Echocardiography in Cardiac Arrest: Incremental Diagnostic and Prognostic Role during Resuscitation Care
Abstract
1. Introduction
2. Role of Ultrasonography in Cardiac Arrest
2.1. Role of Ultrasonography during Cardiopulmonary Resuscitation
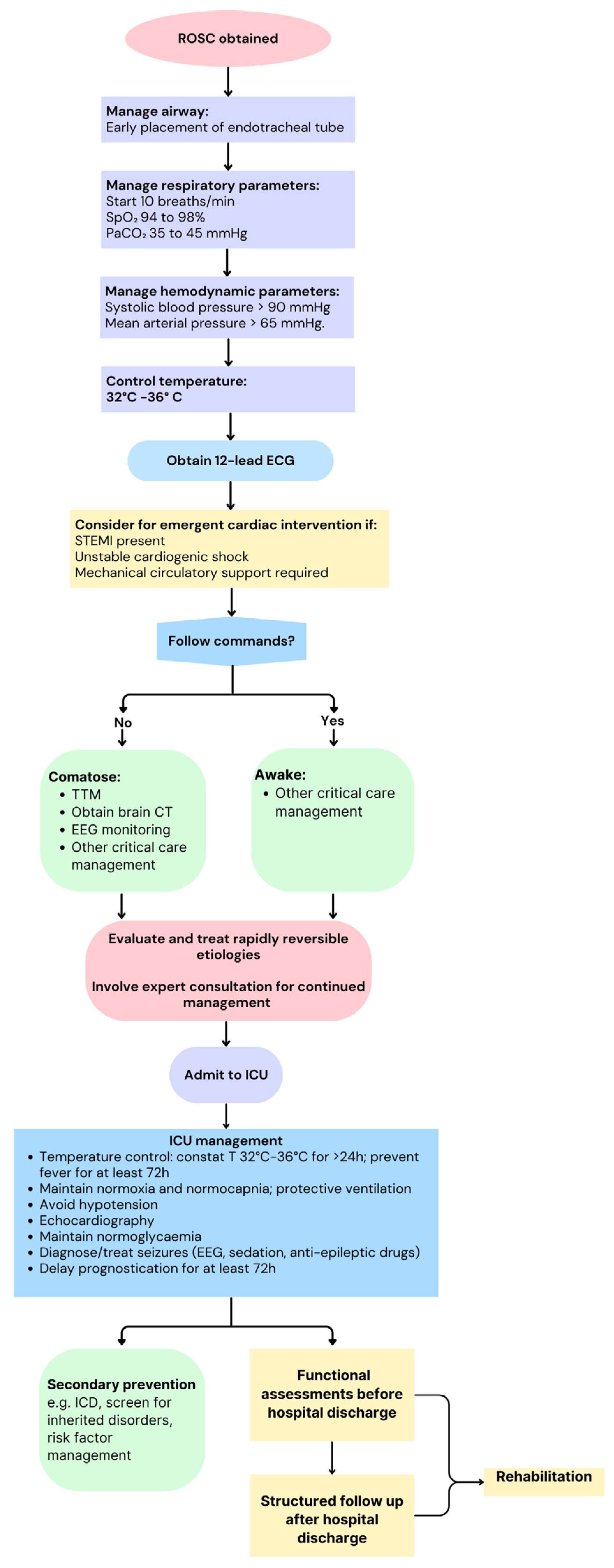
2.2. Role of Ultrasonography in Post-Resuscitation Care
2.2.1. Diagnosis of the Underlying Cause of Cardiac Arrest
2.2.2. Hemodynamic Monitoring and Optimization
2.3. Role of Transesophageal Echocardiography
3. Post-Cardiac Arrest Syndrome: Pathophysiology and Echocardiographic Feature
3.1. Role of Echocardiography in Post-Arrest Management of Suspected ACS/CAD
3.2. Role of Non-Cardiac Ultrasounds
4. Prognostic Role of Echocardiography in Resuscitated CA Patients: A Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yow, A.G.; Rajasurya, V.; Ahmed, I.; Sharma, S. Sudden Cardiac Death; StatPearls: St. Petersburg, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507854/ (accessed on 28 May 2024).
