 38–42. [Google Scholar] [CrossRef]
- Syed, T.; Chadha, N.; Kumar, D.; Gupta, G.; Sterling, R.K. Non-Invasive Assessment of Liver Fibrosis and Steatosis in End-Stage Renal Disease Patients Undergoing Renal Transplant Evaluation. Gastroenterol. Res. 2021, 14, 244–251. [Google Scholar] [CrossRef]
- Schmoyer, C.J.; Kumar, D.; Gupta, G.; Sterling, R.K. Diagnostic Accuracy of Noninvasive Tests to Detect Advanced Hepatic Fibrosis in Patients With Hepatitis C and End-Stage Renal Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2332–2339.e1. [Google Scholar] [CrossRef]
- Lee, J.-J.; Wei, Y.-J.; Lin, M.-Y.; Niu, S.-W.; Hsu, P.-Y.; Huang, J.-C.; Jang, T.-Y.; Yeh, M.-L.; Huang, C.-I.; Liang, P.-C. The applicability of non-invasive methods for assessing liver fibrosis in hemodialysis patients with chronic hepatitis C. PLoS ONE 2020, 15, e0242601. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; McPherson, S. Management of decompensated cirrhosis. Clin. Med. 2018, 18, s60–s65. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, B.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018, 137, 961–972. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gordon, S.; Rupp, L.; Zhang, T.; Boscarino, J.; Vijayadeva, V.; Schmidt, M.; Lu, M.; Investigators, C.H.C.S. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J. Viral Hepat. 2014, 21, 930–937. [Google Scholar] [CrossRef]
- Teshale, E.; Lu, M.; Rupp, L.; Holmberg, S.; Moorman, A.; Spradling, P.; Vijayadeva, V.; Boscarino, J.; Schmidt, M.; Gordon, S. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: The Chronic Hepatitis Cohort Study (CH e CS). J. Viral Hepat. 2014, 21, 917–920. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Holmberg, S.D.; Lu, M.; Rupp, L.B.; Lamerato, L.E.; Moorman, A.C.; Vijayadeva, V.; Boscarino, J.A.; Henkle, E.M.; Gordon, S.C.; Investigators, C.H.C.S. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin. Infect. Dis. 2013, 57, 240–246. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia–Tsao, G.; Bosch, J.; Burroughs, A.K.; Ripoll, C.; Maurer, R.; Planas, R.; Escorsell, A. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin. Gastroenterol. Hepatol. 2009, 7, 689–695. [Google Scholar] [CrossRef]
- Hayashi, H.; Beppu, T.; Shirabe, K.; Maehara, Y.; Baba, H. Management of thrombocytopenia due to liver cirrhosis: A review. World J. Gastroenterol. 2014, 20, 2595. [Google Scholar] [CrossRef]
- Ohki, I.; Dan, K.; Kuriya, S.-I.; Nomura, T. A study on the mechanism of anemia and leukopenia in liver cirrhosis. Jpn. J. Med. 1988, 27, 155–159. [Google Scholar] [CrossRef] [PubMed]
- McHutchison, J.G.; Manns, M.P.; Longo, D.L. Definition and management of anemia in patients infected with hepatitis C virus. Liver Int. 2006, 26, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pagan, J.; De Gottardi, A.; Bosch, J. The modern management of portal hypertension–primary and secondary prophylaxis of variceal bleeding in cirrhotic patients. Aliment. Pharmacol. Ther. 2008, 28, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A. Preventing first variceal hemorrhage in cirrhosis. J. Clin. Gastroenterol. 2007, 41, S305–S311. [Google Scholar] [CrossRef] [PubMed]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J. Gastroenterol. 2021, 56, 593–619. [Google Scholar] [CrossRef]
- Halliday, J.; Ramm, G.; Powell, L.; Brock, J.; Pippard, M. Cellular iron processing and storage: The role of ferritin. In Iron Metabolism in Health and Disease, 7th ed.; Brock, J.H., Halliday, J.W., Pippard, M.J., Powell, L.W., Eds.; WB Saunders: London, UK, 1994; pp. 98–121. [Google Scholar]
