Computed Tomography Evaluation of Coronary Atherosclerosis: The Road Travelled, and What Lies Ahead
Abstract
1. Introduction
2. Coronary Atherosclerosis
3. CCTA in the Context of Imaging Evaluation for CAD
3.1. Reductions in Radiation Exposure
3.2. Transition from Qualitative to Quantitative CCTA Evaluation
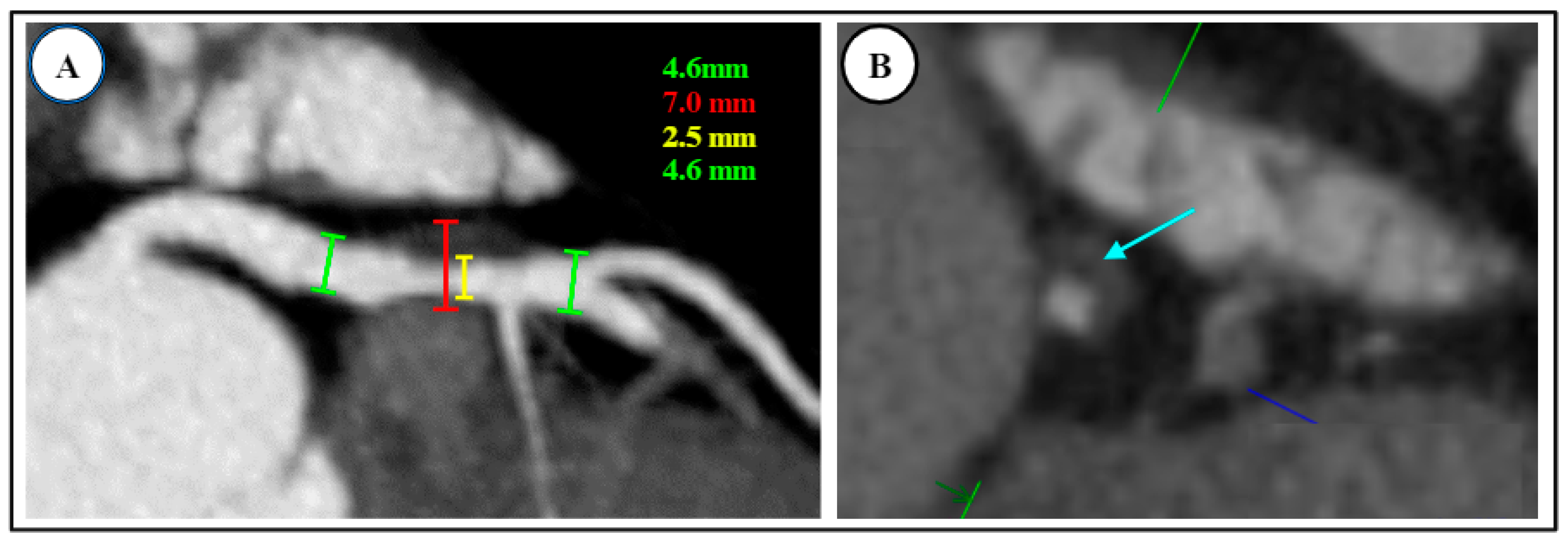
3.3. Plaque Characteristics
4. Functional Evaluation by CT
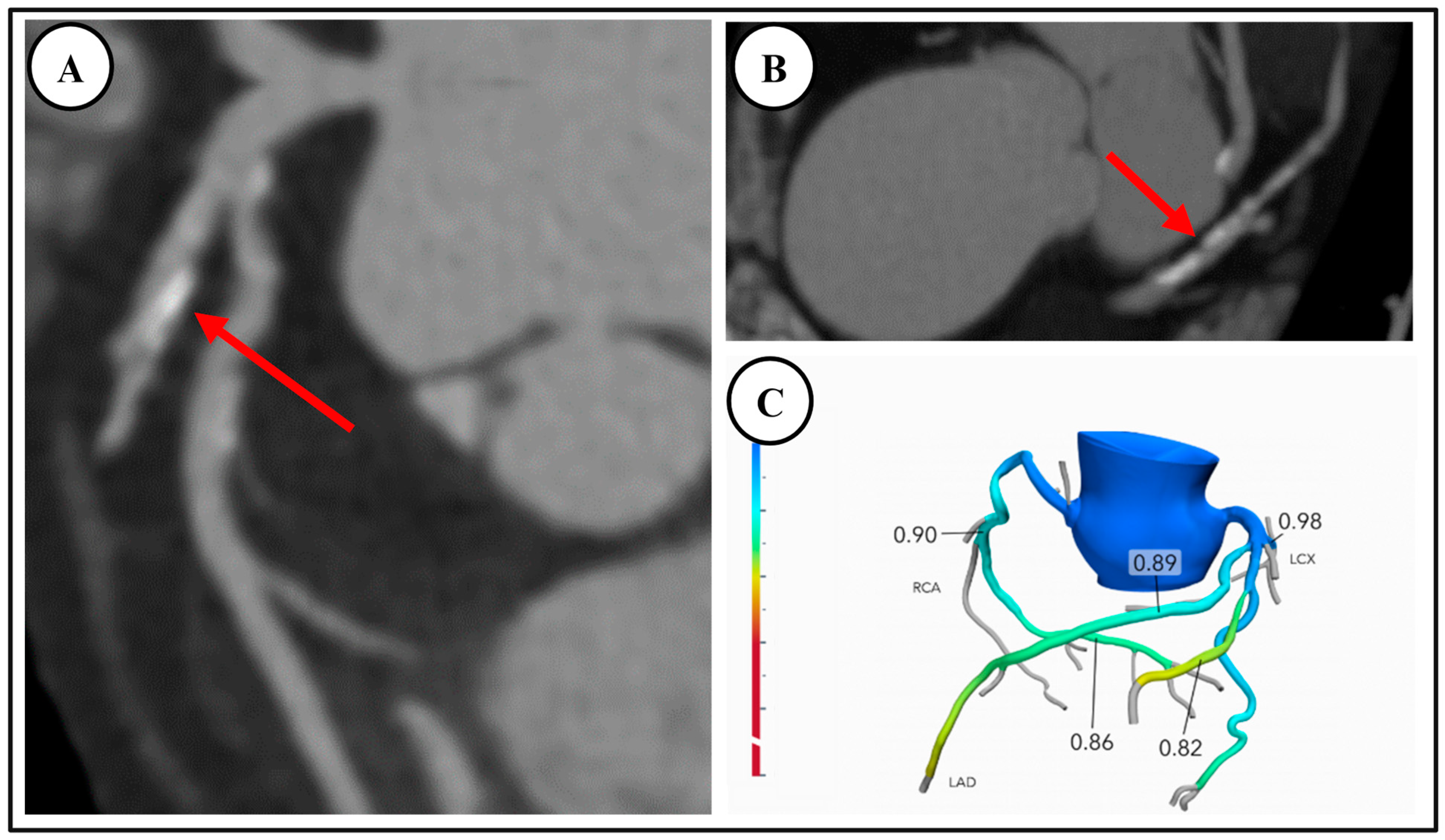
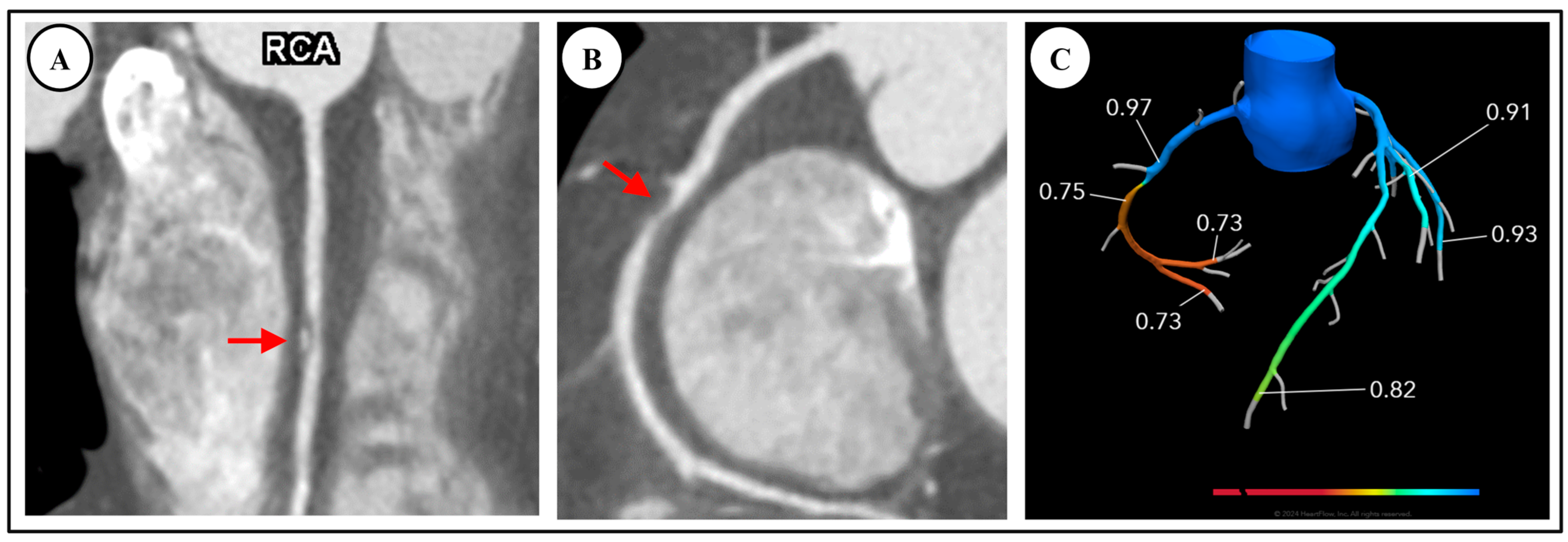
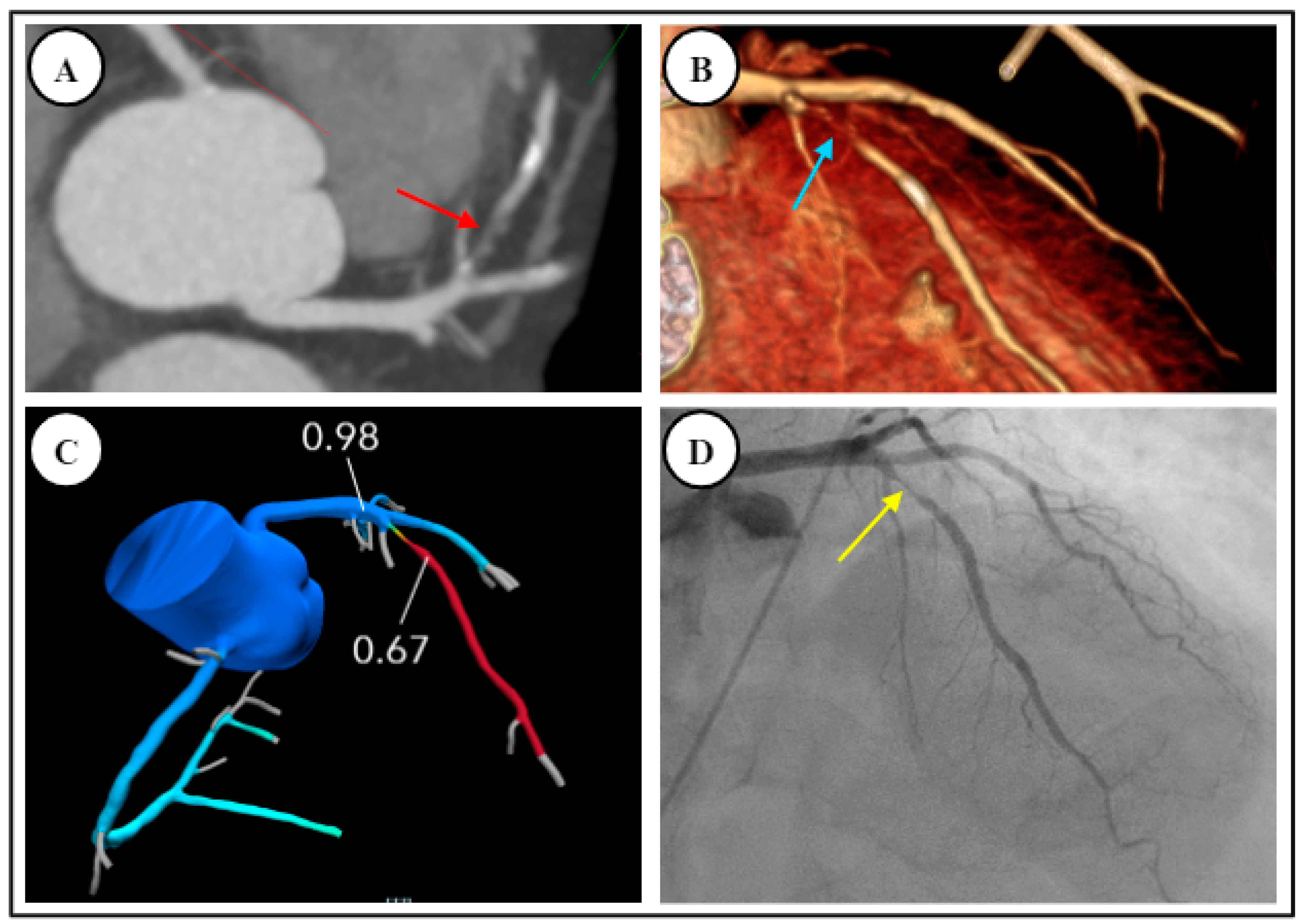
4.1. Fractional Flow Reserve—Computed Tomography (FFR-CT)
4.2. CT Perfusion Imaging (CTP)
5. Subclinical CAD by CCTA
Coronary Artery Calcium Score
6. Clinical Role of CCTA
6.1. CCTA in the Emergency Room
6.2. CCTA in the Outpatient Setting for Stable Chest Pain, Compared to Functional Testing
6.3. Socio-Economic Impact of CCTA on Clinical Management
7. Future Directions
7.1. Photon Counting CT (PCCT) Technology
7.2. Artificial Intelligence Applications in CCTA and CAC
8. Summary of Clinical Practice
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguade-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef]
- Williams, M.C.; Hunter, A.; Shah, A.S.; Assi, V.; Lewis, S.; Smith, J.; Berry, C.; Boon, N.A.; Clark, E.; Flather, M.; et al. Use of Coronary Computed Tomographic Angiography to Guide Management of Patients With Coronary Disease. J. Am. Coll. Cardiol. 2016, 67, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.; Pickett, C.; Bittencourt, M.S.; Villines, T.C.; Petrillo, S.; Di Carli, M.F.; Blankstein, R. Outcomes after coronary computed tomography angiography in the emergency department: A systematic review and meta-analysis of randomized, controlled trials. J. Am. Coll. Cardiol. 2013, 61, 880–892. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Cerrato, E.; Biondi-Zoccai, G.; Omede, P.; Sciuto, F.; Presutti, D.G.; Quadri, G.; Raff, G.L.; Goldstein, J.A.; Litt, H.; et al. Coronary computed tomographic angiography for detection of coronary artery disease in patients presenting to the emergency department with chest pain: A meta-analysis of randomized clinical trials. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Jeon, J.Y.; Kim, Y.; Kim, H.M.; Yoon, Y.E.; Lee, S.P.; Kim, H.K.; Sohn, D.W.; Sung, J.; Kim, Y.J. Statin therapy is associated with lower all-cause mortality in patients with non-obstructive coronary artery disease. Atherosclerosis 2015, 239, 335–342. [Google Scholar] [CrossRef]
- Chow, B.J.; Small, G.; Yam, Y.; Chen, L.; McPherson, R.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F.; et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: Results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter registry) registry. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 981–989. [Google Scholar] [CrossRef]
- Scot-Heart, I. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): An open-label, parallel-group, multicentre trial. Lancet 2015, 385, 2383–2391. [Google Scholar] [CrossRef]
- GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Writing, C.; Kontos, M.C.; de Lemos, J.A.; Deitelzweig, S.B.; Diercks, D.B.; Gore, M.O.; Hess, E.P.; McCarthy, C.P.; McCord, J.K.; Musey, P.I., Jr.; et al. 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1925–1960. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Twisk, J.W.R.; Norgaard, B.L.; Zarins, C.K.; Knaapen, P.; Min, J.K. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur. Heart J. 2017, 38, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Tonino, P.A.; Fearon, W.F.; De Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Ver Lee, P.N.; Maccarthy, P.A.; Van’t Veer, M.; Pijls, N.H. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.F.; Felix, N.; Melo, P.H.C.; Gauza, M.M.; Calomeni, P.; Generoso, G.; Khatri, S.; Altmayer, S.; Blankstein, R.; Bittencourt, M.S.; et al. Coronary Computed Tomography Angiography versus Invasive Coronary Angiography in Stable Chest Pain: A Meta-Analysis of Randomized Controlled Trials. Circ. Cardiovasc. Imaging 2023, 16, e015800. [Google Scholar] [CrossRef]
- Fischer, C.; Hulten, E.; Belur, P.; Smith, R.; Voros, S.; Villines, T.C. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: A meta-analysis. J. Cardiovasc. Comput. Tomogr. 2013, 7, 256–266. [Google Scholar] [CrossRef]
- Nikolaou, K.; Alkadhi, H.; Bamberg, F.; Leschka, S.; Wintersperger, B.J. MRI and CT in the diagnosis of coronary artery disease: Indications and applications. Insights Imaging 2011, 2, 9–24. [Google Scholar] [CrossRef]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatston, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. CAD-RADS: Coronary Artery Disease—Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J. Am. Coll. Radiol. 2016, 13, 1458–1466.e9. [Google Scholar] [CrossRef]
- Rybicki, F.J.; Udelson, J.E.; Peacock, W.F.; Goldhaber, S.Z.; Isselbacher, E.M.; Kazerooni, E.; Kontos, M.C.; Litt, H.; Woodard, P.K. 2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients With Chest Pain: A Joint Document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J. Am. Coll. Cardiol. 2016, 67, 853–879. [Google Scholar] [CrossRef]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.F.; Canan, A.; Xi, Y.; Litt, H.; Diercks, D.B.; Abbara, S.; Kay, F.U. Comparative Effectiveness of Coronary CT Angiography and Standard of Care for Evaluating Acute Chest Pain: A Living Systematic Review and Meta-Analysis. Radiol. Cardiothorac. Imaging 2023, 5, e230022. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Li, D.; Kazerooni, E.A.; Thomas, G.S.; Mieres, J.H.; Shaw, L.J. Diagnostic Accuracy of Noninvasive 64-row Computed Tomographic Coronary Angiography (CCTA) Compared with Myocardial Perfusion Imaging (MPI): The PICTURE Study, A Prospective Multicenter Trial. Acad. Radiol. 2017, 24, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef]
