Diagnostic and Prognostic Utilities of Pancreatic Stone Protein in Patients with Suspected Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of PSP Level
2.3. Statistical Analysis
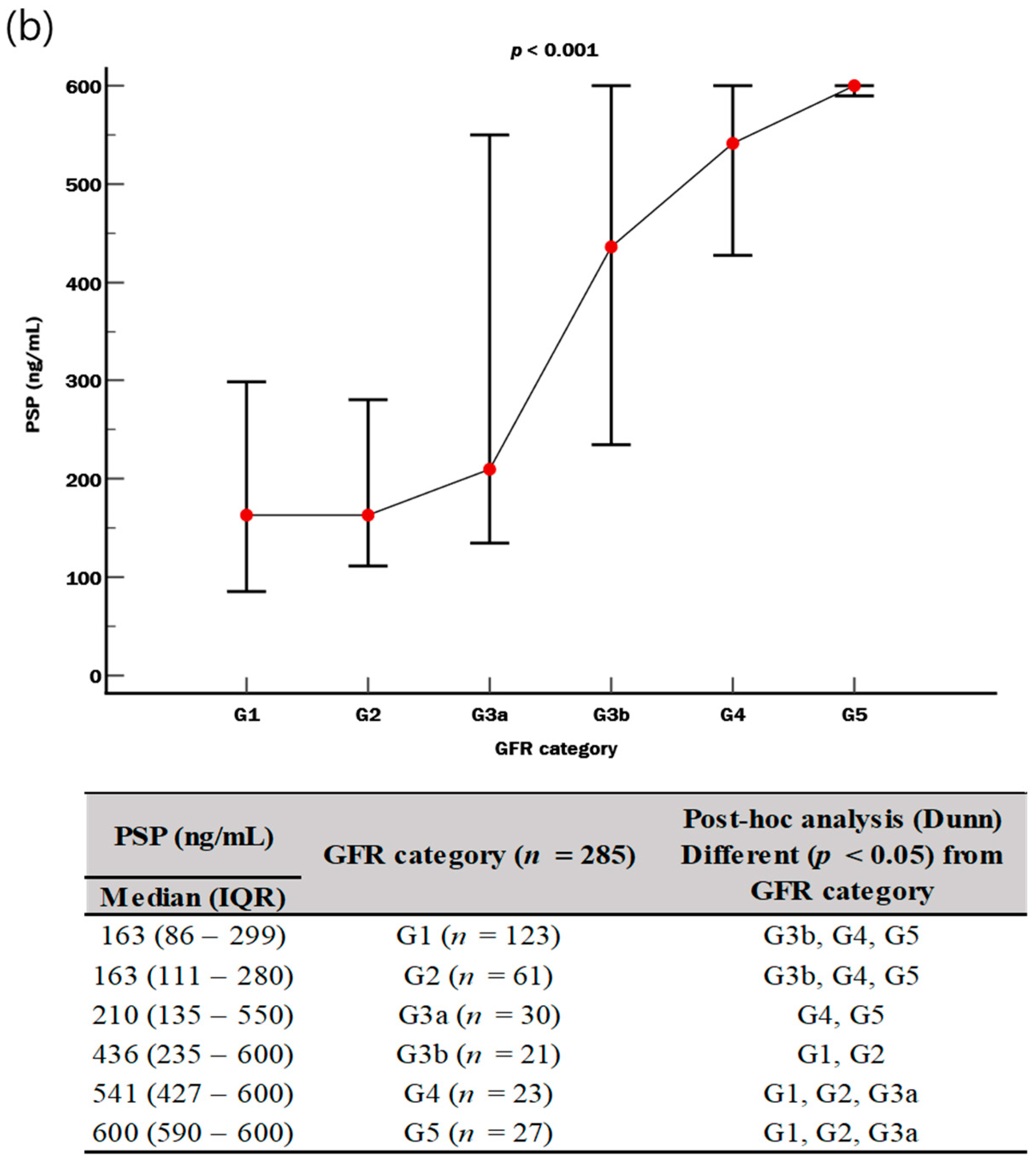
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis—A narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef]
- Gregoriano, C.; Heilmann, E.; Molitor, A.; Schuetz, P. Role of procalcitonin use in the management of sepsis. J. Thorac. Dis. 2020, 12, S5–S15. [Google Scholar] [CrossRef] [PubMed]
- Casserly, B.; Read, R.; Levy, M.M. Multimarker panels in sepsis. Crit. Care Clin. 2011, 27, 391–405. [Google Scholar] [CrossRef]
- Hur, M.; Kim, H.; Lee, S.; Cristofano, F.; Magrini, L.; Marino, R.; Gori, C.S.; Bongiovanni, C.; Zancla, B.; Cardelli, P.; et al. Diagnostic and prognostic utilities of multimarkers approach using procalcitonin, B-type natriuretic peptide, and neutrophil gelatinase-associated lipocalin in critically ill patients with suspected sepsis. BMC Infect. Dis. 2014, 14, 224. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Hur, M.; Moon, H.W.; Yun, Y.M.; Di Somma, S. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann. Intensive Care 2017, 7, 27. [Google Scholar] [CrossRef]
- Lee, C.W.; Kou, H.W.; Chou, H.S.; Chou, H.H.; Huang, S.F.; Chang, C.H.; Wu, C.H.; Yu, M.C.; Tsai, H.I. A combination of SOFA score and biomarkers gives a better prediction of septic AKI and in-hospital mortality in critically ill surgical patients: A pilot study. World J. Emerg. Surg. 2018, 13, 41. [Google Scholar] [CrossRef]
- Song, J.; Moon, S.; Park, D.W.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock: A prospective observational study according to the sepsis-3 definitions. Medicine 2020, 99, e20495. [Google Scholar] [CrossRef]
- Fidalgo, P.; Nora, D.; Coelho, L.; Povoa, P. Pancreatic stone protein: Review of a new biomarker in sepsis. J. Clin. Med. 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Graf, R. Pancreatic stone protein—Sepsis and the riddles of the exocrine pancreas. Pancreatology 2020, 20, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.X.; Hayakawa, T.; Ko, S.B.; Ishiguro, H.; Kitagawa, M. Pancreatic stone protein/regenerating protein family in pancreatic and gastrointestinal diseases. Intern. Med. 2011, 50, 1507–1516. [Google Scholar] [CrossRef]