- Mehta, C.; Brady, W. Pulseless electrical activity in cardiac arrest: Electrocardiographic presentations and management considerations based on the electrocardiogram. Am. J. Emerg. Med. 2012, 30, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Rabjohns, J.; Quan, T.; Boniface, K.; Pourmand, A. Pseudo-pulseless electrical activity in the emergency department, an evidence based approach. Am. J. Emerg. Med. 2020, 38, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Gräsner, J.-T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; Van de Voorde, P.; Madar, J.; Zideman, D.; Mentzelopoulos, S.; et al. European Resuscitation Council Guidelines 2021: Executive summary. Resuscitation 2021, 161, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Hirsch, K.G.; Abella, B.S.; Amorim, E.; Bader, M.K.; Barletta, J.F.; Berg, K.; Callaway, C.W.; Friberg, H.; Gilmore, E.J.; Greer, D.M.; et al. Critical Care Management of Patients After Cardiac Arrest: A Scientific Statement From the American Heart Association and Neurocritical Care Society. Circulation 2024, 149, e168–e200. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Vignon, P.; Robba, C. How I use ultrasound in cardiac arrest. Intensiv. Care Med. 2023, 49, 1531–1534. [Google Scholar] [CrossRef]
- Jensen, M.B.; Sloth, E.; Larsen, K.M.; Schmidt, M.B. Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur. J. Anaesthesiol. 2004, 21, 700–707. [Google Scholar] [CrossRef]
- Niendorff, D.F.; Rassias, A.J.; Palac, R.; Beach, M.L.; Costa, S.; Greenberg, M. Rapid cardiac ultrasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation 2005, 67, 81–87. [Google Scholar] [CrossRef]
- Breitkreutz, R.; Walcher, F.; Seeger, F.H. Focused echocardiographic evaluation in resuscitation management: Concept of an advanced life support–conformed algorithm. Crit. Care Med. 2007, 35, S150–S161. [Google Scholar] [CrossRef]
- Hernandez, C.; Shuler, K.; Hannan, H.; Sonyika, C.; Likourezos, A.; Marshall, J. C.A.U.S.E.: Cardiac arrest ultra-sound exam—A better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation 2008, 76, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Prosen, G.; Križmarić, M.; Završnik, J.; Grmec, Š. Impact of Modified Treatment in Echocardiographically Confirmed Pseudo-Pulseless Electrical Activity in Out-of-Hospital Cardiac Arrest Patients with Constant End-Tidal Carbon Dioxide Pressure during Compression Pauses. J. Int. Med. Res. 2010, 38, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; A Cibinel, G.; Portale, G.; Forte, P.; Giannuzzi, R.; Pignataro, G.; Silveri, N.G. The proposal of an integrated ultrasonographic approach into the ALS algorithm for cardiac arrest: The PEA protocol. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 77–88. [Google Scholar] [PubMed]
- Lichtenstein, D.; Malbrain, M.L. Critical care ultrasound in cardiac arrest. Technological requirements for performing the SESAME-protocol—A holistic approach. Anaesthesiol. Intensiv. Ther. 2015, 47, 471–481. [Google Scholar] [CrossRef]
- Atkinson, P.R.; Beckett, N.; French, J.; Banerjee, A.; Fraser, J.; Lewis, D. Does Point-of-care Ultrasound Use Impact Resuscitation Length, Rates of Intervention, and Clinical Outcomes During Cardiac Arrest? A Study from the Sonography in Hypotension and Cardiac Arrest in the Emergency Department (SHoC-ED) Investigators. Cureus 2019, 11, e4456. [Google Scholar] [CrossRef]