- Stocker, T.J.; Deseive, S.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Heckner, M.; Bax, J.J.; Fang, X.M.; Grove, E.L.; et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: Results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur. Heart J. 2018, 39, 3715–3723. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Knuuti, J.; Bengel, F.; Bax, J.J.; Kaufmann, P.A.; Le Guludec, D.; Perrone Filardi, P.; Marcassa, C.; Ajmone Marsan, N.; Achenbach, S.; Kitsiou, A.; et al. Risks and benefits of cardiac imaging: An analysis of risks related to imaging for coronary artery disease. Eur. Heart J. 2014, 35, 633–638. [Google Scholar] [CrossRef]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADS 2.0—2022 Coronary Artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). JACC Cardiovasc. Imaging 2022, 15, 1974–2001. [Google Scholar] [CrossRef]
- Boster, J.; Hull, R.; Williams, M.U.; Berger, J.; Sharp, A.; Fentanes, E.; Maroules, C.; Cury, R.; Thomas, D. Adoption of the Coronary Artery Disease-reporting and Data System: Reduced Downstream Testing and Cardiology Referral Rates in Patients with Non-obstructive Coronary Artery Disease. Cureus 2019, 11, e5708. [Google Scholar] [CrossRef]
- Thomsen, C.; Abdulla, J. Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kitagawa, T.; Ohashi, N.; Utsunomiya, H.; Kunita, E.; Oka, T.; Urabe, Y.; Tsushima, H.; Awai, K.; Kihara, Y. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J. Cardiovasc. Comput. Tomogr. 2013, 7, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, G.A.; Carrascosa, P.; Bruining, N.; Waksman, R.; Garcia-Garcia, H.M. Defining the non-vulnerable and vulnerable patients with computed tomography coronary angiography: Evaluation of atherosclerotic plaque burden and composition. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 481–491. [Google Scholar] [CrossRef]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Fukuda, S.; Tanaka, A.; Nakanishi, K.; Taguchi, H.; Yoshiyama, M.; Shimada, K.; Yoshikawa, J. Prognosis of vulnerable plaque on computed tomographic coronary angiography with normal myocardial perfusion image. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 332–340. [Google Scholar] [CrossRef]
- Otsuka, K.; Fukuda, S.; Tanaka, A.; Nakanishi, K.; Taguchi, H.; Yoshikawa, J.; Shimada, K.; Yoshiyama, M. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc. Imaging 2013, 6, 448–457. [Google Scholar] [CrossRef]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef]
- Park, H.B.; Heo, R.; o Hartaigh, B.; Cho, I.; Gransar, H.; Nakazato, R.; Leipsic, J.; Mancini, G.B.; Koo, B.K.; Otake, H.; et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: A direct comparison to fractional flow reserve. JACC Cardiovasc. Imaging 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Nakazato, R.; Shalev, A.; Doh, J.H.; Koo, B.K.; Gransar, H.; Gomez, M.J.; Leipsic, J.; Park, H.B.; Berman, D.S.; Min, J.K. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J. Am. Coll. Cardiol. 2013, 62, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Hartaigh, B.O.; Danad, I.; Han, D.; Lee, J.H.; Gransar, H.; Szymonifka, J.; Lin, F.Y.; Min, J.K. Diffuse coronary artery disease among other atherosclerotic plaque characteristics by coronary computed tomography angiography for predicting coronary vessel-specific ischemia by fractional flow reserve. Atherosclerosis 2017, 258, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Dzaye, O.; Steffensen, F.H.; Botker, H.E.; Jensen, J.M.; Ronnow Sand, N.P.; Kragholm, K.H.; Sorensen, H.T.; Leipsic, J.; Maeng, M.; et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2020, 76, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.S.; Hulten, E.; Ghoshhajra, B.; O’Leary, D.; Christman, M.P.; Montana, P.; Truong, Q.A.; Steigner, M.; Murthy, V.L.; Rybicki, F.J.; et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ. Cardiovasc. Imaging 2014, 7, 282–291. [Google Scholar] [CrossRef]
- Min, J.K.; Shaw, L.J.; Berman, D.S. The present state of coronary computed tomography angiography a process in evolution. J. Am. Coll. Cardiol. 2010, 55, 957–965. [Google Scholar] [CrossRef]
- Parikh, R.; Patel, A.; Lu, B.; Senapati, A.; Mahmarian, J.; Chang, S.M. Cardiac Computed Tomography for Comprehensive Coronary Assessment: Beyond Diagnosis of Anatomic Stenosis. Methodist Debakey Cardiovasc. J. 2020, 16, 77–85. [Google Scholar] [CrossRef]
- Ko, B.S.; Wong, D.T.; Norgaard, B.L.; Leong, D.P.; Cameron, J.D.; Gaur, S.; Marwan, M.; Achenbach, S.; Kuribayashi, S.; Kimura, T.; et al. Diagnostic Performance of Transluminal Attenuation Gradient and Noninvasive Fractional Flow Reserve Derived from 320-Detector Row CT Angiography to Diagnose Hemodynamically Significant Coronary Stenosis: An NXT Substudy. Radiology 2016, 279, 75–83. [Google Scholar] [CrossRef]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012, 308, 1237–1245. [Google Scholar] [CrossRef]
- Norgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]
- Koo, B.K.; Erglis, A.; Doh, J.H.; Daniels, D.V.; Jegere, S.; Kim, H.S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Petraco, R.; Shun-Shin, M.J.; Ahmad, Y.; Nijjer, S.; Al-Lamee, R.; Kikuta, Y.; Shiono, Y.; Mayet, J.; Francis, D.P.; et al. Diagnostic Accuracy of Computed Tomography-Derived Fractional Flow Reserve: A Systematic Review. JAMA Cardiol. 2017, 2, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; De Bruyne, B.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Quality-of-Life and Economic Outcomes of Assessing Fractional Flow Reserve With Computed Tomography Angiography: PLATFORM. J. Am. Coll. Cardiol. 2015, 66, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Curzen, N.P.; Nolan, J.; Zaman, A.G.; Norgaard, B.L.; Rajani, R. Does the Routine Availability of CT-Derived FFR Influence Management of Patients With Stable Chest Pain Compared to CT Angiography Alone?: The FFRCT RIPCORD Study. JACC Cardiovasc. Imaging 2016, 9, 1188–1194. [Google Scholar] [CrossRef]