- Keel, M.; Härter, L.; Reding, T.; Sun, L.K.; Hersberger, M.; Seifert, B.; Bimmler, D.; Graf, R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit. Care Med. 2009, 37, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Nata, K.; Noguchi, N.; Narushima, Y.; Akiyama, T.; Ikeda, T.; Nakagawa, K.; Takasawa, S.; Okamoto, H. Production and characterization of Reg knockout mice: Reduced proliferation of pancreatic beta-cells in Reg knockout mice. Diabetes 2002, 51 (Suppl. S3), S478–S483. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Raptis, D.; Li, X.; Li, F.; Chen, B.; He, J.; Graf, R.; Sun, Z. Pancreatic stone protein/regenerating protein (PSP/reg): A novel secreted protein up-regulated in type 2 diabetes mellitus. Endocrine 2015, 48, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dong, B.; Reding, T.; Peng, Y.; Lin, H.; Zhi, M.; Han, M.; Graf, R.; Li, L. Association of serum PSP/REG Iα with renal function in pregnant women. Biomed. Res. Int. 2019, 2019, 6970890. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). QuikRead Go for C-Reactive Protein Testing in Primary Care 2019. Available online: https://www.nice.org.uk/advice/mib78 (accessed on 2 January 2024).
- National Institute for Health and Care Excellence (NICE). Sepsis: Recognition, Diagnosis and Early Management. Available online: https://www.nice.org.uk/guidance/ng51 (accessed on 2 January 2024).
- Unterberg, M.; Rahmel, T.; Rump, K.; Wolf, A.; Haberl, H.; von Busch, A.; Bergmann, L.; Bracht, T.; Zarbock, A.; Ehrentraut, S.F.; et al. The impact of the COVID-19 pandemic on non-COVID induced sepsis survival. BMC Anesthesiol. 2022, 22, 12. [Google Scholar] [CrossRef]
- Abionic. IVD CAPSULE PSP. Available online: https://www.abionic.com/en/products (accessed on 11 January 2024).