- Worwood, M. Laboratory determination of iron status. In Iron Metabolism in Health and Disease; WB Saunders: London, UK, 1994; pp. 449–476. [Google Scholar]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef]
- Yang, W.; Israni, R.K.; Brunelli, S.M.; Joffe, M.M.; Fishbane, S.; Feldman, H.I. Hemoglobin variability and mortality in ESRD. J. Am. Soc. Nephrol. 2007, 18, 3164–3170. [Google Scholar] [CrossRef]
- Ofsthun, N.; Labrecque, J.; Lacson, E.; Keen, M.; Lazarus, J.M. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003, 63, 1908–1914. [Google Scholar] [CrossRef]
- Mazairac, A.H.; de Wit, G.A.; Grooteman, M.P.; Penne, E.L.; van der Weerd, N.C.; van den Dorpel, M.A.; Nubé, M.J.; Lévesque, R.; Ter Wee, P.M.; Bots, M.L.; et al. A composite score of protein-energy nutritional status predicts mortality in haemodialysis patients no better than its individual components. Nephrol. Dial. Transpl. 2011, 26, 1962–1967. [Google Scholar] [CrossRef][Green Version]
- Chung, S.H.; Lindholm, B.; Lee, H.B. Influence of initial nutritional status on continuous ambulatory peritoneal dialysis patient survival. Perit. Dial. Int. 2000, 20, 19–26. [Google Scholar] [CrossRef]
- Li, S.; Collins, A.J. Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodialysis patients. Kidney Int. 2004, 65, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Regidor, D.L.; Kopple, J.D.; Kovesdy, C.P.; Kilpatrick, R.D.; McAllister, C.J.; Aronovitz, J.; Greenland, S.; Kalantar-Zadeh, K. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J. Am. Soc. Nephrol. 2006, 17, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.M.; Joffe, M.M.; Berns, J.S.; Pisoni, R.L.; Port, F.K.; Feldman, H.I. Anemia and mortality in hemodialysis patients: Accounting for morbidity and treatment variables updated over time. Kidney Int. 2005, 68, 2323–2330. [Google Scholar] [CrossRef]
- de Seigneux, S.; Martin, P.-Y. Management of patients with nephrotic syndrome. Swiss Med. Wkly. 2009, 139, 416. [Google Scholar]
- Djoussé, L.; Rothman, K.J.; Cupples, L.A.; Levy, D.; Ellison, R.C. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 2002, 106, 2919–2924. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.; Oratz, M.; Schreiber, S. Effects of nutrition and alcohol on albumin synthesis. Alcohol. Clin. Exp. Res. 1983, 7, 28–30. [Google Scholar] [CrossRef]
- Kuzuya, M.; Izawa, S.; Enoki, H.; Okada, K.; Iguchi, A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? Clin. Nutr. 2007, 26, 84–90. [Google Scholar] [CrossRef]
- Herrmann, F.R.; Safran, C.; Levkoff, S.E.; Minaker, K.L. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch. Intern. Med. 1992, 152, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, P.; Feldman, J. Association of serum albumin and mortality risk. J. Clin. Epidemiol. 1997, 50, 693–703. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef]
- Angulo, P.; Bugianesi, E.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Barrera, F.; Haflidadottir, S.; Day, C.P.; George, J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 782–789.e4. [Google Scholar] [CrossRef]