- Atkinson, P.; Bowra, J.; Milne, J.; Lewis, D.; Lambert, M.; Jarman, B.; Noble, V.E.; Lamprecht, H.; Harris, T.; Connolly, J.; et al. International Federation for Emergency Medicine Consensus Statement: Sonography in hypotension and cardiac arrest (SHoC): An international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest. CJEM 2017, 19, 459–470. [Google Scholar] [CrossRef]
- Ávila-Reyes, D.; Acevedo-Cardona, A.O.; Gómez-González, J.F.; Echeverry-Piedrahita, D.R.; Aguirre-Flórez, M.; Giraldo-Diaconeasa, A. Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): Narrative review article. Ultrasound J. 2021, 13, 46. [Google Scholar] [CrossRef]
- Blanco, P.; Buendía, C.M. Point-of-care ultrasound in cardiopulmonary resuscitation: A concise review. J. Ultrasound 2017, 20, 193–198. [Google Scholar] [CrossRef]
- White, J. The Value of Focused Echocardiography During Cardiac Arrest. J. Diagn. Med. Sonogr. 2019, 35, 484–490. [Google Scholar] [CrossRef]
- Osman, A.; Sum, K.M. Role of upper airway ultrasound in airway management. J. Intensiv. Care 2016, 4, 52. [Google Scholar] [CrossRef]
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic ultrasonography: A narrative review. Intensiv. Care Med. 2019, 45, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Teran, F.; Prats, M.I.; Nelson, B.P.; Kessler, R.; Blaivas, M.; Peberdy, M.A.; Shillcutt, S.K.; Arntfield, R.T.; Bahner, D. Focused Transesophageal Echocardiography During Cardiac Arrest Resuscitation. Circ. 2020, 76, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, G.R.; Teran, F.; Lecluyse, V.; Costescu, A.; Belliveau, M.; Desjardins, G.; Denault, A. Transesophageal Echocardiography in Patients in Cardiac Arrest: The Heart and Beyond. Can. J. Cardiol. 2023, 39, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.; Rehman, M.A.; Jelic, T.; Berdnikov, A.; Teran, F.; Richards, S.; Askin, N.; Jarman, R. Transoesophageal echocardiography in cardiac arrest: A systematic review. Resuscitation 2021, 168, 167–175. [Google Scholar] [CrossRef]
- Borde, D.; Kumar, C.; Jasapara, A.; Shetty, V.; Juvekar, N.; Desurkar, V.; Gaidu, J.; Joshi, P.; Asegaonkar, B.; Kp, U.; et al. Use of a Video Laryngoscope to Reduce Complications of Transesophageal Echocardiography Probe Insertion: A Multicenter Randomized Study. J. Cardiothorac. Vasc. Anesthesia 2022, 36, 4289–4295. [Google Scholar] [CrossRef]
- Buschmann, C.T.; Tsokos, M. Frequent and rare complications of resuscitation attempts. Intensiv. Care Med. 2009, 35, 397–404. [Google Scholar] [CrossRef]
- Liu, L.; Karatasakis, A.; Kudenchuk, P.J.; Kirkpatrick, J.N.; Sayre, M.R.; Carlbom, D.J.; Johnson, N.J.; Probstfield, J.L.; Counts, C.; Branch, K.R.H. Scoping review of echocardiographic parameters associated with diagnosis and prognosis after resuscitated sudden cardiac arrest. Resuscitation 2023, 184, 109719. [Google Scholar] [CrossRef]