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 64, 1270–1275. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Nagashima, K.; Noma, H.; Sato, Y.; Gosho, M. Sample size calculations for single-arm survival studies using transformations of the Kaplan-Meier estimator. Pharm. Stat. 2021, 20, 499–511. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Deo, S.V.; Deo, V.; Sundaram, V. Survival analysis—Part 2: Cox proportional hazards model. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 229–233. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Szabo, B.G.; Kiss, R.; Lenart, K.S.; Marosi, B.; Vad, E.; Lakatos, B.; Ostorhazi, E. Clinical and microbiological characteristics and outcomes of community-acquired sepsis among adults: A single center, 1-year retrospective observational cohort study from Hungary. BMC Infect. Dis. 2019, 19, 584. [Google Scholar] [CrossRef]
- Kuitunen, I.; Ponkilainen, V.T.; Uimonen, M.M.; Eskelinen, A.; Reito, A. Testing the proportional hazards assumption in cox regression and dealing with possible non-proportionality in total joint arthroplasty research: Methodological perspectives and review. BMC Musculoskelet. Disord. 2021, 22, 489. [Google Scholar] [CrossRef]
- Yu, S.; Song, S.A.; Jun, K.R.; Park, H.Y.; Lee, J.N. Clinical performance of monocyte distribution width for early detection of sepsis in emergency department patients: A prospective study. Ann. Lab. Med. 2022, 42, 286–289. [Google Scholar] [CrossRef]
- Kim, H.; Hur, M.; Struck, J.; Bergmann, A.; Di Somma, S. Proenkephalin predicts organ failure, renal replacement therapy, and mortality in patients with sepsis. Ann. Lab. Med. 2020, 40, 466–473. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, H.; Niwa, T.; Shikano, M.; Naruse, S.; Kitagawa, M.; Nakae, Y.; Ishiguro, H.; Kondo, T.; Hayakawa, T. Urinary excretion of pancreatic stone protein in diabetic nephropathy. Intern. Med. 1998, 37, 500–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vonzun, L.; Brun, R.; Gadient-Limani, N.; Schneider, M.A.; Reding, T.; Graf, R.; Limani, P.; Ochsenbein-Kölble, N. Serum pancreatic stone protein reference values in healthy pregnant women: A prospective cohort study. J. Clin. Med. 2023, 12, 3200. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.C.; Pickinpaugh, J. Physiologic changes in pregnancy. Surg. Clin. N. Am. 2008, 88, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Giannoni, E.; Wellmann, S.; Stocker, M.; Ammann, R.A.; Graf, R. Normal values for pancreatic stone protein in different age groups. BMC Anesthesiol. 2015, 15, 168. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Jia, D.; Graf, R.; Yang, J. Elevated serum level of pancreatic stone protein/regenerating protein (PSP/reg) is observed in diabetic kidney disease. Oncotarget 2017, 8, 38145–38151. [Google Scholar] [CrossRef]