- Unalp-Arida, A.; CE, R. PNPLA3 I148M and liver fat and fibrosis scores predict liver disease mortality in the United States population. Hepatology 2020, 71, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Cohen, D.E.; Fisher, E.A. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2013; Volume 33, pp. 380–388. [Google Scholar]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Orlic, L.; Mikolasevic, I.; Lukenda, V.; Anic, K.; Jelic, I.; Racki, S. Nonalcoholic fatty liver disease and the renin-angiotensin system blockers in the patients with chronic kidney disease. Wien. Klin. Wochenschr. 2015, 127, 355–362. [Google Scholar] [CrossRef]
- Goh, G.B.; Pagadala, M.R.; Dasarathy, J.; Unalp-Arida, A.; Sargent, R.; Hawkins, C.; Sourianarayanane, A.; Khiyami, A.; Yerian, L.; Pai, R. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015, 35, 979–985. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Miele, L.; Zoppini, G.; Pichiri, I.; Muggeo, M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. In Seminars in Thrombosis and Hemostasis; © Thieme Medical Publishers: New York, NY, USA, 2009; Volume 35, pp. 277–287. [Google Scholar]
- Vlassara, H.; Torreggiani, M.; Post, J.B.; Zheng, F.; Uribarri, J.; Striker, G.E. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. 2009, 76, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshihisa, A.; Kanno, Y.; Watanabe, S.; Yokokawa, T.; Abe, S.; Misaka, T.; Sato, T.; Suzuki, S.; Oikawa, M. Liver stiffness assessed by Fibrosis-4 index predicts mortality in patients with heart failure. Open Heart 2017, 4, e000598. [Google Scholar] [CrossRef]
- Vieira Barbosa, J.; Milligan, S.; Frick, A.; Broestl, J.; Younossi, Z.; Afdhal, N.H.; Lai, M. Fibrosis-4 Index as an Independent Predictor of Mortality and Liver-Related Outcomes in NAFLD. Hepatol. Commun. 2022, 6, 765–779. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.Y.; Jung, S.M.; Song, J.J.; Park, Y.-B.; Lee, S.-W. Fibrosis-4 index at diagnosis is associated with all-cause mortality in patients with microscopic polyangiitis and granulomatosis with polyangiitis. BMC Gastroenterol. 2019, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Yonas, E.; Huang, I.; Lim, M.A.; Nasution, S.A.; Kuswardhani, R.A.T. Fibrosis-4 index and mortality in coronavirus disease 2019: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e368–e374. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | Low-FIB-4 Group (<1.030) (n = 66) | Middle-FIB-4 Group (1.030~2.071) (n = 66) | High-FIB-4 Group (>2.071) (n = 66) | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, year | 56.91 ± 11.72 | 49.94 ± 10.80 | 57.50 ± 10.39 | 63.38 ± 9.97 | <0.001 |
| Male sex | 105 (53.03) | 42 (63.64) | 32 (48.48) | 31 (46.97) | 0.118 |
| Body weight after hemodialysis, kg | 57.68 ± 12.19 | 60.24± 12.36 | 57.05 ± 11.86 | 55.71 ± 12.09 | 0.093 |
| Comorbidity | |||||
| Diabetes mellitus, yes | 58 (29.29) | 21 (31.82) | 22 (33.33) | 15 (22.73) | 0.381 |
| Hypertension, yes | 121 (61.11) | 41 (62.12) | 39 (59.09) | 41 (62.12) | 0.887 |
| Dialysis-related data | |||||
| Hemodialysis duration, year | 10.26 ± 8.17 | 10.15 ± 7.63 | 10.81 ± 8.13 | 9.82 ± 8.80 | 0.778 |
| Cardiothoracic ratio, % | 0.50 ± 0.07 | 0.49 ± 0.06 | 0.48 ± 0.05 | 0.53 ± 0.08 | <0.001 |
| Urea reduction ratio (URR), % | 0.77 ± 0.07 | 0.77 ± 0.08 | 0.78 ± 0.07 | 0.78 ± 0.07 | 0.809 |
| KT/V Daugirdas | 1.83 ± 0.40 | 1.81 ± 0.38 | 1.85 ± 0.44 | 1.83 ± 0.37 | 0.829 |
| Normalized protein catabolic rate (nPCR), g/(kg·d) | 1.21 ± 0.48 | 1.17 ± 0.39 | 1.20 ± 0.46 | 1.27 ± 0.57 | 0.442 |
| Time-averaged concentration of blood urea nitrogen (TACurea), mmol/L | 38.22 ± 10.35 | 39.63 ± 9.71 | 38.36 ± 10.21 | 36.62 ± 11.05 | 0.251 |
| Hematological data | |||||
