Exploring the Application of the Artificial-Intelligence-Integrated Platform 3D Slicer in Medical Imaging Education
Abstract
:1. Introduction
- -
- RQ 1: What are the applications of 3D Slicer in the field of clinical medical imaging?
- -
- RQ 2: What are the potential applications of the 3D Slicer platform in medical imaging education?
- -
- RQ 3: What key limitations are militating against the inclusion of the 3D Slicer platform in medical imaging education?
2. Methodology
3. Findings
3.1. Application of 3D Slicer Platform in Medical Image Analysis
| Author | Reference | Raw Data | Specific Application | Implication |
|---|---|---|---|---|
| Yuan et al., 2021 | [35] | CT, MRI, 3D models, and the original image | Transforming 2D information from various imaging techniques into 3D information. | Optimizes medical-imaging-teaching activities for the development of modern medical education. |
| Hadi et al., 2022 | [36] | SPECT and CT | Image segmentation, registration, and visualization. | Assists students in navigating intricate steps required in the importation of tomographic data. |
| Durnea et al., 2021 | [21] | MRI | Creating 3D models base on cross-sectional image sets. | Provides a comprehensive and 3D display of anatomical structures and lesions. |
| Bindschadler et al., 2022 | [37] | CT | Turning 4D Cardiac CT into VR/AR | 4D imaging and related workflows will be useful for educational purposes. |
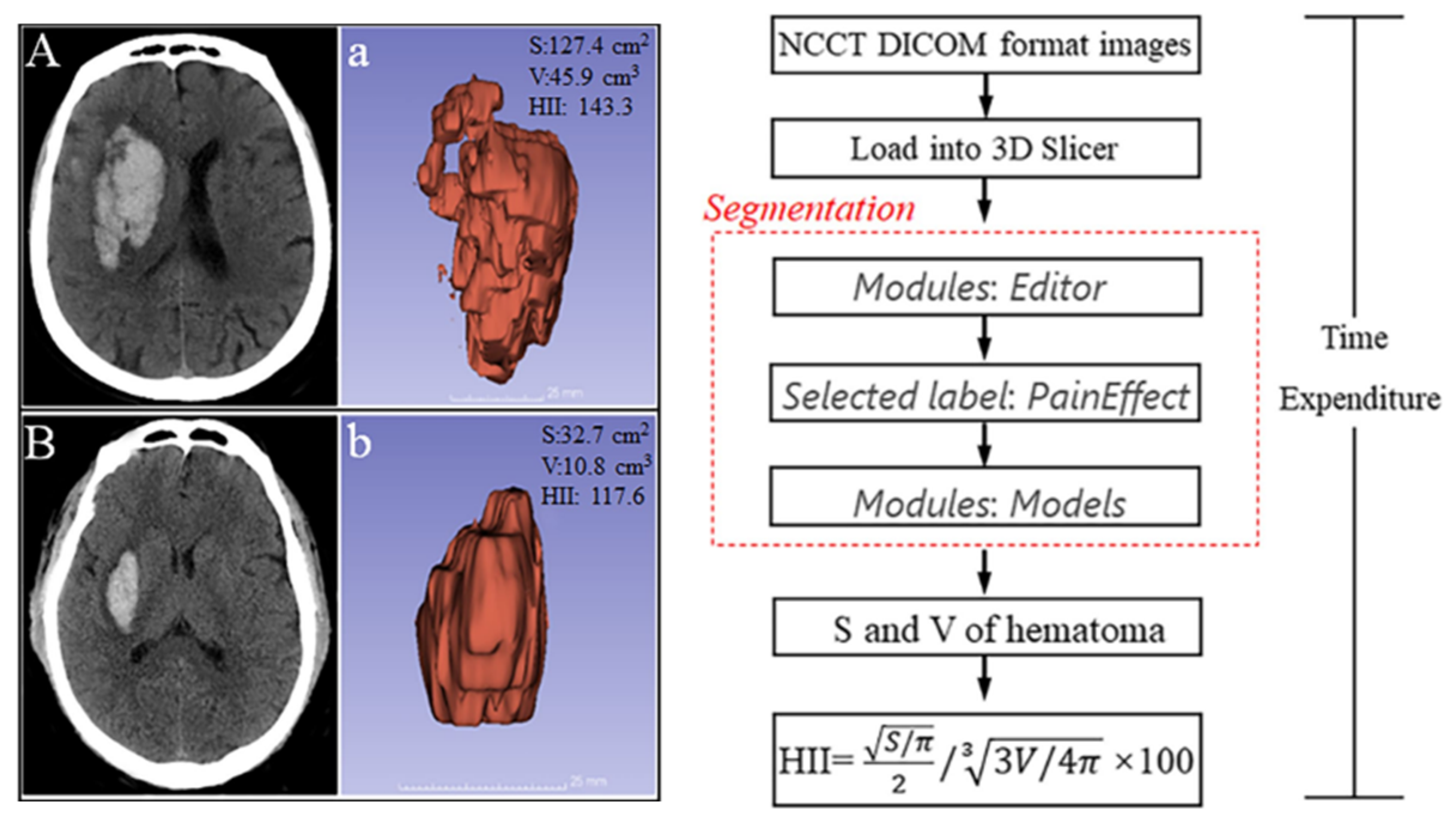
| Cao et al., 2022 | [38] | CT | Calculating the surface area (S) and volume (V) of hematoma. | Providing quantitative indicators for student clinical practice. |
| Levine et al., 2020 | [39] | CT and X-ray | Computationally simulating an X-ray-type image from CT data. | Provides an easy way for students to make digitally reconstructed radiographs |
| Yang et al., 2019 | [40] | MRI | Aiding neurosurgeons in planning the location of an anticipated craniotomy relative to a preoperatively imaged tumor in a physical-to-virtual setup. | Provides more intuitive and interactive teaching tools. |
| Eskandari et al., 2020 | [41] | CT | Assessing the irradiated volumes and both displacement magnitudes and vectors for the heart and left lung. | Enables assessment of the irradiation field in radiographic examination and estimation of radiation dose. |
| You et al., 2022 | [42] | CT | Transforming medical images into digital models to prepare for 3D printing. | Compensates for the lack of skills in 3D design and modeling software. |
| Chen et al., 2022 | [43] | CT | Automatically measuring the morphological parameters of the distal femur. | Enables automatic measurement of bones and other body parts. |