- Pugin, J.; Daix, T.; Pagani, J.L.; Morri, D.; Giacomucci, A.; Dequin, P.F.; Guitton, C.; Que, Y.A.; Zani, G.; Brealey, D.; et al. Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: A prospective multicentric study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef]
- Van Singer, M.; Brahier, T.; Brochu Vez, M.J.; Gerhard Donnet, H.; Hugli, O.; Boillat-Blanco, N. Pancreatic stone protein for early mortality prediction in COVID-19 patients. Crit. Care 2021, 25, 267. [Google Scholar] [CrossRef]
- Melegari, G.; Giuliani, E.; Di Pietro, G.; Alberti, F.; Campitiello, M.; Bertellini, E.; Consortium; Barbieri, A. Point-of-care pancreatic stone protein measurement in critically ill COVID-19 patients. BMC Anesthesiol. 2023, 23, 226. [Google Scholar] [CrossRef]
- Bottari, G.; Caruso, M.; Paionni, E.; De Luca, M.; Romani, L.; Pisani, M.; Grandin, A.; Gargiullo, L.; Zampini, G.; Gagliardi, C.; et al. Accuracy of pancreatic stone protein for diagnosis of sepsis in children admitted to pediatric intensive care or high-dependency care: A pilot study. Ital. J. Pediatr. 2023, 49, 134. [Google Scholar] [CrossRef]
- Rowe, T.A.; McKoy, J.M. Sepsis in older adults. Infect. Dis. Clin. N. Am. 2017, 31, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Zamanipoor Najafabadi, A.H.; Ramspek, C.L.; Dekker, F.W.; Heus, P.; Hooft, L.; Moons, K.G.M.; Peul, W.C.; Collins, G.S.; Steyerberg, E.W.; van Diepen, M. TRIPOD statement: A preliminary pre-post analysis of reporting and methods of prediction models. BMJ Open 2020, 10, e041537. [Google Scholar] [CrossRef] [PubMed]
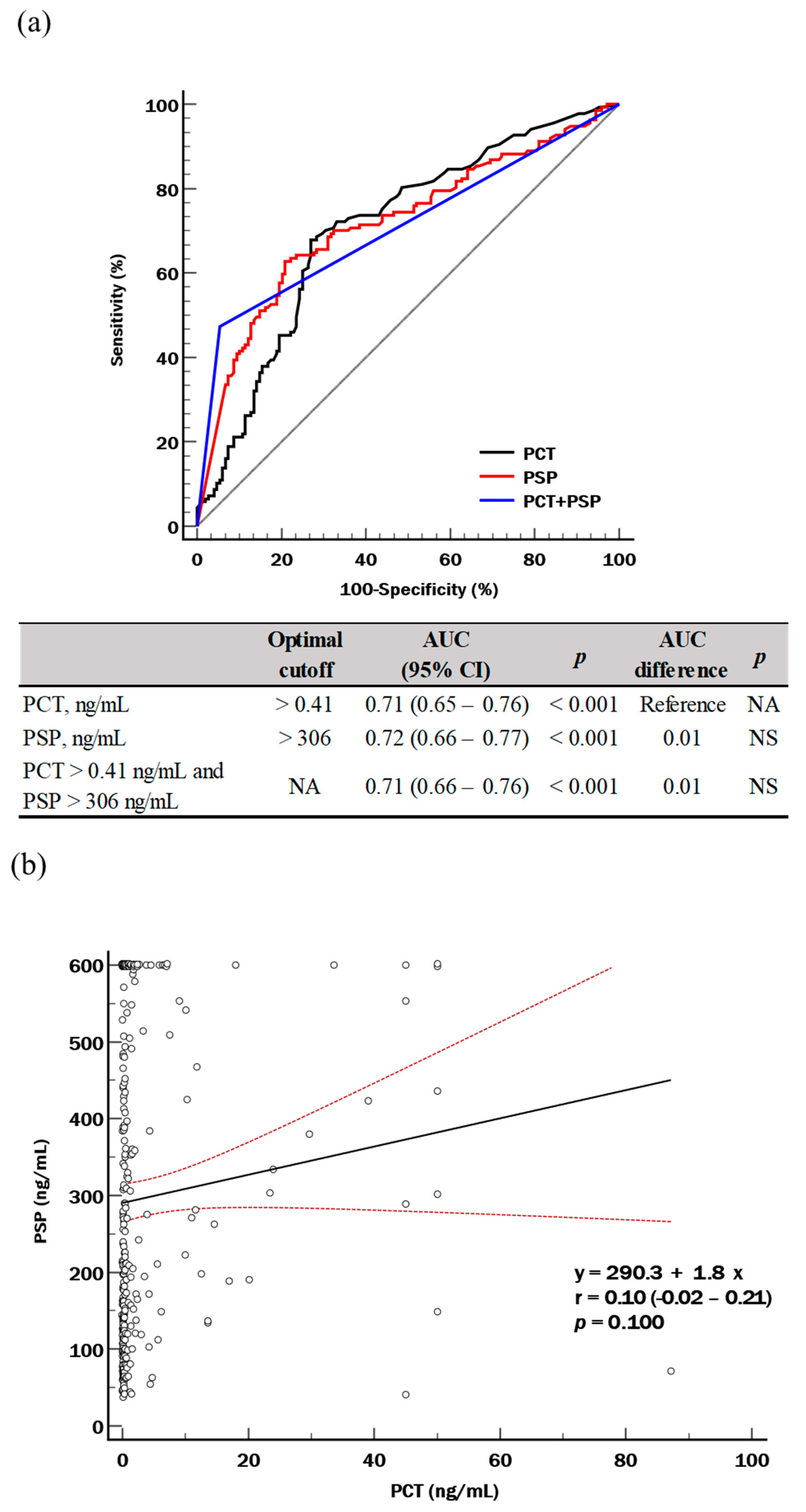
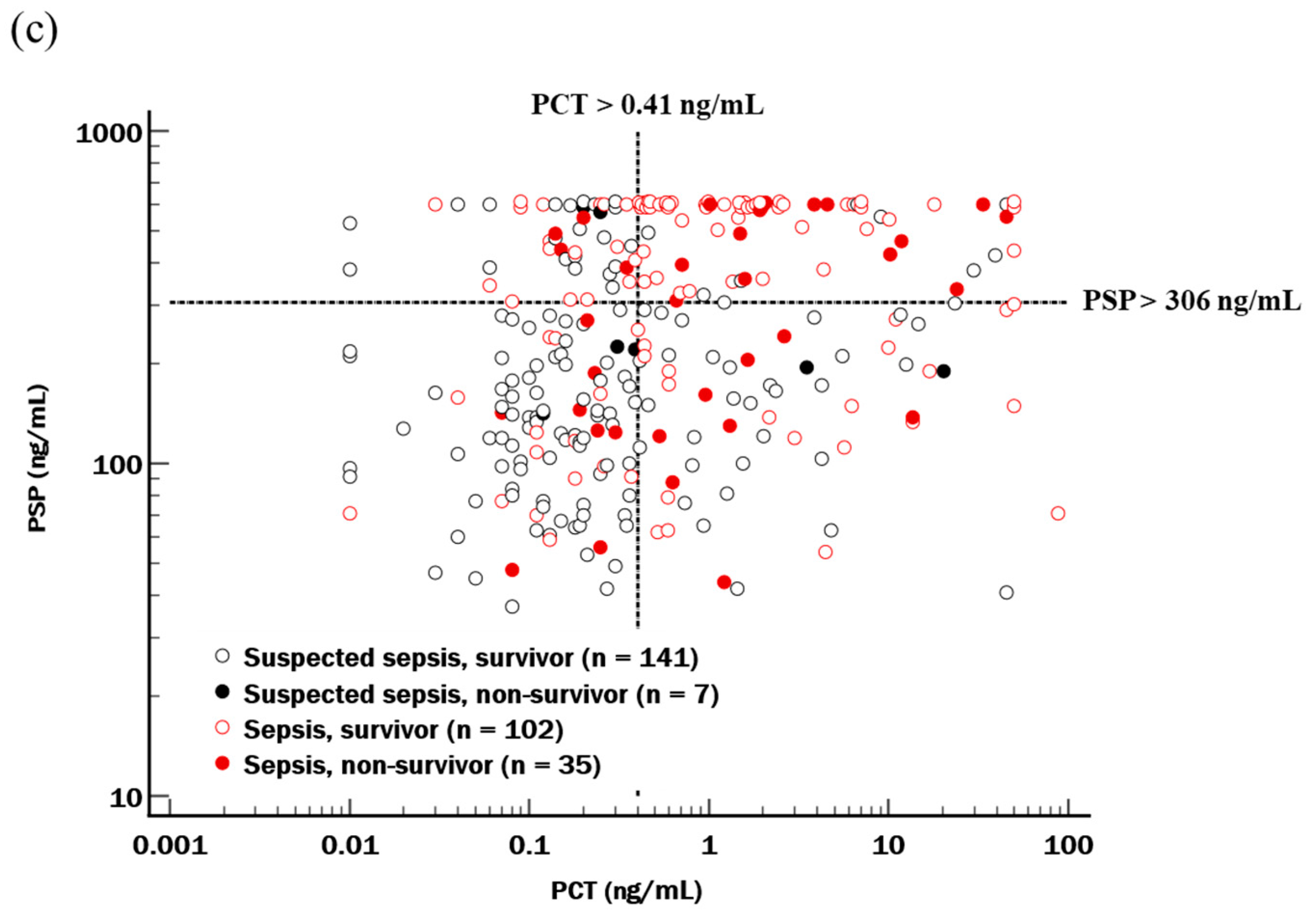

| Total (n = 285) | Suspected Sepsis (n = 148) | Sepsis * (n = 137) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 68 (59–79) | 64 (53–75) | 74 (64–83) | <0.001 |
| Female, n (%) | 114 (40.0) | 64 (43.2) | 50 (36.5) | NS |
| Comorbidities, n (%) | ||||