- Elfwén, L.; Hildebrand, K.; Schierbeck, S.; Sundqvist, M.; Ringh, M.; Claesson, A.; Olsson, J.; Nordberg, P. Focused cardiac ultrasound after return of spontaneous circulation in cardiac-arrest patients. Resuscitation 2019, 142, 16–22. [Google Scholar] [CrossRef]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.-C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Nasser, M.F.; Jabri, A.; Limaye, S.; Sharma, S.; Hamade, H.; Mhanna, M.; Aneja, A.; Gandhi, S. Echocardiographic Evaluation of Pulmonary Embolism: A Review. J. Am. Soc. Echocardiogr. 2023, 36, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Helviz, Y.; Einav, S. Maternal cardiac arrest. Curr. Opin. Anaesthesiol. 2019, 32, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European So-ciety of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Anavekar, N.S.; Mankad, S.V.; White, R.D.; Kashani, K.B.; Barsness, G.W.; Rabinstein, A.A.; Pislaru, S.V. Changes in left ventricular systolic and diastolic function on serial echocardiography after out-of-hospital cardiac arrest. Resuscitation 2018, 126, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Flint, N.; Siegel, R.J. Echo-Guided Pericardiocentesis: When and How Should It Be Performed? Curr. Cardiol. Rep. 2020, 22, 71. [Google Scholar] [CrossRef]
- Swol, J.; Darocha, T.; Paal, P.; Brugger, H.; Podsiadło, P.; Kosiński, S.; Puślecki, M.; Ligowski, M.; Pasquier, M. Extracorporeal Life Support in Accidental Hypothermia with Cardiac Arrest—A Narrative Review. Asaio J. 2022, 68, 153–162. [Google Scholar] [CrossRef]
- Nordmark, J.; Johansson, J.; Sandberg, D.; Granstam, S.-O.; Huzevka, T.; Covaciu, L.; Mörtberg, E.; Rubertsson, S. Assessment of intravascular volume by transthoracic echocardiography during therapeutic hypothermia and rewarming in cardiac arrest survivors. Resuscitation 2009, 80, 1234–1239. [Google Scholar] [CrossRef]
- Pastore, M.C.; Ilardi, F.; Stefanini, A.; Mandoli, G.E.; Palermi, S.; Bandera, F.; Benfari, G.; Esposito, R.; Lisi, M.; Pasquini, A.; et al. Bedside Ultrasound for Hemodynamic Monitoring in Cardiac Intensive Care Unit. J. Clin. Med. 2022, 11, 7538. [Google Scholar] [CrossRef]
- Boyd, J.H.; Sirounis, D.; Maizel, J.; Slama, M. Echocardiography as a guide for fluid management. Crit. Care 2016, 20, 274. [Google Scholar] [CrossRef]
- Pellicori, P.; Hunter, D.; Khin, H.H.E.; Cleland, J.G.F. How to diagnose and treat venous congestion in heart failure. Eur. Hear. J. 2024, 45, 1295–1297. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Spiegel, R.; Rola, P.; Price, S.; Corradi, F.; Hockstein, M. Multiorgan evaluation of perfusion and congestion using ultrasound in patients with shock. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, M.; Resseguier, N.; Thuny, F.; Paganelli, F.; Cautela, J.; Pinto, J.; Ammar, C.; Laine, M.; Bonello, L. Doppler echocardiography for assessment of systemic vascular resistances in cardiogenic shock patients. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.; Söderberg, S.; Gonzalez, M.C.; Tossavainen, E.; Henein, M.Y. Echocardiography based estimation of pulmonary vascular resistance in patients with pulmonary hypertension: A simultaneous Doppler echocardiography and cardiac catheterization study. Eur. Heart J.-Cardiovasc. Imaging 2011, 12, 961–966. [Google Scholar] [CrossRef][Green Version]
- Gamarra, A.; Díez-Villanueva, P.; Salamanca, J.; Aguilar, R.; Mahía, P.; Alfonso, F. Development and Clinical Application of Left Ventricular–Arterial Coupling Non-Invasive Assessment Methods. J. Cardiovasc. Dev. Dis. 2024, 11, 141. [Google Scholar] [CrossRef]