- Lu, M.T.; Ferencik, M.; Roberts, R.S.; Lee, K.L.; Ivanov, A.; Adami, E.; Mark, D.B.; Jaffer, F.A.; Leipsic, J.A.; Douglas, P.S.; et al. Noninvasive FFR Derived From Coronary CT Angiography: Management and Outcomes in the PROMISE Trial. JACC Cardiovasc. Imaging 2017, 10, 1350–1358. [Google Scholar] [CrossRef]
- Kitabata, H.; Leipsic, J.; Patel, M.R.; Nieman, K.; De Bruyne, B.; Rogers, C.; Pontone, G.; Norgaard, B.L.; Bax, J.J.; Raff, G.; et al. Incidence and predictors of lesion-specific ischemia by FFRCT: Learnings from the international ADVANCE registry. J. Cardiovasc. Comput. Tomogr. 2018, 12, 95–100. [Google Scholar] [CrossRef]
- Ko, B.S.; Cameron, J.D.; Munnur, R.K.; Wong, D.T.L.; Fujisawa, Y.; Sakaguchi, T.; Hirohata, K.; Hislop-Jambrich, J.; Fujimoto, S.; Takamura, K.; et al. Noninvasive CT-Derived FFR Based on Structural and Fluid Analysis: A Comparison With Invasive FFR for Detection of Functionally Significant Stenosis. JACC Cardiovasc. Imaging 2017, 10, 663–673. [Google Scholar] [CrossRef]
- Cademartiri, F.; Seitun, S.; Clemente, A.; La Grutta, L.; Toia, P.; Runza, G.; Midiri, M.; Maffei, E. Myocardial blood flow quantification for evaluation of coronary artery disease by computed tomography. Cardiovasc. Diagn. Ther. 2017, 7, 129–150. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Schulman-Marcus, J.; Min, J.K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 836–844. [Google Scholar] [CrossRef]
- George, R.T.; Mehra, V.C.; Chen, M.Y.; Kitagawa, K.; Arbab-Zadeh, A.; Miller, J.M.; Matheson, M.B.; Vavere, A.L.; Kofoed, K.F.; Rochitte, C.E.; et al. Myocardial CT perfusion imaging and SPECT for the diagnosis of coronary artery disease: A head-to-head comparison from the CORE320 multicenter diagnostic performance study. Radiology 2014, 272, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, F.; Becker, A.; Schwarz, F.; Marcus, R.P.; Greif, M.; von Ziegler, F.; Blankstein, R.; Hoffmann, U.; Sommer, W.H.; Hoffmann, V.S.; et al. Detection of hemodynamically significant coronary artery stenosis: Incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology 2011, 260, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Cameron, J.D.; Leung, M.; Meredith, I.T.; Leong, D.P.; Antonis, P.R.; Crossett, M.; Troupis, J.; Harper, R.; Malaiapan, Y.; et al. Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: A comparison with fractional flow reserve. JACC Cardiovasc. Imaging 2012, 5, 1097–1111. [Google Scholar] [CrossRef]
- Bettencourt, N.; Ferreira, N.D.; Leite, D.; Carvalho, M.; Ferreira Wda, S.; Schuster, A.; Chiribiri, A.; Leite-Moreira, A.; Silva-Cardoso, J.; Nagel, E.; et al. CAD detection in patients with intermediate-high pre-test probability: Low-dose CT delayed enhancement detects ischemic myocardial scar with moderate accuracy but does not improve performance of a stress-rest CT perfusion protocol. JACC Cardiovasc. Imaging 2013, 6, 1062–1071. [Google Scholar] [CrossRef]
- Huber, A.M.; Leber, V.; Gramer, B.M.; Muenzel, D.; Leber, A.; Rieber, J.; Schmidt, M.; Vembar, M.; Hoffmann, E.; Rummeny, E. Myocardium: Dynamic versus single-shot CT perfusion imaging. Radiology 2013, 269, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dharampal, A.; Wragg, A.; Davies, L.C.; van Geuns, R.J.; Anagnostopoulos, C.; Klotz, E.; Kitslaar, P.; Broersen, A.; Mathur, A.; et al. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: Does it predict functionally significant coronary lesions? Eur. Heart J. Cardiovasc. Imaging 2014, 15, 85–94. [Google Scholar] [CrossRef]
- Greif, M.; von Ziegler, F.; Bamberg, F.; Tittus, J.; Schwarz, F.; D’Anastasi, M.; Marcus, R.P.; Schenzle, J.; Becker, C.; Nikolaou, K.; et al. CT stress perfusion imaging for detection of haemodynamically relevant coronary stenosis as defined by FFR. Heart 2013, 99, 1004–1011. [Google Scholar] [CrossRef]
- Pelgrim, G.J.; Dorrius, M.; Xie, X.; den Dekker, M.A.; Schoepf, U.J.; Henzler, T.; Oudkerk, M.; Vliegenthart, R. The dream of a one-stop-shop: Meta-analysis on myocardial perfusion CT. Eur. J. Radiol. 2015, 84, 2411–2420. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Del Torto, A.; Mushtaq, S.; et al. Diagnostic accuracy of simultaneous evaluation of coronary arteries and myocardial perfusion with single stress cardiac computed tomography acquisition compared to invasive coronary angiography plus invasive fractional flow reserve. Int. J. Cardiol. 2018, 273, 263–268. [Google Scholar] [CrossRef]
- Sliwicka, O.; Sechopoulos, I.; Baggiano, A.; Pontone, G.; Nijveldt, R.; Habets, J. Dynamic myocardial CT perfusion imaging-state of the art. Eur. Radiol. 2023, 33, 5509–5525. [Google Scholar] [CrossRef]
- Cury, R.C.; Kitt, T.M.; Feaheny, K.; Blankstein, R.; Ghoshhajra, B.B.; Budoff, M.J.; Leipsic, J.; Min, J.K.; Akin, J.; George, R.T. A randomized, multicenter, multivendor study of myocardial perfusion imaging with regadenoson CT perfusion vs single photon emission CT. J. Cardiovasc. Comput. Tomogr. 2015, 9, 103–112.e2. [Google Scholar] [CrossRef] [PubMed]
- Rochitte, C.E.; George, R.T.; Chen, M.Y.; Arbab-Zadeh, A.; Dewey, M.; Miller, J.M.; Niinuma, H.; Yoshioka, K.; Kitagawa, K.; Nakamori, S.; et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: The CORE320 study. Eur. Heart J. 2014, 35, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, M.; Coenen, A.; Kofflard, M.; Bruning, T.; Kietselaer, B.; Galema, T.; Kock, M.; Niezen, A.; Das, M.; van Gent, M.; et al. Comprehensive Cardiac CT With Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease: The Multicenter, Randomized CRESCENT-II Trial. JACC Cardiovasc. Imaging 2017, 11, 1625–1636. [Google Scholar] [CrossRef]