| Shi et al., 2022 | [44] | MRI | Determining the preoperative evaluation value before microsurgical vascular decompression. | More accurate than MRI in preoperative evaluation of neurovascular relationship and offending vessel. |
| Liao et al., 2022 | [45] | CT | Assisting endoscopic treatment for patients with hypertensive cerebral hemorrhage. | Better demonstrates and explains pathological changes and anatomical structures |
| Huie et al., 2022 | [46] | CT | Iterating slice-by-slice through 3D structures to calculate second moment of area and other cross-sectional properties. | Analyzing the internal geometric structure of biological structures provides more possibilities. |
| Huang et al., 2022 | [47] | CT | A fly-through visualization of both the inside and outside of the duct. | Provides more insight into pancreatic diseases than a fly-through visualization. |
| Briend et al., 2020 | [48] | MRI | Medical image visualization and analysis. | Allows medical imaging to be presented to students in a more intuitive and clear manner. |
| Zaffino et al., 2020 | [49] | CT | Providing and a basic input–output mechanism. | Providing diverse learning resources for medical students. |
| Liu et al., 2022 | [50] | PET, MRI, and CT | Predicting epileptic lesions according to signals and morphology of images | Medical students can gain a deeper understanding of the characteristics and manifestations of epilepsy. |
3.2. Potential Applications of the 3D Slicer Platform in Medical Imaging Education
3.2.1. Application of 3D Slicer in Image Segmentation and Reconstruction
3.2.2. Application of 3D Slicer in Computer-Aided Diagnosis and Research
3.2.3. Application of 3D Slicer in Quantitative Analysis of Medical Imaging
4. Discussion
- The development of teaching methods and materials for medical image education based on 3D Slicer in order to improve the effectiveness and quality of medical image education.
- Simplifying the interface and operations of 3D Slicer to make it more user-friendly and accessible to more educational practitioners and students.
- Conducting educational experiments and evaluations to determine the effectiveness and feasibility of 3D Slicer in medical image education.
- Integration with other educational resources and tools. Medical imaging education requires the comprehensive use of various teaching resources and tools, such as textbooks, virtual laboratories, and simulators.
- The expansion of applications for the platform involves the further integration of imaging technology and diagnostic imaging needs, constituting a path for future research.