| Malignancy | 125 (43.9) | 65 (43.9) | 60 (43.8) | NS |
| Hypertension | 121 (42.5) | 52 (35.1) | 69 (50.4) | 0.012 |
| Diabetes mellitus | 89 (31.2) | 41 (27.7) | 48 (35.0) | NS |
| Chronic kidney disease | 43 (15.1) | 8 (5.4) | 35 (25.5) | 0.012 |
| Cerebrovascular accident | 39 (13.7) | 9 (6.1) | 30 (21.9) | <0.001 |
| Chronic heart failure | 25 (8.8) | 7 (4.7) | 18 (13.1) | 0.020 |
| Dementia | 19 (6.7) | 4 (2.7) | 15 (10.9) | 0.001 |
| AIDS | 2 (0.7) | 2 (1.3) | 0 (0.0) | NS |
| Patient enrollment ** | ||||
| Admission to sampling (day) | 3 (1–12) | 3 (1–9) | 5 (1–21) | 0.011 |
| Postoperative state, n (%) | 6 (2.1) | 0 (0.0) | 6 (4.3) | NA |
| General ward, n (%) | 239 (83.9) | 132 (89.7) | 107 (78.1) | <0.001 |
| Emergency room, n (%) | 27 (16.1) | 14 (9.5) | 13 (9.5) | NS |
| Intensive care unit, n (%) | 19 (6.7) | 2 (1.4) | 17 (12.4) | <0.001 |
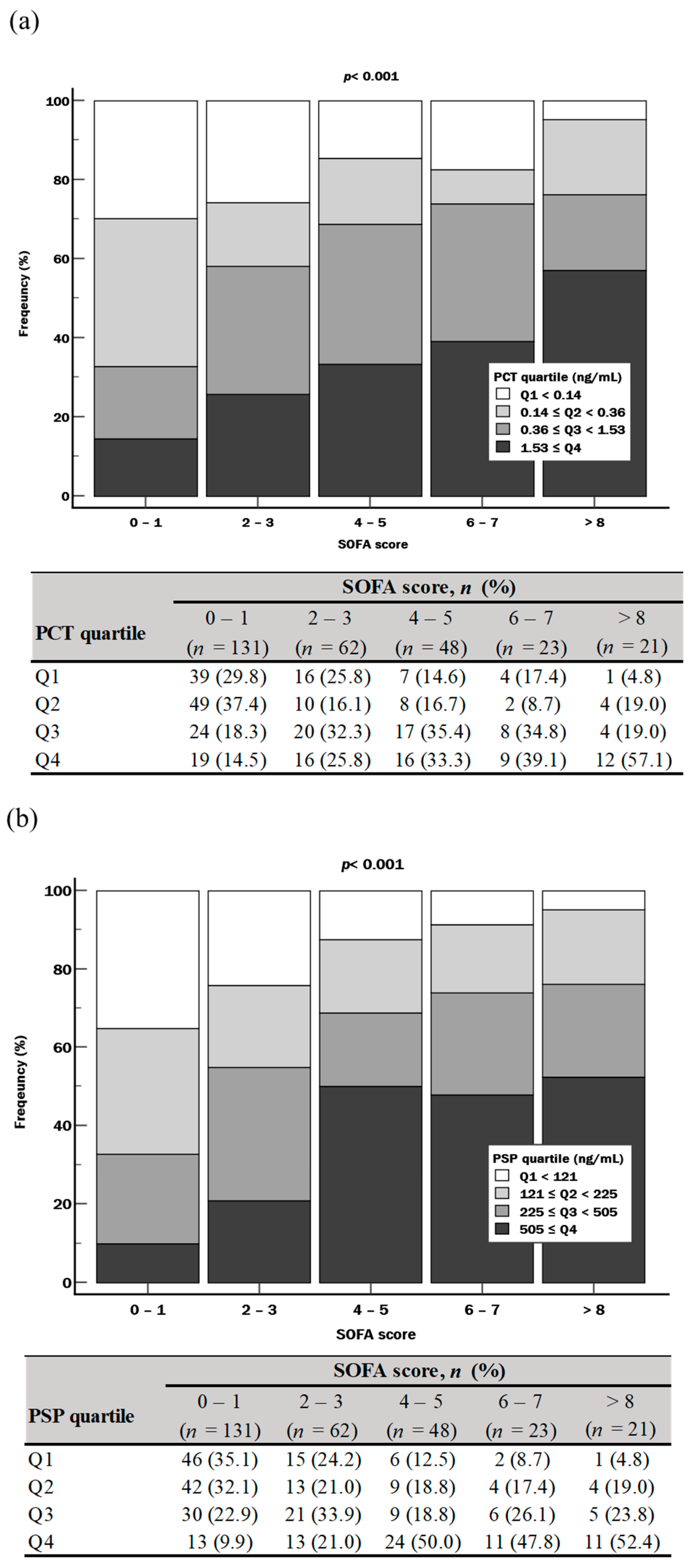
| SOFA score at enrollment | ||||
| Total | 2 (0–4) | 1 (0–1) | 4 (3–6) | <0.001 |
| Central nervous system | 0 (0–1) | 0 (0–0) | 1 (0–2) | <0.001 |
| Renal | 0 (0–1) | 0 (0–0) | 1 (0–2) | <0.001 |
| Respiratory | 0 (0–1) | 0 (0–0) | 1 (0–1) | <0.001 |
| Coagulation | 0 (0–0) | 0 (0–0) | 0 (0–1) | <0.001 |
| Circulatory | 0 (0–0) | 0 (0–0) | 0 (0–0) | <0.001 |
| Liver | 0 (0–0) | 0 (0–0) | 0 (0–1) | <0.001 |
| Clinical outcomes | ||||
| ICU admission, n (%) | 87 (29.8) | 29 (19.6) | 52 (40.9) | <0.001 |
| ICU stay (day) | 4 (2–19) | 2 (1–6) | 5 (2–34) | NS |
| Hospital stay (day) | 20 (10–46) | 13 (7–27) | 29 (13–54) | <0.001 |
| In-hospital mortality, n (%) | 42 (14.7) | 7 (4.7) | 35 (25.5) | <0.001 |
| 30-day mortality, n (%) | 21 (7.4) | 5 (3.4) | 16 (11.7) | <0.001 |
| Vasopressor use, n (%) | 34 (11.9) | 6 (4.1) | 28 (20.4) | <0.001 |
| KRT, n (%) | 24 (8.4) | 1 (0.7) | 23 (18.1) | <0.001 |
| Laboratory values at enrollment | ||||
| WBC (×109/L) | 10.6 (7.8–14.4) | 10.2 (7.4–14.1) | 11.0 (8.6–14.9) | NS |
| Hb (g/dL) | 10.1 (9.0–11.7) | 10.5 (9.3–12.7) | 10.0 (8.7–10.9) | <0.001 |
| PLT (×109/L) | 224 (157–289) | 249 (193–319) | 187 (128–259) | <0.001 |
| Lactate (mmol/L) *** | 1.8 (1.3–2.5) | 1.7 (1.2–2.3) | 1.8 (1.4–2.8) | NS |
| Total bilirubin (umol/L) | 0.7 (0.5–1.1) | 0.6 (0.4–0.9) | 0.8 (0.5–1.5) | <0.001 |