- Gaur, S.; Taylor, C.A.; Jensen, J.M.; Botker, H.E.; Christiansen, E.H.; Kaltoft, A.K.; Holm, N.R.; Leipsic, J.; Zarins, C.K.; Achenbach, S.; et al. FFR Derived From Coronary CT Angiography in Nonculprit Lesions of Patients With Recent STEMI. JACC Cardiovasc. Imaging 2017, 10, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Huang, B.T.; Lv, W.Y.; Liu, W.; Peng, Y.; Xia, T.L.; Wang, P.J.; Zuo, Z.L.; Liu, R.S.; Zhang, C.; et al. The Prognosis of Patients With Nonobstructive Coronary Artery Disease Versus Normal Arteries Determined by Invasive Coronary Angiography or Computed Tomography Coronary Angiography: A Systematic Review. Medicine 2016, 95, e3117. [Google Scholar] [CrossRef]
- Lin, F.Y.; Shaw, L.J.; Dunning, A.M.; Labounty, T.M.; Choi, J.H.; Weinsaft, J.W.; Koduru, S.; Gomez, M.J.; Delago, A.J.; Callister, T.Q.; et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: A prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J. Am. Coll. Cardiol. 2011, 58, 510–519. [Google Scholar] [CrossRef]
- Min, J.K.; Dunning, A.; Lin, F.Y.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.J.; Cheng, V.; et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J. Am. Coll. Cardiol. 2011, 58, 849–860. [Google Scholar] [CrossRef]
- Chow, B.J.; Wells, G.A.; Chen, L.; Yam, Y.; Galiwango, P.; Abraham, A.; Sheth, T.; Dennie, C.; Beanlands, R.S.; Ruddy, T.D. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J. Am. Coll. Cardiol. 2010, 55, 1017–1028. [Google Scholar] [CrossRef]
- Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; MacLean, L.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef]
- LaBounty, T.M.; Devereux, R.B.; Lin, F.Y.; Weinsaft, J.W.; Min, J.K. Impact of coronary computed tomographic angiography findings on the medical treatment and control of coronary artery disease and its risk factors. Am. J. Cardiol. 2009, 104, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.; Erthal, F.; Abdelsalam, M.A.; Murad, M.H.; Wang, Z.; Erwin, P.J.; Hillis, G.S.; Kritharides, L.; Chow, B.J.W. Prognostic value of segment involvement score compared to other measures of coronary atherosclerosis by computed tomography: A systematic review and meta-analysis. J. Cardiovasc. Comput. Tomogr. 2017, 11, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.T.; Horne, A.; Martin, S.S.; Blaha, M.J.; Blankstein, R.; Budoff, M.J.; Sibley, C.; Polak, J.F.; Frick, K.D.; Blumenthal, R.S.; et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: The Multi-Ethnic Study of Atherosclerosis (MESA). PLoS ONE 2015, 10, e0116377. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Polonsky, T.S.; Young, R.; McClelland, R.L.; Delaney, J.C.; Dawood, F.; Blaha, M.J.; Miedema, M.D.; Sibley, C.T.; Carr, J.J.; et al. Utility of Nontraditional Risk Markers in Individuals Ineligible for Statin Therapy According to the 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines. Circulation 2015, 132, 916–922. [Google Scholar] [CrossRef][Green Version]
- Yeboah, J.; Young, R.; McClelland, R.L.; Delaney, J.C.; Polonsky, T.S.; Dawood, F.Z.; Blaha, M.J.; Miedema, M.D.; Sibley, C.T.; Carr, J.J.; et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J. Am. Coll. Cardiol. 2016, 67, 139–147. [Google Scholar] [CrossRef]
- Cook, N.R.; Ridker, P.M. Further insight into the cardiovascular risk calculator: The roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern. Med. 2014, 174, 1964–1971. [Google Scholar] [CrossRef]
- Blaha, M.J.; Cainzos-Achirica, M.; Greenland, P.; McEvoy, J.W.; Blankstein, R.; Budoff, M.J.; Dardari, Z.; Sibley, C.T.; Burke, G.L.; Kronmal, R.A.; et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016, 133, 849–858. [Google Scholar] [CrossRef]
- Nasir, K.; Bittencourt, M.S.; Blaha, M.J.; Blankstein, R.; Agatson, A.S.; Rivera, J.J.; Miedema, M.D.; Sibley, C.T.; Shaw, L.J.; Blumenthal, R.S.; et al. Implications of Coronary Artery Calcium Testing among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2015, 66, 1657–1668. [Google Scholar] [CrossRef]
- Rozanski, A.; Gransar, H.; Shaw, L.J.; Kim, J.; Miranda-Peats, L.; Wong, N.D.; Rana, J.S.; Orakzai, R.; Hayes, S.W.; Friedman, J.D.; et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J. Am. Coll. Cardiol. 2011, 57, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Mamudu, H.M.; Paul, T.K.; Veeranki, S.P.; Budoff, M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: A systematic review. Atherosclerosis 2014, 236, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Blaha, M.J.; Berman, D.S.; Nasir, K.; Budoff, M.; Leipsic, J.; Blankstein, R.; Narula, J.; Rumberger, J.; Shaw, L.J. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.S.; Cronin, P.; Blaha, M.J.; Budoff, M.J.; Kazerooni, E.A.; Narula, J.; Yankelevitz, D.; Abbara, S. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J. Cardiovasc. Comput. Tomogr. 2017, 11, 74–84. [Google Scholar] [CrossRef]
- Gaine, S.P.; Blumenthal, R.S.; Sharma, G. Coronary Artery Calcium Score as a Graded Decision Tool. JACC Adv. 2023, 2, 100664. [Google Scholar] [CrossRef]
- McEvoy, J.W.; Martin, S.S.; Dardari, Z.A.; Miedema, M.D.; Sandfort, V.; Yeboah, J.; Budoff, M.J.; Goff, D.C., Jr.; Psaty, B.M.; Post, W.S.; et al. Coronary Artery Calcium to Guide a Personalized Risk-Based Approach to Initiation and Intensification of Antihypertensive Therapy. Circulation 2017, 135, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Peterson, E. The New 2017 ACC/AHA Guidelines “Up the Pressure” on Diagnosis and Treatment of Hypertension. JAMA 2017, 318, 2083–2084. [Google Scholar] [CrossRef]
- Grandhi, G.R.; Mszar, R.; Cainzos-Achirica, M.; Rajan, T.; Latif, M.A.; Bittencourt, M.S.; Shaw, L.J.; Batlle, J.C.; Blankstein, R.; Blaha, M.J.; et al. Coronary Calcium to Rule Out Obstructive Coronary Artery Disease in Patients With Acute Chest Pain. JACC Cardiovasc. Imaging 2022, 15, 271–280. [Google Scholar] [CrossRef]