- Kazimierczyk, R.; Kazimierczyk, E.; Knapp, M.; Sobkowicz, B.; Malek, L.A.; Blaszczak, P.; Ptaszynska-Kopczynska, K.; Grzywna, R.; Kaminski, K.A. Echocardiographic Assessment of Right Ventricular–Arterial Coupling in Predicting Prognosis of Pulmonary Arterial Hypertension Patients. J. Clin. Med. 2021, 10, 2995. [Google Scholar] [CrossRef]
- Arntfield, R.; Pace, J.; McLeod, S.; Granton, J.; Hegazy, A.; Lingard, L. Focused transesophageal echocardiography for emergency physicians—Description and results from simulation training of a structured four-view examination. Ultrasound J. 2015, 7, 27. [Google Scholar] [CrossRef]
- Arntfield, R.; Pace, J.; Hewak, M.; Thompson, D. Focused Transesophageal Echocardiography by Emergency Physicians is Feasible and Clinically Influential: Observational Results from a Novel Ultrasound Program. J. Emerg. Med. 2016, 50, 286–294. [Google Scholar] [CrossRef]
- Arntfield, R.; Lau, V.; Landry, Y.; Priestap, F.; Ball, I. Impact of Critical Care Transesophageal Echocardiography in Medical–Surgical ICU Patients: Characteristics and Results From 274 Consecutive Examinations. J. Intensiv. Care Med. 2020, 35, 896–902. [Google Scholar] [CrossRef]
- Teran, F.; West, F.M.; Jelic, T.; Taylor, L.; Jafry, Z.M.; Burns, K.M.; Owyang, C.G.; Emt, C.C.; Abella, B.S.; Andrus, P.; et al. Resuscitative transesophageal echocardiography in emergency departments in the United States and Canada: A cross-sectional survey. Am. J. Emerg. Med. 2024, 76, 164–172. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Girard, M.; Desjardins, G.; Denault, A.Y. Transesophageal lung ultrasonography: A novel technique for investigating hypoxemia. Can. J. Anaesth. 2016, 63, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Denault, A.Y.; Roberts, M.; Cios, T.; Malhotra, A.; Paquin, S.C.; Tan, S.; Cavayas, Y.A.; Desjardins, G.; Klick, J. Transgastric Abdominal Ultrasonography in Anesthesia and Critical Care: Review and Proposed Approach. Anesthesia Analg. 2021, 133, 630–647. [Google Scholar] [CrossRef]
- Lazzarin, T.; Tonon, C.R.; Martins, D.; Fávero, E.L.; Baumgratz, T.D.; Pereira, F.W.L.; Pinheiro, V.R.; Ballarin, R.S.; Queiroz, D.A.R.; Azevedo, P.S.; et al. Post-Cardiac Arrest: Mechanisms, Management, and Future Perspectives. J. Clin. Med. 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Elfwén, L.; Lagedal, R.; Rubertsson, S.; James, S.; Oldgren, J.; Olsson, J.; Hollenberg, J.; Jensen, U.; Ringh, M.; Svensson, L.; et al. Post-resuscitation myocardial dysfunction in out-of-hospital cardiac arrest patients randomized to immediate coronary angiography versus standard of care. IJC Heart Vasc. 2020, 27, 100483. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villar, S.; Kraut, J.; Arévalo-Serrano, J.; Sakka, S.; Harris, C.; Awad, I.; Toolan, M.; Vanapalli, S.; Collins, A.; Spataru, A.; et al. Systemic acidemia impairs cardiac function in critically Ill patients. eClinicalMedicine 2021, 37, 100956. [Google Scholar] [CrossRef] [PubMed]
- Frasch, M.G.; Giussani, D.A. Heart during acidosis: Etiology and early detection of cardiac dysfunction. eClinicalMedicine 2021, 37, 100994. [Google Scholar] [CrossRef]