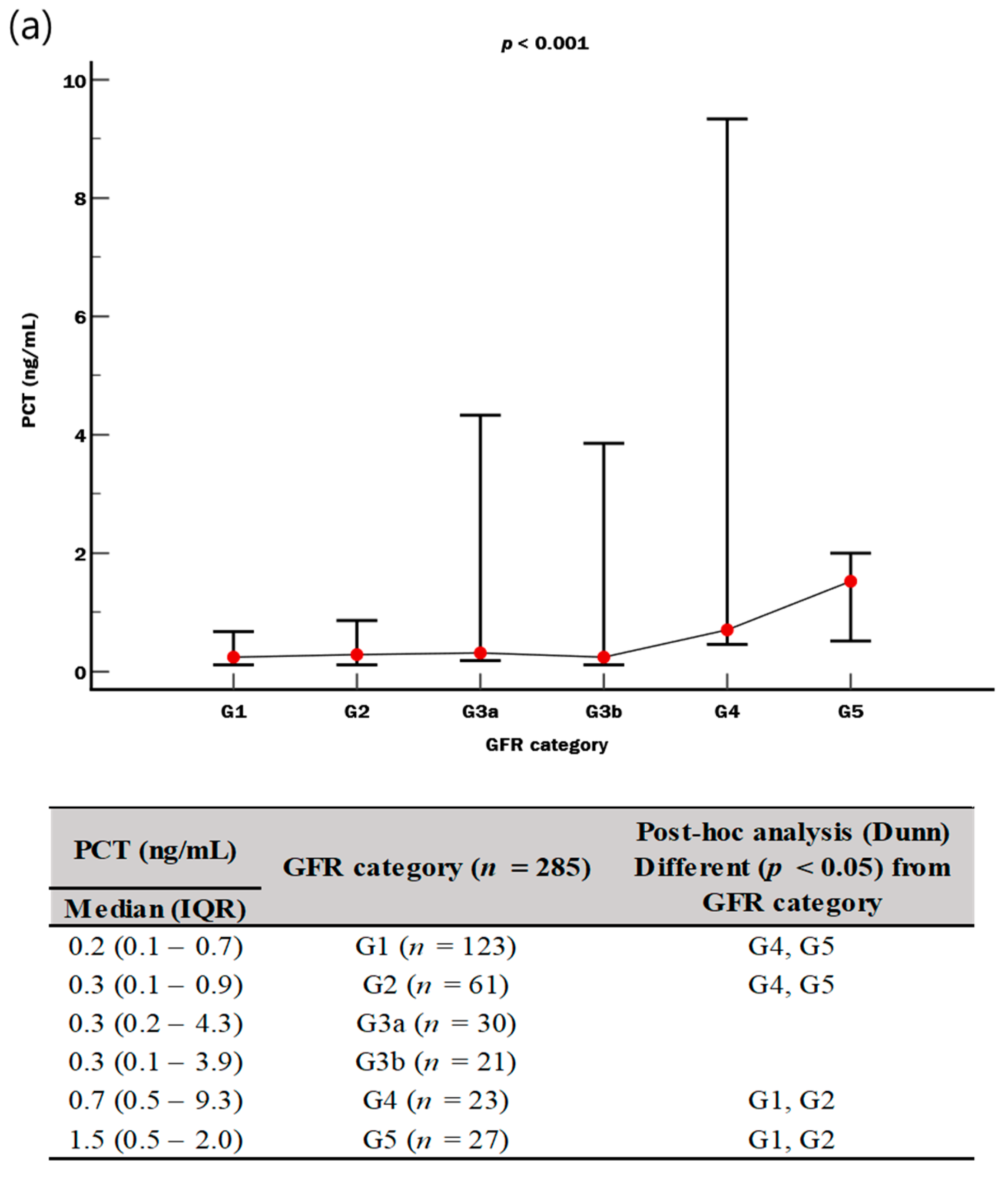
| Cr (μmol/L) | 0.9 (0.6–1.3) | 0.8 (0.6–1.0) | 1.3 (0.7–2.4) | <0.001 |
| eGFR (mL/min/1.73 m2) | 83 (46–101) | 93 (70–104) | 55 (20–94) | <0.001 |
| CRP (mg/L) | 15.9 (12.5–21.3) | 15.7 (12.2–22.1) | 15.8 (12.5–21.0) | NS |
| PCT (ng/mL) | 0.36 (0.14–1.53) | 0.20 (0.11–0.50) | 0.60 (0.26–2.50) | <0.001 |
| PSP (ng/mL) | 225 (121–506) | 164 (101–280) | 408 (162–730) | <0.001 |
| PCT Quartile, n (%) | PSP Quartile, n (%) | PCT and PSP Combination, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCT Q1 (n = 67) | PCT Q2–Q4 (n = 218) | p | PSP Q1 (n = 70) | PSP Q2–Q4 (n = 215) | p | PCT Q1 PSP Q1 (n = 27) | PCT Q2–Q4 PSP Q1 (n = 43) | PCT Q1 PSP Q2–Q4 (n = 40) | PCT Q2–Q4 PSP Q2–Q4 (n = 175) | p | |
| ICU admission (n = 87) | |||||||||||
| No | 43 (64.2) | 155 (71.1) | - | 53 (75.7) | 145 (67.4) | - | 20 (74.1) | 33 (76.7) | 23 (57.5) | 122 (69.7) | - |
| Yes | 24 (35.8) | 63 (28.9) | NS | 17 (24.3) | 70 (32.6) | NS | 7 (25.9) | 10 (23.3) | 17 (42.5) | 53 (30.3) | NS |
| In-hospital mortality (n = 42) | |||||||||||
| No | 64 (95.5) | 179 (82.1) | - | 66 (94.3) | 177 (82.3) | - | 25 (96.2) | 40 (93.0) | 38 (95.0) | 139 (79.4) | - |
| Yes | 3 (4.5) | 39 (17.9) | 0.007 | 4 (5.7) | 38 (17.7) | 0.014 | 1 (3.8) | 3 (7.0) | 2 (5.0) | 36 (20.6) | 0.002 |
| 30-day mortality (n = 21) | |||||||||||
| No | 66 (98.5) | 198 (90.8) | - | 69 (98.6) | 195 (90.7) | - | 27 (100.0) | 42 (97.7) | 39 (97.5) | 156 (89.1) | - |
| Yes | 1 (1.5) | 20 (9.2) | 0.036 | 1 (1.4) | 20 (9.3) | 0.029 | 0 (0.0) | 1 (2.3) | 1 (2.5) | 19 (10.9) | 0.041 |
| Vasopressor use (n = 34) | |||||||||||
| No | 56 (83.6) | 195 (89.4) | - | 64 (91.4) | 187 (87.0) | - | 23 (85.2) | 41 (95.3) | 33 (82.5) | 154 (88.0) | - |
| Yes | 11 (16.4) | 23 (10.6) | NS | 6 (8.6) | 28 (13.0) | NS | 4 (14.8) | 2 (4.7) | 7 (17.5) | 21 (12.0) | NS |
| KRT (n = 24) | |||||||||||
| No | 66 (98.5) | 195 (89.4) | - | 69 (98.6) | 192 (89.3) | - | 26 (96.3) | 43 (100.0) | 40 (100.0) | 152 (86.9) | - |
| Yes | 1 (1.5) | 23 (10.6) | 0.020 | 1 (1.4) | 23 (10.7) | 0.016 | 1 (3.7) | 0 (0.0) | 0 (0.0) | 23 (13.1) | 0.003 |
| * Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| No in-hospital mortality (n = 243) vs. In-hospital mortality (n = 42) | ||||||||||||
| Age > 65 | NS | NS | NS | - | - | - | NS | NS | NS | NS | NS | NS |
| Male | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Comorbidity number > 1 | NS | NS | NS | - | - | - | NS | NS | NS | NS | NS | NS |
| SOFA score > 1 | 3.676 | 1.279–10.562 | 0.016 | 3.787 | 1.191–9.941 | 0.022 | 3.466 | 1.197–10.031 | 0.022 | 3.441 | 1.191–9.941 | 0.022 |
| PCT > Q1 | NS | NS | NS | - | - | - | NS | NS | NS | - | - | - |
| PSP > Q1 | - | - | - | NS | NS | NS | NS | NS | NS | - | - | - |
| PCT > Q1 and PSP > Q1 | - | - | - | - | - | - | - | - | - | NS | NS | NS |
| log(t)*(age > 65) | - | - | - | - | - | - | - | - | - | 0.209 | 0.072–0.609 | 0.004 |
| log(t)*(male) | - | - | - | - | - | - | - | - | - | 0.078 | 0.015–0.406 | 0.002 |
| log(t)*(comorbidity number > 1) | - | - | - | - | - | - | - | - | - | 0.021 | 0.009–0.683 | 0.021 |
| log(t)*(SOFA score > 1) | - | - | - | - | - | - | - | - | - | NS | NS | NS |
| log(t)*(PCT > Q1 and PSP > Q1) | - | - | - | - | - | - | - | - | - | <0.001 | <0.001–0.001 | <0.001 |
| No 30-day mortality (n = 264) vs. 30-day mortality (n = 21) | ||||||||||||
| Age > 65 | NS | NS | NS | - | - | - | NS | NS | NS | NS | NS | NS |
| Male | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Comorbidity number > 1 | NS | NS | NS | - | - | - | NS | NS | NS | NS | NS | NS |
| SOFA score > 1 | 6.209 | 1.699–22.695 | 0.006 | 6.342 | 1.782–22.568 | 0.004 | 6.155 | 1.700–22.279 | 0.006 | 6.209 | 1.699–22.695 | 0.006 |
| PCT > Q1 | NS | NS | NS | - | - | - | NS | NS | NS | - | - | - |
| PSP > Q1 | - | - | - | NS | NS | NS | NS | NS | NS | - | - | - |
| PCT > Q1 and PSP > Q1 | - | - | - | - | - | - | - | - | - | NS | NS | NS |
| log(t)*(age > 65) | - | - | - | - | - | - | - | - | - | 0.057 | 0.013–0.242 | 0.004 |
| log(t)*(male) | - | - | - | - | - | - | - | - | - | 0.033 | 0.001–0.753 | 0.002 |
| log(t)*(comorbidity number > 1) | - | - | - | - | - | - | - | - | - | <0.001 | 0.002–0.130 | 0.021 |
| log(t)*(SOFA score > 1) | - | - | - | - | - | - | - | - | - | NS | NS | NS |
| log(t)*(PCT > Q1 and PSP > Q1) | - | - | - | - | - | - | - | - | - | 0.002 | <0.001–0.001 | <0.001 |