- Agha, A.M.; Pacor, J.; Grandhi, G.R.; Mszar, R.; Khan, S.U.; Parikh, R.; Agrawal, T.; Burt, J.; Blankstein, R.; Blaha, M.J.; et al. The Prognostic Value of CAC Zero among Individuals Presenting with Chest Pain: A Meta-Analysis. JACC Cardiovasc. Imaging 2022, 15, 1745–1757. [Google Scholar] [CrossRef]
- Mowatt, G.; Cook, J.A.; Hillis, G.S.; Walker, S.; Fraser, C.; Jia, X.; Waugh, N. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: Systematic review and meta-analysis. Heart 2008, 94, 1386–1393. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Wilson, G.T.; Tak, T. Accuracy of multislice computed tomography coronary angiography: A pooled estimate. Cardiol. Rev. 2008, 16, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Biondi-Zoccai, G.G.; Malagutti, P.; Agostoni, P.; Morello, R.; Valgimigli, M.; Hamon, M. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: A meta-analysis. J. Am. Coll. Cardiol. 2006, 48, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, T.; Zhang, R.; Xu, L.; Peng, Z.; Ding, J.; Wang, S.; Li, M.; Sun, G. Meta-analysis: Diagnostic accuracy of coronary CT angiography with prospective ECG gating based on step-and-shoot, Flash and volume modes for detection of coronary artery disease. Eur. Radiol. 2014, 24, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Meijboom, W.B.; Meijs, M.F.; Schuijf, J.D.; Cramer, M.J.; Mollet, N.R.; van Mieghem, C.A.; Nieman, K.; van Werkhoven, J.M.; Pundziute, G.; Weustink, A.C.; et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: A prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 2008, 52, 2135–2144. [Google Scholar] [CrossRef]
- Miller, J.M.; Rochitte, C.E.; Dewey, M.; Arbab-Zadeh, A.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 2008, 359, 2324–2336. [Google Scholar] [CrossRef]
- Habib, P.J.; Green, J.; Butterfield, R.C.; Kuntz, G.M.; Murthy, R.; Kraemer, D.F.; Percy, R.F.; Miller, A.B.; Strom, J.A. Association of cardiac events with coronary artery disease detected by 64-slice or greater coronary CT angiography: A systematic review and meta-analysis. Int. J. Cardiol. 2013, 169, 112–120. [Google Scholar] [CrossRef]
- Hulten, E.A.; Carbonaro, S.; Petrillo, S.P.; Mitchell, J.D.; Villines, T.C. Prognostic value of cardiac computed tomography angiography: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 57, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, J.; Lv, X.; Cai, W. Prognostic value of cardiac computed tomography angiography in patients with suspected coronary artery disease: A meta-analysis. Cardiology 2014, 128, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.J.; Small, G.; Yam, Y.; Chen, L.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: An InteRnational Multicenter registry. Circ. Cardiovasc. Imaging 2011, 4, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Anderson, J.L.; Brinker, J.A.; Brophy, J.A.; Casey, D.E., Jr.; Cross, R.R.; Edmundowicz, D.; Hachamovitch, R.; Hlatky, M.A.; Jacobs, J.E.; et al. ACC/AHA/ASE/ASNC/HRS/IAC/Mended Hearts/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SNMMI 2014 health policy statement on use of noninvasive cardiovascular imaging: A report of the American College of Cardiology Clinical Quality Committee. J. Am. Coll. Cardiol. 2014, 63, 698–721. [Google Scholar] [CrossRef]
- Cho, I.; Chang, H.J.; Sung, J.M.; Pencina, M.J.; Lin, F.Y.; Dunning, A.M.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; et al. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (coronary CT angiography evaluation for clinical outcomes: An international multicenter registry). Circulation 2012, 126, 304–313. [Google Scholar] [CrossRef]
- Cho, I.; Al’Aref, S.J.; Berger, A.; Ó Hartaigh, B.; Gransar, H.; Valenti, V.; Lin, F.Y.; Achenbach, S.; Berman, D.S.; Budoff, M.J.; et al. Prognostic value of coronary computed tomographic angiography findings in asymptomatic individuals: A 6-year follow-up from the prospective multicentre international CONFIRM study. Eur. Heart J. 2018, 39, 934–941. [Google Scholar] [CrossRef]
- McDermott, M.; Meah, M.N.; Khaing, P.; Wang, K.L.; Ramsay, J.; Scott, G.; Rickman, H.; Burt, T.; McGowan, I.; Fairbairn, T.; et al. Rationale and Design of SCOT-HEART 2 Trial: CT Angiography for the Prevention of Myocardial Infarction. JACC Cardiovasc. Imaging 2024, 17, 1101–1112. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Chen, M.Y.; Dey, A.K.; Virmani, R.; Finn, A.V.; Khamis, R.Y.; Choi, A.D.; Min, J.K.; Williams, M.C.; Buckler, A.J.; et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Rovera, C.; Moretti, C.; Bisanti, F.; De Zan, G.; Guglielmo, M. Myocardial Bridging: Review on the Role of Coronary Computed Tomography Angiography. J. Clin. Med. 2023, 12, 5949. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2010, 56, 1864–1894. [Google Scholar] [CrossRef]
- Samad, Z.; Hakeem, A.; Mahmood, S.S.; Pieper, K.; Patel, M.R.; Simel, D.L.; Douglas, P.S. A meta-analysis and systematic review of computed tomography angiography as a diagnostic triage tool for patients with chest pain presenting to the emergency department. J. Nucl. Cardiol. 2012, 19, 364–376. [Google Scholar] [CrossRef] [PubMed]