- Cha, K.-C.; Kim, H.I.; Kim, O.H.; Cha, Y.S.; Lee, K.H.; Hwang, S.O. Echocardiographic patterns of postresuscitation myocardial dysfunction. Resuscitation 2018, 124, 90–95. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Chonde, M.D.; Dezfulian, C. Myocardial Dysfunction and Shock after Cardiac Arrest. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Ruiz-Bailén, M.; de Hoyos, E.A.; Ruiz-Navarro, S.; Díaz-Castellanos, M.; Rucabado-Aguilar, L.; Gómez-Jiménez, F.J.; Martínez-Escobar, S.; Moreno, R.M.; Fierro-Rosón, J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation 2005, 66, 175–181. [Google Scholar] [CrossRef]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. New Engl. J. Med. 2021, 385, 2544–2553. [Google Scholar] [CrossRef]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. New Engl. J. Med. 2019, 380, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Laursen, C.B.; Davidsen, J.R.; Madsen, P.H. Utility of lung ultrasound in near-drowning victims. BMJ Case Rep. 2012, 2012, bcr0120125687. [Google Scholar] [CrossRef] [PubMed]
- D’andrea, A.; Conte, M.; Cavallaro, M.; Scarafile, R.; Riegler, L.; Cocchia, R.; Pezzullo, E.; Carbone, A.; Natale, F.; Santoro, G.; et al. Transcranial Doppler ultrasonography: From methodology to major clinical applications. World J. Cardiol. 2016, 8, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Chonde, M.D.; Shafton, A.; Abu-Daya, H.; Chalhoub, D.; Althouse, A.D.; Rittenberger, J.C. Echocardiographic left ventricular systolic dysfunction early after resuscitation from cardiac arrest does not predict mortality or vasopressor requirements. Resuscitation 2016, 106, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ramjee, V.; Grossestreuer, A.V.; Yao, Y.; Perman, S.M.; Leary, M.; Kirkpatrick, J.N.; Forfia, P.R.; Kolansky, D.M.; Abella, B.S.; Gaieski, D.F. Right ventricular dysfunction after resuscitation predicts poor outcomes in cardiac arrest patients independent of left ventricular function. Resuscitation 2015, 96, 186–191. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Anavekar, N.S.; Mankad, S.V.; Khasawneh, M.; White, R.D.; Barsness, G.W.; Rabinstein, A.A.; Kashani, K.B.; Pislaru, S.V. Echocardiographic left ventricular diastolic dysfunction predicts hospital mortality after out-of-hospital cardiac arrest. J. Crit. Care 2018, 47, 114–120. [Google Scholar] [CrossRef]
- Chang, W.-T.; Ma, M.H.-M.; Chien, K.-L.; Huang, C.-H.; Tsai, M.-S.; Shih, F.-Y.; Yuan, A.; Tsai, K.-C.; Lin, F.-Y.; Lee, Y.-T.; et al. Postresuscitation myocardial dysfunction: Correlated factors and prognostic implications. Intensive Care Med. 2007, 33, 88–95. [Google Scholar] [CrossRef]
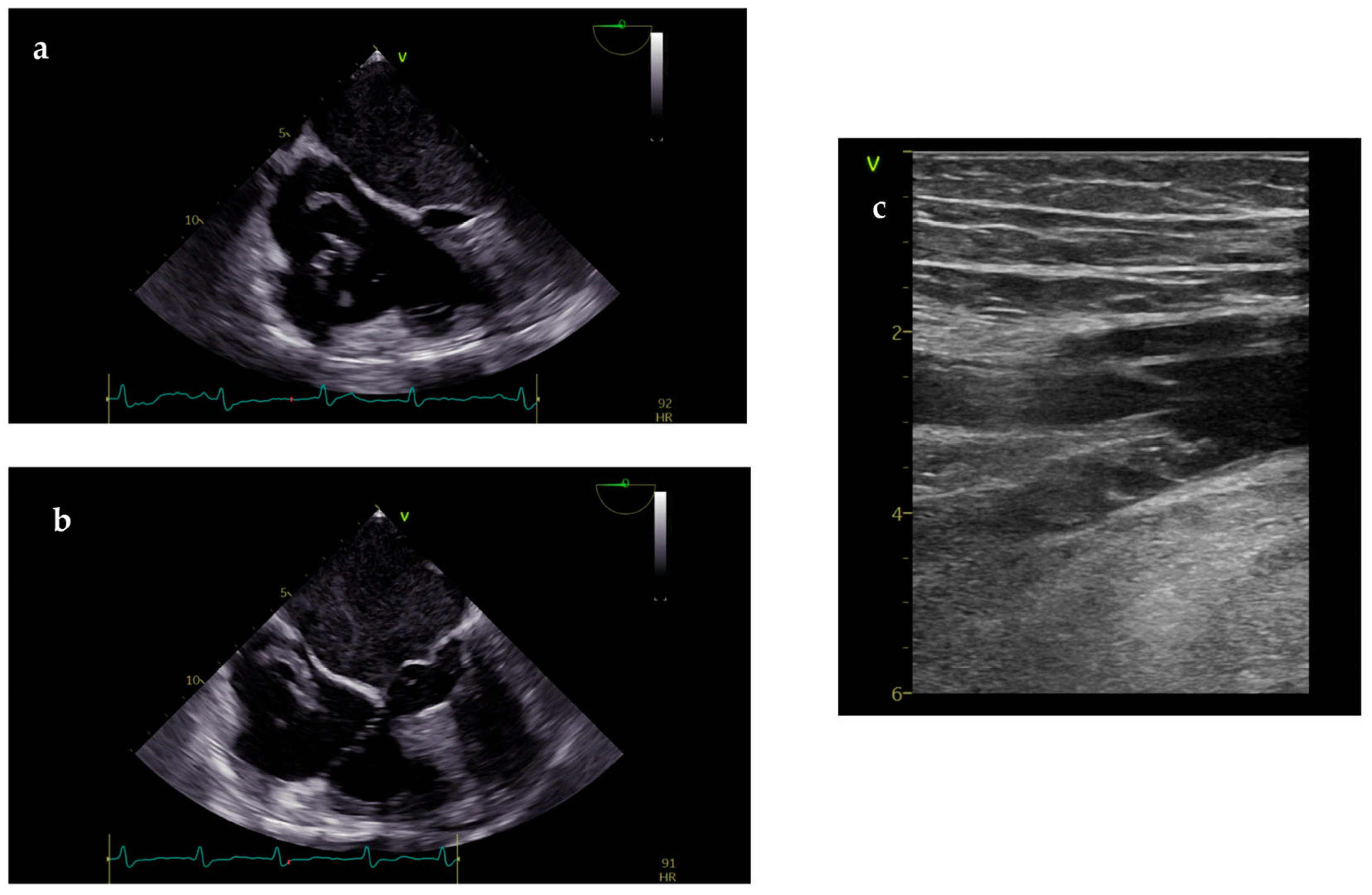
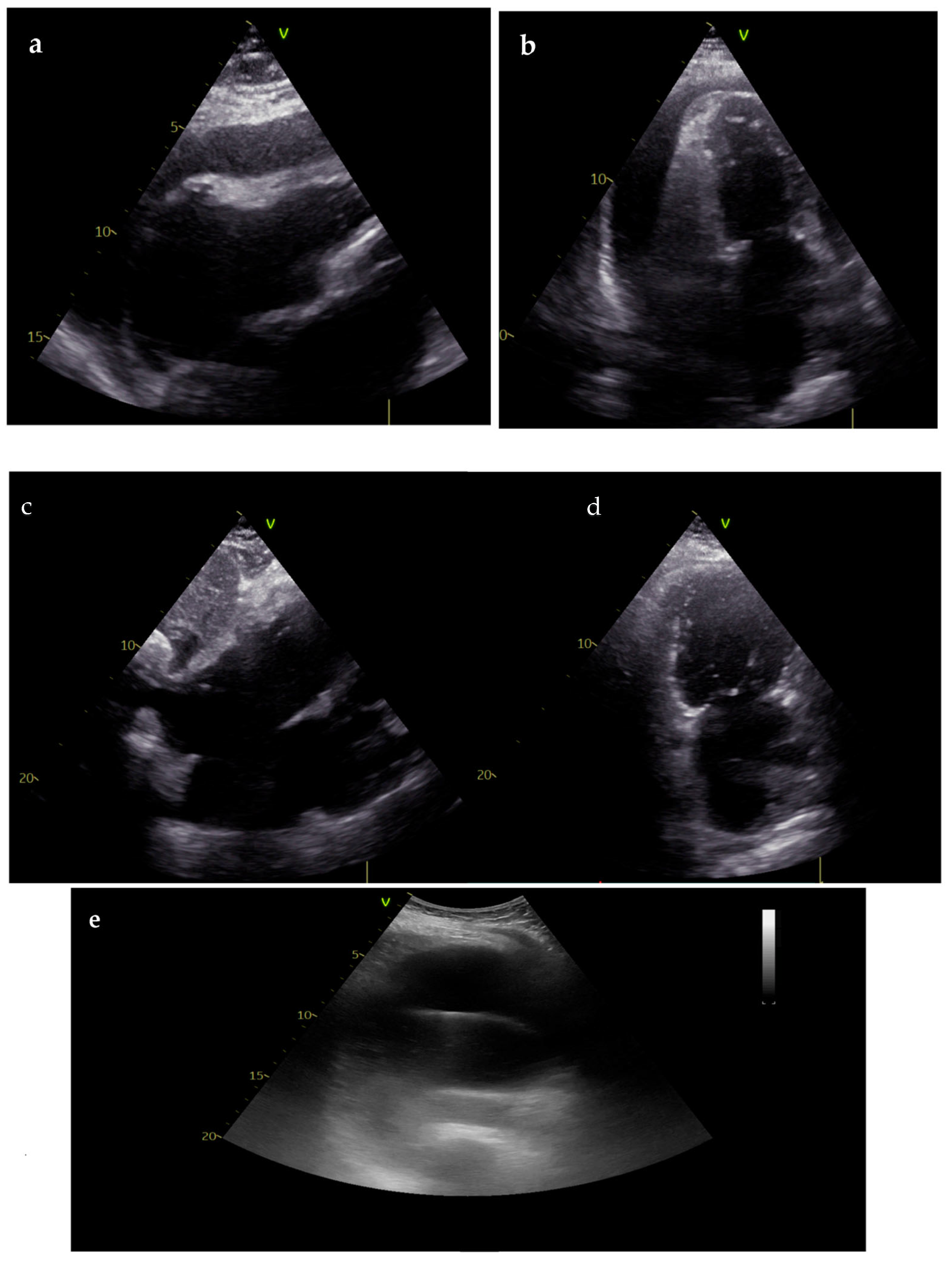
| Goals of the US During CPR | Goals of the US in Post-Resuscitation Care |
|---|---|
| Diagnosis of reversible causes | Diagnosis of the underlying cause of CA |
| Confirm the effectiveness of chest compressions | Hemodynamic monitoring and optimization |
| Determine the presence of cardiac contractions or “standstill” | Assist ventilatory support |
| Confirm bilateral ventilation after intubation | Assessment of CPR complication |
| Assist invasive procedures (pericardiocentesis, vascular cannulation, extracorporeal CPR) | Assessment of multiorgan function (prognosis) |
| Assist invasive procedures |
| Potential Cause | US Views | Suggestive Findings | Intervention |
|---|---|---|---|
| Profound hypovolemia | Subcostal Abdomen | Small LV and RV cavity size Near end-systolic obliteration (“kissing ventricle”) Collapsed IVC (<10 mm) Massive bleeding in the abdomen | Fluid administration; assess response |