| No KRT (n = 261) vs. KRT (n = 24) | ||||||||||||
| Age > 65 | NS | NS | NS | - | - | - | NS | NS | NS | NS | NS | NS |
| Male | 0.224 | 0.066–0.757 | 0.016 | 0.223 | 0.066–0.752 | 0.016 | 0.225 | 0.067–0.756 | 0.016 | 0.225 | 0.067–0.756 | 0.016 |
| Comorbidity number > 1 | NS | NS | NS | - | - | - | NS | NS | NS | NS | NS | NS |
| SOFA score > 1 | 11.894 | 1.580–89.540 | 0.016 | 12.424 | 1.659–93.074 | 0.014 | 11.249 | 1.494–84.673 | 0.019 | 11.894 | 1.580–89.540 | 0.016 |
| PCT > Q1 | NS | NS | NS | - | - | - | NS | NS | NS | - | - | - |
| PSP > Q1 | - | - | - | NS | NS | NS | NS | NS | NS | - | - | - |
| PCT > Q1 and PSP > Q1 | - | - | - | - | - | - | - | - | - | NS | NS | NS |
| log(t)*(age > 65) | - | - | - | - | - | - | - | - | - | 0.020 | 0.023–0.165 | 0.005 |
| log(t)*(male) | - | - | - | - | - | - | - | - | - | 0.008 | 0.006–0.107 | 0.008 |
| log(t)*(comorbidity number > 1) | - | - | - | - | - | - | - | - | - | 0.043 | 0.002–0.656 | 0.021 |
| log(t)*(SOFA score > 1) | - | - | - | - | - | - | - | - | - | NS | NS | NS |
| log(t)*(PCT > Q1 and PSP > Q1) | - | - | - | - | - | - | - | - | - | <0.001 | <0.001–0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-H.; Kim, H.; Moon, H.-W.; Yun, Y.-M.; Park, M.; Lee, S.; Hur, M. Diagnostic and Prognostic Utilities of Pancreatic Stone Protein in Patients with Suspected Sepsis. Diagnostics 2024, 14, 2076. https://doi.org/10.3390/diagnostics14182076
Lee G-H, Kim H, Moon H-W, Yun Y-M, Park M, Lee S, Hur M. Diagnostic and Prognostic Utilities of Pancreatic Stone Protein in Patients with Suspected Sepsis. Diagnostics. 2024; 14(18):2076. https://doi.org/10.3390/diagnostics14182076
Chicago/Turabian StyleLee, Gun-Hyuk, Hanah Kim, Hee-Won Moon, Yeo-Min Yun, Mikyoung Park, Seungho Lee, and Mina Hur. 2024. "Diagnostic and Prognostic Utilities of Pancreatic Stone Protein in Patients with Suspected Sepsis" Diagnostics 14, no. 18: 2076. https://doi.org/10.3390/diagnostics14182076
APA StyleLee, G.-H., Kim, H., Moon, H.-W., Yun, Y.-M., Park, M., Lee, S., & Hur, M. (2024). Diagnostic and Prognostic Utilities of Pancreatic Stone Protein in Patients with Suspected Sepsis. Diagnostics, 14(18), 2076. https://doi.org/10.3390/diagnostics14182076