- El-Hayek, G.; Benjo, A.; Uretsky, S.; Al-Mallah, M.; Cohen, R.; Bamira, D.; Chavez, P.; Nascimento, F.; Santana, O.; Patel, R.; et al. Meta-analysis of coronary computed tomography angiography versus standard of care strategy for the evaluation of low risk chest pain: Are randomized controlled trials and cohort studies showing the same evidence? Int. J. Cardiol. 2014, 177, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Linde, J.J.; Hove, J.D.; Sorgaard, M.; Kelbaek, H.; Jensen, G.B.; Kuhl, J.T.; Hindso, L.; Kober, L.; Nielsen, W.B.; Kofoed, K.F. Long-Term Clinical Impact of Coronary CT Angiography in Patients With Recent Acute-Onset Chest Pain: The Randomized Controlled CATCH Trial. JACC Cardiovasc. Imaging 2015, 8, 1404–1413. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Chinnaiyan, K.M.; Abidov, A.; Achenbach, S.; Berman, D.S.; Hayes, S.W.; Hoffmann, U.; Lesser, J.R.; Mikati, I.A.; O’Neil, B.J.; et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J. Am. Coll. Cardiol. 2011, 58, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Litt, H.I.; Gatsonis, C.; Snyder, B.; Singh, H.; Miller, C.D.; Entrikin, D.W.; Leaming, J.M.; Gavin, L.J.; Pacella, C.B.; Hollander, J.E. CT angiography for safe discharge of patients with possible acute coronary syndromes. N. Engl. J. Med. 2012, 366, 1393–1403. [Google Scholar] [CrossRef]
- Hoffmann, U.; Truong, Q.A.; Schoenfeld, D.A.; Chou, E.T.; Woodard, P.K.; Nagurney, J.T.; Pope, J.H.; Hauser, T.H.; White, C.S.; Weiner, S.G.; et al. Coronary CT angiography versus standard evaluation in acute chest pain. N. Engl. J. Med. 2012, 367, 299–308. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Abbara, S.; Shaw, L.J. Past, Present, and Future of CTA. Circulation 2024, 150, 87–90. [Google Scholar] [CrossRef]
- Hadamitzky, M.; Freissmuth, B.; Meyer, T.; Hein, F.; Kastrati, A.; Martinoff, S.; Schomig, A.; Hausleiter, J. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc. Imaging 2009, 2, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Fazel, P.; Peterman, M.A.; Schussler, J.M. Three-year outcomes and cost analysis in patients receiving 64-slice computed tomographic coronary angiography for chest pain. Am. J. Cardiol. 2009, 104, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Danciu, S.C.; Herrera, C.J.; Stecy, P.J.; Carell, E.; Saltiel, F.; Hines, J.L. Usefulness of multislice computed tomographic coronary angiography to identify patients with abnormal myocardial perfusion stress in whom diagnostic catheterization may be safely avoided. Am. J. Cardiol. 2007, 100, 1605–1608. [Google Scholar] [CrossRef]
- Abidov, A.; Gallagher, M.J.; Chinnaiyan, K.M.; Mehta, L.S.; Wegner, J.H.; Raff, G.L. Clinical effectiveness of coronary computed tomographic angiography in the triage of patients to cardiac catheterization and revascularization after inconclusive stress testing: Results of a 2-year prospective trial. J. Nucl. Cardiol. 2009, 16, 701–713. [Google Scholar] [CrossRef]
- Group, D.T.; Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Stechovsky, C.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef]
- Shaw, L.J.; Hausleiter, J.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.J.; Kim, Y.J.; et al. Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: Results from the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J. Am. Coll. Cardiol. 2012, 60, 2103–2114. [Google Scholar] [CrossRef]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef]
- Min, J.K.; Berman, D.S.; Dunning, A.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.J.; Cheng, V.; et al. All-cause mortality benefit of coronary revascularization vs. medical therapy in patients without known coronary artery disease undergoing coronary computed tomographic angiography: Results from CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). Eur. Heart J. 2012, 33, 3088–3097. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Dai, D.; Hernandez, A.F.; Douglas, P.S.; Messenger, J.; Garratt, K.N.; Maddox, T.M.; Peterson, E.D.; Roe, M.T. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am. Heart J. 2014, 167, 846–852.e2. [Google Scholar] [CrossRef]
- Lubbers, M.; Dedic, A.; Coenen, A.; Galema, T.; Akkerhuis, J.; Bruning, T.; Krenning, B.; Musters, P.; Ouhlous, M.; Liem, A.; et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: The multicentre, randomized CRESCENT trial. Eur. Heart J. 2016, 37, 1232–1243. [Google Scholar] [CrossRef]
- McKavanagh, P.; Lusk, L.; Ball, P.A.; Verghis, R.M.; Agus, A.M.; Trinick, T.R.; Duly, E.; Walls, G.M.; Stevenson, M.; James, B.; et al. A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: The clinical results of the CAPP randomized prospective trial. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, ehae177. [Google Scholar] [CrossRef]
- Mark, D.B.; Federspiel, J.J.; Cowper, P.A.; Anstrom, K.J.; Hoffmann, U.; Patel, M.R.; Davidson-Ray, L.; Daniels, M.R.; Cooper, L.S.; Knight, J.D.; et al. Economic Outcomes With Anatomical Versus Functional Diagnostic Testing for Coronary Artery Disease. Ann. Intern. Med. 2016, 165, 94–102. [Google Scholar] [CrossRef]
- Agus, A.M.; McKavanagh, P.; Lusk, L.; Verghis, R.M.; Walls, G.M.; Ball, P.A.; Trinick, T.R.; Harbinson, M.T.; Donnelly, P.M. The cost-effectiveness of cardiac computed tomography for patients with stable chest pain. Heart 2016, 102, 356–362. [Google Scholar] [CrossRef]
- Chang, H.J.; Lin, F.Y.; Gebow, D.; An, H.Y.; Andreini, D.; Bathina, R.; Baggiano, A.; Beltrama, V.; Cerci, R.; Choi, E.Y.; et al. Selective Referral Using CCTA Versus Direct Referral for Individuals Referred to Invasive Coronary Angiography for Suspected CAD: A Randomized, Controlled, Open-Label Trial. JACC Cardiovasc. Imaging 2019, 12, 1303–1312. [Google Scholar] [CrossRef]
- Sturts, A.; Ruzieh, M.; Dhruva, S.S.; Peterson, B.; Mandrola, J.M.; Liu, G.; Redberg, R.F.; Foy, A.J. Resource Utilization Following Coronary Computed Tomographic Angiography and Stress Echocardiography in Patients Presenting to the Emergency Department With Chest Pain. Am. J. Cardiol. 2022, 163, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.; Schmidt, B.; Ulzheimer, S.; Alkadhi, H. Cardiac imaging with photon counting CT. Br. J. Radiol. 2023, 96, 20230407. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.J.; O’Doherty, J.; Schoepf, U.J.; Ellison, B.; Byrne, J.; Fink, N.; Zsarnoczay, E.; Wolf, E.V.; Allmendinger, T.; Schmidt, B.; et al. Myocardial Characterization with Extracellular Volume Mapping with a First-Generation Photon-counting Detector CT with MRI Reference. Radiology 2023, 307, e222030. [Google Scholar] [CrossRef]