| White blood cell counts, 1000/μL | 6.41 ± 1.88 | 7.38 ± 1.89 | 6.45 ± 1.57 | 5.39 ± 1.65 | <0.001 |
| Hemoglobin, g/dL | 10.81 ± 1.35 | 11.07 ± 1.14 | 10.91 ± 1.44 | 10.43 ± 1.38 | 0.017 |
| Platelets, 1000/μL | 182.76 ± 64.28 | 232.48 ± 61.76 | 183.73 ± 46.95 | 131.29 ± 36.05 | <0.001 |
| Biochemical data | |||||
| Aspartate aminotransferase, U/L | 21.22 ± 13.56 | 13.77 ± 7.01 | 20.00 ± 7.14 | 30.03 ± 18.00 | <0.001 |
| Alanine aminotransferase, U/L | 20.42 ± 13.71 | 18.17 ± 12.30 | 19.77 ± 11.43 | 23.37 ± 16.57 | 0.084 |
| Total bilirubin, mg/dL | 0.30 ± 0.17 | 0.27 ± 0.14 | 0.31 ± 0.16 | 0.33 ± 0.21 | 0.106 |
| Albumin, g/dL | 4.03 ± 0.42 | 4.12 ± 0.36 | 4.08 ± 0.33 | 3.89 ± 0.52 | 0.003 |
| Glucose, AC, mg/dL | 112.48 ± 50.66 | 113.83 ± 45.91 | 111.65 ± 44.81 | 111.95 ± 60.64 | 0.965 |
| Glycated hemoglobin A1c, % | 7.56 ± 1.51 | 7.89 ± 1.23 | 7.66 ± 1.63 | 7.01 ± 1.60 | 0.183 |
| Blood urea nitrogen before hemodialysis, mg/dL | 65.29 ± 16.14 | 67.81 ± 13.97 | 67.02 ± 15.82 | 60.98 ± 17.79 | 0.029 |
| Creatinine, mg/dL | 10.72 ± 2.57 | 11.60 ± 2.88 | 10.88 ± 2.28 | 9.66 ± 2.16 | <0.001 |
| Uric acid, mg/dL | 7.01 ± 1.39 | 7.22 ± 1.39 | 7.12 ± 1.24 | 6.67 ± 1.50 | 0.055 |
| Sodium, mEq/L | 137.77 ± 2.88 | 137.47 ± 2.71 | 138.21 ± 2.94 | 137.63 ± 2.99 | 0.300 |
| Potassium, mEq/L | 4.78 ± 0.69 | 4.95 ± 0.68 | 4.78 ± 0.66 | 4.60 ± 0.69 | 0.015 |
| Corrected calcium, mg/dL | 10.00 ± 1.01 | 10.16 ± 0.98 | 10.00 ± 1.06 | 9.82 ± 0.97 | 0.146 |
| Phosphate, mg/dL | 4.84 ± 1.44 | 5.36 ± 1.38 | 5.11 ± 1.35 | 4.04 ± 1.24 | <0.001 |
| Aluminum, g/dL | 1.10 ± 1.17 | 1.17 ± 1.38 | 0.98 ± 0.71 | 1.16 ± 1.32 | 0.555 |
| Iron, μg/dL | 70.52 ± 30.22 | 69.61 ± 28.85 | 72.28 ± 33.28 | 69.47 ± 28.30 | 0.844 |
| Total iron-binding capacity, μg/dL | 262.03 ± 53.85 | 278.48 ± 51.75 | 260.88 ± 45.43 | 246.32 ± 60.43 | 0.005 |
| Ferritin, ng/mL | 246.68 ± 258.11 | 168.83 ± 174.81 | 230.80 ± 237.54 | 345.39 ± 318.45 | 0.001 |
| Intact parathyroid hormone, pg/mL | 297.47 ± 384.64 | 342.29 ± 286.70 | 351.61 ± 565.74 | 197.00 ± 170.87 | 0.035 |
| High-sensitivity C-reactive protein, mg/L | 5.86 ± 11.26 | 6.03 ± 9.62 | 5.50 ± 12.40 | 6.04 ± 11.74 | 0.952 |
| Total cholesterol, mg/dL | 162.61 ± 35.33 | 173.70 ± 36.46 | 165.67 ± 33.03 | 148.25 ± 31.91 | <0.001 |
| Triglyceride, mg/dL | 133.34 ± 75.85 | 159.62 ± 89.82 | 123.35 ± 65.86 | 116.78 ± 62.66 | 0.002 |
| High-density lipoprotein, mg/dL | 45.23 ± 13.20 | 44.80 ± 14.10 | 47.77 ± 11.70 | 43.09 ± 13.45 | 0.121 |
| Low-density lipoprotein, mg/dL | 90.71 ± 29.46 | 96.98 ± 32.56 | 93.18 ± 27.90 | 81.82 ± 25.78 | 0.009 |
| Significant Variables | Cox Univariate Analysis | Cox Multivariate Hazards Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | aHR (95% CI) | p | |
| Body weight after hemodialysis, kg (each increase of 1 kg) | 1.038 (1.007–1.069) | 0.015 | ||
| Diabetes mellitus, yes | 7.297 (2.829–18.819) | <0.001 | 6.455 (2.466–16.893) | <0.001 |
| Hemodialysis duration, year (each increase of 1 year) | 0.925 (0.861–0.992) | 0.030 | ||
| Urea reduction ratio (URR), % (each increase of 1%) | 0.005 (0.000–0.342) | 0.013 | ||
| KT/V Daugirdas | 0.256 (0.082–0.801) | 0.019 | ||
| Time-averaged concentration of blood urea nitrogen (TACurea), mmol/L (each increase of 1 mmol/L) | 1.045 (1.005–1.086) | 0.027 | 1.056 (1.010–1.104) | 0.016 |
| White blood cell counts, 1000/μL (each increase of 1000/μL) | 1.301 (1.083–1.563) | 0.005 | ||