| Cardiac tamponade | Subcostal | Pericardial effusion Collapsed cardiac chambers Congested IVC | Pericardiocentesis; guide the procedure and assess the response |
| Massive pulmonary embolism | Subcostal Lower limbs | Markedly dilated RV Pressure overload of RV Thrombus-in-transit Congested IVC Presence of DVT (positive CUS) | Consideration of thrombolysis |
| Tension pneumothorax | Lung | Absence of lung sliding during ventilation | Needle decompression, assess response |
| View | Goals and Diagnosis |
|---|---|
| Tamponade Evaluation of LV/RV contractility Signs of PE Signs of profound hypovolemia Signs of compression due to pneumothorax |
| Determine AMC Optimization of chest compression avoiding LVOT obstruction Evaluation of AscAo |
| Tamponade Evaluation of LV/RV contractility Signs of PE Signs of profound hypovolemia; |
| Evaluation of intravascular volume (SCV) Thrombus in transit Assist venous procedures |
| Evaluation of DescAo Assist arterial procedures |
| Echocardiographic Findings | Parameters |
|---|---|
| RV dilatation | RV/LV ratio > 1 RV basal diameter > 41 mm RV mid diameter > 35 mm |
| RV systolic disfunction | TAPSE < 17 mm S’ wave (TDI) < 10 cm/s RV-FAC < 35% RV Tei index (PW) > 0.43 RV Tei index (TDI) > 0.54 RV free wall strain > −20% |
| McConnell Sign | RV basal and mid-free wall akinesia and normal motion of the RV apex |
| RV pressure overload | TR Vmax > 2.9 m/s Pulmonary flow AcT < 60 msec Pulmonary flow mid-systolic notch Paradoxical IVS motion Flattened IVS with D-shaped LV Dilated PA (>25 mm) TAPSE: PASP ratio < 0.4 Dilated IVC (>21 mm) and/or diminished collapsibility |
| 60/60 Sign | TR jet gradient < 60 mmHg and Pulmonary AcT < 60 ms |
| Thrombus in transit | Thrombus in RV, RA or PA |
| Parameter | Utility | How to Calculate | Normal Values and Interpretation | |
|---|---|---|---|---|
| Perfusion parameters | LVOT VTI | The distance that blood travels across the LVOT during the cardiac cycle | Tracing the PWD spectral display of the LVOT | LVOT-VTI > 18 cm |
| SV | The volume of blood pumped during each systolic cardiac contraction | SV = LVOT area * x LVOT-VTI SVi= SV/BSA | SV > 70 mL SVi >35 mL/mq | |
| CO and CI | Amount of blood pumped by the heart in a minute; | CO = SV × HR CI = CO/BSA | CO > 4 l/min CI < 2.5 l/min/mq | |
| Preload parameters (fluid responsiveness and fluid tolerance) | IVC diameter and collapsibility | Used to estimate RA pressure and volemic status | Diameters of IVC at end-expiration and inspiration in subcostal view | IVC < 21 mm that collapses > 50% (RAP 0–5 mmHg); IVC > 21 mm that collapses > 50% or IVC < 21 mm that collapses < 50% (RAP 5–10 mmHg); IVC > 21 mm that collapses < 50% (RAP 10–20 mmHg) |
| JVD ratio | Used to estimate RA pressure and volemic status | JVD during Valsalva/JVD at rest | JVD ratio < 3 suggests elevated RAP and fluid overload | |
| LVOT-VTI variability | Dynamic parameters that suggest fluid responsiveness | Evaluation of LVOT-VTI in different respiratory phases during MV, after PLR or fluid challenge | Change in LVOT-VTI < 10–15% indicates fluid responsiveness | |
| VExUS score | Evaluation of systemic congestion in four grades | Combined evaluation of IVC diameter and venous flow pattern using PWD in HV, PV, and IRV | VExUS score 0 = no congestion; VExUS score 3 = severe congestion | |