- Mergen, V.; Sartoretti, T.; Klotz, E.; Schmidt, B.; Jungblut, L.; Higashigaito, K.; Manka, R.; Euler, A.; Kasel, M.; Eberhard, M.; et al. Extracellular Volume Quantification With Cardiac Late Enhancement Scanning Using Dual-Source Photon-Counting Detector CT. Invest. Radiol. 2022, 57, 406–411. [Google Scholar] [CrossRef]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Rau, A.; Taron, J.; Weiss, J.; Stein, T.; Faby, S.; von Zur Muehlen, C.; Ruile, P.; et al. Accuracy of Ultrahigh-Resolution Photon-counting CT for Detecting Coronary Artery Disease in a High-Risk Population. Radiology 2023, 307, e223305. [Google Scholar] [CrossRef]
- Kolossvary, M.; De Cecco, C.N.; Feuchtner, G.; Maurovich-Horvat, P. Advanced atherosclerosis imaging by CT: Radiomics, machine learning and deep learning. J. Cardiovasc. Comput. Tomogr. 2019, 13, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, L.; Qu, M.; Chen, B.; Wang, G. Artificial Intelligence in Coronary CT Angiography: Current Status and Future Prospects. Front. Cardiovasc. Med. 2022, 9, 896366. [Google Scholar] [CrossRef] [PubMed]
- Massalha, S.; Clarkin, O.; Thornhill, R.; Wells, G.; Chow, B.J.W. Decision Support Tools, Systems, and Artificial Intelligence in Cardiac Imaging. Can. J. Cardiol. 2018, 34, 827–838. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Van Assen, M.; Tesche, C.; De Cecco, C.N.; Chiesa, M.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Guaricci, A.I.; et al. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. BioMed Res. Int. 2020, 2020, 6649410. [Google Scholar] [CrossRef]
- Zreik, M.; van Hamersvelt, R.W.; Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. A Recurrent CNN for Automatic Detection and Classification of Coronary Artery Plaque and Stenosis in Coronary CT Angiography. IEEE Trans. Med. Imaging 2019, 38, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Nakajima, K.; Taki, J.; Wakabayashi, H.; Matsuo, S.; Konishi, T.; Okuda, K.; Shibutani, T.; Onoguchi, M.; Kinuya, S. Ability of artificial intelligence to diagnose coronary artery stenosis using hybrid images of coronary computed tomography angiography and myocardial perfusion SPECT. Eur. J. Hybrid. Imaging 2019, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Gotz, M.; Antoniades, C.; Heidenreich, J.F.; Leiner, T.; Beer, M. Artificial intelligence in coronary computed tomography angiography: Demands and solutions from a clinical perspective. Front. Cardiovasc. Med. 2023, 10, 1120361. [Google Scholar] [CrossRef]
- Kolossvary, M.; Karady, J.; Szilveszter, B.; Kitslaar, P.; Hoffmann, U.; Merkely, B.; Maurovich-Horvat, P. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques with Napkin-Ring Sign. Circ. Cardiovasc. Imaging 2017, 10, e006843. [Google Scholar] [CrossRef]
- Coenen, A.; Kim, Y.H.; Kruk, M.; Tesche, C.; De Geer, J.; Kurata, A.; Lubbers, M.L.; Daemen, J.; Itu, L.; Rapaka, S.; et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the Machine Consortium. Circ. Cardiovasc. Imaging 2018, 11, e007217. [Google Scholar] [CrossRef]
- Itu, L.; Rapaka, S.; Passerini, T.; Georgescu, B.; Schwemmer, C.; Schoebinger, M.; Flohr, T.; Sharma, P.; Comaniciu, D. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J. Appl. Physiol. 2016, 121, 42–52. [Google Scholar] [CrossRef]
- Tesche, C.; De Cecco, C.N.; Baumann, S.; Renker, M.; McLaurin, T.W.; Duguay, T.M.; Bayer, R.R., 2nd; Steinberg, D.H.; Grant, K.L.; Canstein, C.; et al. Coronary CT Angiography-derived Fractional Flow Reserve: Machine Learning Algorithm versus Computational Fluid Dynamics Modeling. Radiology 2018, 288, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Eng, D.; Chute, C.; Khandwala, N.; Rajpurkar, P.; Long, J.; Shleifer, S.; Khalaf, M.H.; Sandhu, A.T.; Rodriguez, F.; Maron, D.J.; et al. Automated coronary calcium scoring using deep learning with multicenter external validation. NPJ Digit. Med. 2021, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Van Assen, M.; Martin, S.S.; Varga-Szemes, A.; Rapaka, S.; Cimen, S.; Sharma, P.; Sahbaee, P.; De Cecco, C.N.; Vliegenthart, R.; Leonard, T.J.; et al. Automatic coronary calcium scoring in chest CT using a deep neural network in direct comparison with non-contrast cardiac CT: A validation study. Eur. J. Radiol. 2021, 134, 109428. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, S.G.M.; Lessmann, N.; Velthuis, B.K.; Bank, I.E.M.; van den Bongard, D.; Leiner, T.; de Jong, P.A.; Veldhuis, W.B.; Correa, A.; Terry, J.G.; et al. Deep Learning for Automatic Calcium Scoring in CT: Validation Using Multiple Cardiac CT and Chest CT Protocols. Radiology 2020, 295, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xiao, J.; Xie, Y.; Hu, Y.; Zhang, S.; Li, X.; Wang, Z.; Li, Z.; Wang, X. The correlation of deep learning-based CAD-RADS evaluated by coronary computed tomography angiography with breast arterial calcification on mammography. Sci. Rep. 2020, 10, 11532. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chiesa, M.; Trotta, M.; Gatti, M.; Palmisano, V.; Dell’Aversana, S.; Baessato, F.; Cavaliere, A.; Cicala, G.; Loffreno, A.; et al. Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA. Atherosclerosis 2020, 294, 25–32. [Google Scholar] [CrossRef]
- Johnson, K.M.; Johnson, H.E.; Zhao, Y.; Dowe, D.A.; Staib, L.H. Scoring of Coronary Artery Disease Characteristics on Coronary CT Angiograms by Using Machine Learning. Radiology 2019, 292, 354–362. [Google Scholar] [CrossRef]
- Motwani, M.; Dey, D.; Berman, D.S.; Germano, G.; Achenbach, S.; Al-Mallah, M.H.; Andreini, D.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: A 5-year multicentre prospective registry analysis. Eur. Heart J. 2017, 38, 500–507. [Google Scholar] [CrossRef]