| Total bilirubin, mg/dL (each increase of 1 mg/dL) | 7.871 (1.345–46.071) | 0.022 | 19.360 (2.619–143.086) | 0.04 |
| Glucose, AC, mg/dL (each increase of 1 mg/dL) | 1.009 (1.003–1.016) | 0.005 | ||
| Corrected calcium, mg/dL (each increase of 1 mg/dL) | 0.550 (0.338–0.893) | 0.016 | ||
| High-density lipoprotein, mg/dL (each increase of 1 mg/dL) | 0.962 (0.925–1.000) | 0.048 | ||
| FIB-4 | 1.222 (0.865–1.728) | 0.256 | ||
| Significant Variables | Cox Univariate Analysis | Cox Multivariate Hazards Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | aHR (95% CI) | p | |
| Age, year (each increase of 1 year) | 1.064 (1.031–1.097) | <0.001 | ||
| Diabetes mellitus, yes | 3.427 (1.703–6.893) | 0.001 | 5.688 (2.358–13.720) | <0.001 |
| Hemoglobin, g/dL (each increase of 1 g/dL) | 0.542 (0.395–0.743) | <0.001 | 0.524 (0.369–0.745) | <0.001 |
| Aspartate aminotransferase, U/L (each increase of 1 U/L) | 1.021 (1.006–1.035) | 0.005 | ||
| Total bilirubin, mg/dL (each increase of 1 mg/dL) | 6.575 (1.425–30.344) | 0.016 | ||
| Albumin, g/dL (each increase of 1 g/dL) | 0.304 (0.192–0.481) | <0.001 | 0.538 (0.296–0.978) | 0.042 |
| Glucose, AC, mg/dL (each increase of 1 mg/dL) | 1.007 (1.004–1.011) | <0.001 | ||
| Blood urea nitrogen before hemodialysis, mg/dL (each increase of 1 mg/dL) | 0.968 (0.945–0.992) | 0.009 | ||
| Creatinine, mg/dL (each increase of 1 mg/dL) | 0.684 (0.585–0.800) | <0.001 | ||
| Sodium, mEq/L (each increase of 1 mEq/L) | 0.862 (0.769–0.967) | 0.011 | ||
| Potassium, mEq/L (each increase of 1 mEq/L) | 0.430 (0.239–0.774) | 0.005 | ||
| Corrected calcium, mg/dL (each increase of 1 mg/dL) | 0.588 (0.398–0.868) | 0.008 | ||
| Phosphate, mg/dL (each increase of 1 mg/dL) | 0.689 (0.520–0.913) | 0.010 | ||
| Total iron-binding capacity, μg/dL (each increase of 1 μg/dL) | 0.992 (0.985–1.000) | 0.049 | ||
| Ferritin, ng/mL (each increase of 1 ng/mL) | 1.002 (1.001–1.003) | 0.001 | ||
| High-sensitivity C-reactive protein, mg/L (each increase of 1 mg/L) | 1.020 (1.002–1.038) | 0.029 | ||
| Total cholesterol, mg/dL (each increase of 1 mg/dL) | 0.988 (0.977–0.999) | 0.026 | ||
| High-density lipoprotein, mg/dL (each increase of 1 mg/dL) | 0.962 (0.933–0.993) | 0.015 | ||
| FIB-4 | 1.683 (1.414–2.003) | <0.001 | 1.589 (1.262–2.001) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-H.; Yen, C.-L.; Jeng, W.-J.; Hung, C.-C.; Hsiao, C.-C.; Tian, Y.-C.; Chen, K.-H. Fibrosis-4 Score Is Associated with Mortality in Hemodialysis Patients with Chronic Viral Hepatitis: A Retrospective Study. Diagnostics 2024, 14, 2048. https://doi.org/10.3390/diagnostics14182048
Liu H-H, Yen C-L, Jeng W-J, Hung C-C, Hsiao C-C, Tian Y-C, Chen K-H. Fibrosis-4 Score Is Associated with Mortality in Hemodialysis Patients with Chronic Viral Hepatitis: A Retrospective Study. Diagnostics. 2024; 14(18):2048. https://doi.org/10.3390/diagnostics14182048
Chicago/Turabian StyleLiu, Hao-Hsuan, Chieh-Li Yen, Wen-Juei Jeng, Cheng-Chieh Hung, Ching-Chung Hsiao, Ya-Chung Tian, and Kuan-Hsing Chen. 2024. "Fibrosis-4 Score Is Associated with Mortality in Hemodialysis Patients with Chronic Viral Hepatitis: A Retrospective Study" Diagnostics 14, no. 18: 2048. https://doi.org/10.3390/diagnostics14182048
APA StyleLiu, H.-H., Yen, C.-L., Jeng, W.-J., Hung, C.-C., Hsiao, C.-C., Tian, Y.-C., & Chen, K.-H. (2024). Fibrosis-4 Score Is Associated with Mortality in Hemodialysis Patients with Chronic Viral Hepatitis: A Retrospective Study. Diagnostics, 14(18), 2048. https://doi.org/10.3390/diagnostics14182048