| LUS B-lines | Evaluation of pulmonary congestion | Evaluation of B-lines in 8 to 12 zones | B-lines < 3 for scanning zone = normal; Multiple and diffuse B-lines = severe congestion | |
| E/e’ | Marker of LV filling pressure that correlates with PCWP [ PCWP ≈ 1.24 × (E/e) + 1.9] | The ratio between mitral inflow E velocity using PWD and e’ lateral and medial velocity using TDI | E/e’ < 7 = normal filling pressure; E/e’ > 15 = elevated filling pressure | |
| Afterload parameters | SVR | Determinant of LV afterload and reflects the tone of systemic blood vessels. | MAP-CVP/CO ** | SVR 800–1200 dynes·s/cm5= 10–15 WU |
| PASS | Estimation of pulmonary artery systolic pressure | PASP = 4 × (TRV2) + RAP | PASP < 35 mmHg | |
| PAMP | Estimation of pulmonary artery mean pressure | PAPM = 0.61 × PASP + 2 or PAPM = 4 × (PRV2) + RAP | PAMP < 20 mmHg | |
| PVR | Determinant of RV afterload and reflect the tone of pulmonary blood vessels | PVR = (PAMP-PCWP)/CO | PVR < 2 WU | |
| TRV/RVOT-VTI ratio | Parameter to estimate PVR and PAP | The ratio between TRV and RVOT-VTI was calculated tracing the PWD spectral display of the RVOT. | TRV/RVOT-VTI ratio < 0.45 |
| Parameters | THE | Notes | |
|---|---|---|---|
| Systolic function | Serial LVEF assessment | LVEF evaluated through the Biplane method | Dynamic changes in systolic function are associated with outcomes after OHCA more than single static measurements. |
| RV function | RV FAC and 3D RV ejection fraction * | Reduced RV systolic function (RV FAC < 35% or 3D RV ejection fraction < 45%) associated with worse outcome | |
| Diastolic function | LV diastolic function and filling pressures | Ratio of early mitral Doppler filling and mitral annular excursion (E/e’) * | LV diastolic dysfunction (E/e’ > 14) is associated with increased mortality after OHCA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauriello, A.; Marrazzo, G.; Del Vecchio, G.E.; Ascrizzi, A.; Roma, A.S.; Correra, A.; Sabatella, F.; Gioia, R.; Desiderio, A.; Russo, V.; et al. Echocardiography in Cardiac Arrest: Incremental Diagnostic and Prognostic Role during Resuscitation Care. Diagnostics 2024, 14, 2107. https://doi.org/10.3390/diagnostics14182107
Mauriello A, Marrazzo G, Del Vecchio GE, Ascrizzi A, Roma AS, Correra A, Sabatella F, Gioia R, Desiderio A, Russo V, et al. Echocardiography in Cardiac Arrest: Incremental Diagnostic and Prognostic Role during Resuscitation Care. Diagnostics. 2024; 14(18):2107. https://doi.org/10.3390/diagnostics14182107
Chicago/Turabian StyleMauriello, Alfredo, Gemma Marrazzo, Gerardo Elia Del Vecchio, Antonia Ascrizzi, Anna Selvaggia Roma, Adriana Correra, Francesco Sabatella, Renato Gioia, Alfonso Desiderio, Vincenzo Russo, and et al. 2024. "Echocardiography in Cardiac Arrest: Incremental Diagnostic and Prognostic Role during Resuscitation Care" Diagnostics 14, no. 18: 2107. https://doi.org/10.3390/diagnostics14182107
APA StyleMauriello, A., Marrazzo, G., Del Vecchio, G. E., Ascrizzi, A., Roma, A. S., Correra, A., Sabatella, F., Gioia, R., Desiderio, A., Russo, V., & D’Andrea, A. (2024). Echocardiography in Cardiac Arrest: Incremental Diagnostic and Prognostic Role during Resuscitation Care. Diagnostics, 14(18), 2107. https://doi.org/10.3390/diagnostics14182107