- Singh, Y.; Shakyawar, D.; Hu, W. Non-ischemic endocardial scar geometric remodeling toward topological machine learning. Proc. Inst. Mech. Eng. H 2020, 234, 1029–1035. [Google Scholar] [CrossRef]


| Advantages | Limitations | |
|---|---|---|
| Functional | ||
| Stress ECG | Non-invasive, no radiation, readily available, low cost | Poor localization, requires exercise capacity to achieve adequate stress |
| Stress TTE | No radiation, high availability, localizes ischemia, concurrent assessment of other parameters such as valvular function or diastology | Body habitus, requires technical expertise, requires adequate exercise capacity or tolerance of dobutamine |
| SPECT (stress/rest imaging) | High availability, localizes ischemia, highly reproducible, cost effective | Attenuation artifacts in obesity, false positives, balanced ischemia, exposure to radiation |
| PET (stress/rest imaging) | High accuracy (particularly in patients with high BMI), can assess myocardial blood flow | Limited availability, expense, exposure to radiation |
| Stress MRI perfusion | High accuracy, no ionizing radiation exposure, assessment of myocardial scar | Low availability, time consuming, device incompatibilities, expense, contraindicated in severe renal dysfunction |
| Anatomical | ||
| Invasive coronary angiography | High accuracy, widely available, allows for contemporaneous intervention | Invasive, requires intravenous contrast |
| Coronary Computed Tomography Angiography | High accuracy, rapid assessment, identification of subclinical disease | Arrhythmias, high heart rates requiring beta-blocker therapy, stents, renal impairment, severe coronary calcification may cause blooming artifact, exposure to radiation, contraindicated in severe renal dysfunction |
| MRI angiography | High spatial resolution, no ionizing radiation, highly reproducible | Low availability, time consuming, device incompatibilities, contraindicated in severe renal dysfunction |
| Degree of Maximal Coronary Artery Stenosis | Interpretation | Further Investigation | Further Management Considerations (Incorporates Plaque Burden Score from CAC Scoring, Refer to Table 3) | |
|---|---|---|---|---|
| CAD-RADS 0 | 0% | CAD absent (no plaque) | None | Reassurance |
| CAD-RADS 1 | 1–24% | Minimal non-obstructive plaque | None | P1: Consider risk factor modification and preventative therapy P2: Risk factor modification and preventative therapy P3: Aggressive risk factor modification and preventative therapy |
| CAD-RADS 2 | 25–49% | Mild non-obstructive CAD | None | P1/2: Risk factor modification and preventative therapy P3/4: Aggressive risk factor modification and preventative therapy |
| CAD-RADS 3 | 50–69% | Moderate stenosis | Consider functional assessment | P1–4: Aggressive risk factor modification and preventative therapy Consider anti-anginal therapy Consider ICA if positive perfusion testing (I+) |
| CAD-RADS 4 | A. 70–99% B. LM ≥ 50% or three vessel disease ≥ 70% | Severe stenosis | A: Consider ICA or functional test B: ICA recommended | P1–4: Aggressive risk factor modification and preventative therapy Consider anti-anginal therapy +/− revascularization |
| CAD-RADS 5 | 100% | Total coronary occlusion | Consider ICA and/or viability assessment | P1–4: Aggressive risk factor modification and preventative therapy Consider anti-anginal therapy +/− revascularization |
| CAD-RADS N | Non-diagnostic study | Study cannot exclude significant obstructive CAD |
| CAC Score | CAD-RADS 2.0 Classification of Plaque Burden | Statin Recommendation and Intensity | Aspirin for Primary Prevention | Blood Pressure Targets in Intermediate-Risk Patients * |
|---|---|---|---|---|
| 0 | Statin likely of limited value (except for those with suspected familial hypercholesteremia), assess other risk factors | Harm likely > benefits, not recommended | Goal BP < 130/80 mmHg | |
| 1–99 | P1 Mild overall coronary plaque burden | CAC < 75th%: Moderate-intensity statin CAC ≥ 75th%: Moderate- to high-intensity statin | Considering clinical risk, unlikely to benefit from aspirin | Goal BP < 130/80 mmHg |
| 100–299 | P2 Moderate overall coronary plaque burden | Moderate to high intensity | Aspirin recommended following assessment of bleeding risk | Goal BP < 130/80 mmHg |
| 300–999 | P3 Severe overall coronary plaque burden | High-intensity statin | Aspirin recommended following assessment of bleeding risk | Goal BP < 130/80 mmHg |
| >1000 | P4 Extensive overall coronary plaque burden |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, C.; Scalia, I.G.; Anavekar, N.S.; Arsanjani, R.; Jokerst, C.E.; Chow, B.J.W.; Kritharides, L. Computed Tomography Evaluation of Coronary Atherosclerosis: The Road Travelled, and What Lies Ahead. Diagnostics 2024, 14, 2096. https://doi.org/10.3390/diagnostics14182096
Ayoub C, Scalia IG, Anavekar NS, Arsanjani R, Jokerst CE, Chow BJW, Kritharides L. Computed Tomography Evaluation of Coronary Atherosclerosis: The Road Travelled, and What Lies Ahead. Diagnostics. 2024; 14(18):2096. https://doi.org/10.3390/diagnostics14182096
Chicago/Turabian StyleAyoub, Chadi, Isabel G. Scalia, Nandan S. Anavekar, Reza Arsanjani, Clinton E. Jokerst, Benjamin J. W. Chow, and Leonard Kritharides. 2024. "Computed Tomography Evaluation of Coronary Atherosclerosis: The Road Travelled, and What Lies Ahead" Diagnostics 14, no. 18: 2096. https://doi.org/10.3390/diagnostics14182096
APA StyleAyoub, C., Scalia, I. G., Anavekar, N. S., Arsanjani, R., Jokerst, C. E., Chow, B. J. W., & Kritharides, L. (2024). Computed Tomography Evaluation of Coronary Atherosclerosis: The Road Travelled, and What Lies Ahead. Diagnostics, 14(18), 2096. https://doi.org/10.3390/diagnostics14182096