- Developing and expanding modules related to specific educational objectives to meet teaching requirements and enhance this software’s accessibility.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uppot, R.N.; Laguna, B.; Mccarthy, C.J.; Novi, G.D.; Phelps, A.; Siegel, E.; Courtier, J. Implementing Virtual and Augmented Reality Tools for Radiology Education and Training, Communication, and Clinical Care. Radiology 2019, 291, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Li, X.; Fatehi, M.; Hamarneh, G. Guidelines and evaluation of clinical explainable AI in medical image analysis. Med. Image Anal. 2022, 84, 102684. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.M.A.; Naqvi, R.A.; Biswas, M.; Loh, W.K. Deep Perceptual Enhancement for Medical Image Analysis. IEEE J. Biomed. Health Inform. 2022, 26, 4826–4836. [Google Scholar] [CrossRef]
- Rahman, H.; Khan, A.R.; Sadiq, T.; Farooqi, A.H.; Khan, I.U.; Lim, W.H. A Systematic Literature Review of 3D Deep Learning Techniques in Computed Tomography Reconstruction. Tomography 2023, 9, 2158–2189. [Google Scholar] [CrossRef] [PubMed]
- Trullàs, J.C.; Blay, C.; Sarri, E.; Pujol, R. Effectiveness of problem-based learning methodology in undergraduate medical education: A scoping review. BMC Med. Educ. 2022, 22, 104. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, L.; Bu, H.; Feng, M.; Yang, Y.; Zhang, Y.; Liu, F.; Liu, Q.; Li, X.; Jiao, X. Effect of hybrid teaching incorporating problem-based learning on student performance in pathophysiology. J. Int. Med. Res. 2020, 48, 300060520949402. [Google Scholar] [CrossRef] [PubMed]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. 3D Slicer: A Platform for Subject-Specific Image Analysis, Visualization, and Clinical Support. In Intraoperative Imaging and Image-Guided Therapy; Jolesz, F.A., Ed.; Springer: New York, NY, USA, 2014; pp. 277–289. [Google Scholar]
- Kapur, T.; Pieper, S.; Fedorov, A.; Fillion-Robin, J.C.; Halle, M.; O’donnell, L.; Lasso, A.; Ungi, T.; Pinter, C.; Finet, J.; et al. Increasing the impact of medical image computing using community-based open-access hackathons: The NA-MIC and 3D Slicer experience. Med. Image Anal. 2016, 33, 176–180. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Ari, A.P.; Akkurt, B.H.; Musigmann, M.; Mammadov, O.; Blomer, D.A.; Kasap, D.N.G.; Henssen, D.J.H.A.; Nacul, N.G.; Sartoretti, E.; Sartoretti, T.; et al. Pseudoprogression prediction in high grade primary CNS tumors by use of radiomics. Sci. Rep. 2022, 12, 5915. [Google Scholar] [CrossRef]
- Brown, K.H.H.; Illyuk, J.; Ghita, M.; Walls, G.M.M.; Mcgarry, C.K.K.; Butterworth, K.T.T. Assessment of Variabilities in Lung-Contouring Methods on CBCT Preclinical Radiomics Outputs. Cancers 2023, 15, 2677. [Google Scholar] [CrossRef]
- Dudurych, I.; Garcia-Uceda, A.; Saghir, Z.; Tiddens, H.a.W.M.; Vliegenthart, R.; De Bruijne, M. Creating a training set for artificial intelligence from initial segmentations of airways. Eur. Radiol. Exp. 2021, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Risoli, C.; Nicolo, M.; Colombi, D.; Moia, M.; Rapacioli, F.; Anselmi, P.; Michieletti, E.; Ambrosini, R.; Di Terlizzi, M.; Grazioli, L.; et al. Different Lung Parenchyma Quantification Using Dissimilar Segmentation Software: A Multi-Center Study for COVID-19 Patients. Diagnostics 2022, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- De Geer, A.F.; Van Alphen, M.J.A.; Zuur, C.L.; Loeve, A.J.; Van Veen, R.L.P.; Karakullukcu, M.B. A hybrid registration method using the mandibular bone surface for electromagnetic navigation in mandibular surgery. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Huang, T.; Tang, J.; Zhang, W.; Xu, W.; Wang, N.; Deng, Y.; Yu, X.; Xu, L. Wearable Mixed-Reality Holographic Navigation Guiding the Management of Penetrating Intracranial Injury Caused by a Nail. J. Digit. Imaging 2021, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Rajani, K.; Olson, I.; Jacobs, J.J.; Riviere-Cazaux, C.; Burns, K.; Carlstrom, L.; Schroeder, M.; Oh, J.; Howe, C.L.; Rahman, M.; et al. Methods for intratumoral microdialysis probe targeting and validation in murine brain tumor models. J. Neurosci. Methods 2021, 363, 109321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Li, Z.; Wei, H.; Cai, Q.; Chen, Q.; Liu, Z.; Ye, H.; Song, P.; Cheng, L.; et al. Clinical application of 3D-Slicer + 3D printing guide combined with transcranial neuroendoscopic in minimally invasive neurosurgery. Sci. Rep. 2022, 12, 20421. [Google Scholar] [CrossRef] [PubMed]
- Torres Berdeguez, M.B.; Thomas, S.; Oliveira, S.M.; Vasconcellos De Sa, L.; Lopes De Souza, S.A.; Milian, F.M.; Xavier Da Silva, A. Individual dose planning in radiosynoviorthesis treatment: Step by step. Appl. Radiat. Isot. 2020, 163, 109177. [Google Scholar] [CrossRef]
- Whyne, C.M.; Underwood, G.; Davidson, S.R.H.; Robert, N.; Huang, C.; Akens, M.K.; Fichtinger, G.; Yee, A.J.M.; Hardisty, M. Development and validation of a radiofrequency ablation treatment planning system for vertebral metastases. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 2339–2347. [Google Scholar] [CrossRef]
- Connolly, L.; Deguet, A.; Leonard, S.; Tokuda, J.; Ungi, T.; Krieger, A.; Kazanzides, P.; Mousavi, P.; Fichtinger, G.; Taylor, R.H. Bridging 3D Slicer and ROS2 for Image-Guided Robotic Interventions. Sensors 2022, 22, 5336. [Google Scholar] [CrossRef]
- Durnea, C.M.; Siddiqi, S.; Nazarian, D.; Munneke, G.; Sedgwick, P.M.; Doumouchtsis, S.K. 3D-Volume Rendering of the Pelvis with Emphasis on Paraurethral Structures Based on MRI Scans and Comparisons between 3D Slicer and OsiriX (R). J. Med. Syst. 2021, 45, 27. [Google Scholar] [CrossRef]
- Wasserthal, J.; Breit, H.C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust segmentation of 104 anatomic structures in CT images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef] [PubMed]
- Locastro, E.; Veeraraghavan, H.; Kapur, T.; Iyer, A.; Pinter, C.; Sharp, G.; Deasy, J.; Apte, A. Slicer: An Extension for 3D Slicer to Execute CERR Analysis Routines. Med. Phys. 2022, 49, E676. [Google Scholar]
- Huang, H.; Zheng, H.; Lin, L.; Cai, M.; Hu, H.; Zhang, Q.; Chen, Q.; Iwamoto, Y.; Han, X.; Chen, Y.W.; et al. Medical Image Segmentation with Deep Atlas Prior. IEEE Trans. Med. Imaging 2021, 40, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Sareen, K. Development of a navigable 3D virtual model of temporal bone anatomy. J. Vis. Commun. Med. 2023, 46, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Pujol, S.; Baldwin, M.; Nassiri, J.; Kikinis, R.; Shaffer, K. Using 3D Modeling Techniques to Enhance Teaching of Difficult Anatomical Concepts. Acad. Radiol. 2016, 23, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.E.; Schleicher, R.; Laguna, S.; Billot, B.; Schaefer, P.; Mckaig, B.; Goldstein, J.N.; Sheth, K.N.; Rosen, M.S.; Kimberly, W.T. Quantitative Brain Morphometry of Portable Low-Field-Strength MRI Using Super-Resolution Machine Learning. Radiology 2023, 306, e220522. [Google Scholar] [CrossRef]
- Erdem, H.; Tekeli, M.; Cevik, Y.; Kilic Safak, N.; Kaya, O.; Boyan, N.; Oguz, O. Three-Dimensional (3D) Analysis of Orbital Morphometry in Healthy Anatolian Adults: Sex, Side Discrepancies, and Clinical Relevance. Cureus 2023, 15, e45208. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, J.; Li, R.; Zhou, J. Learning multi-modal brain tumor segmentation from privileged semi-paired MRI images with curriculum disentanglement learning. Comput. Biol. Med. 2023, 159, 106927. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, J.; Ma, J. MURF: Mutually Reinforcing Multi-modal Image Registration and Fusion. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 12148–12166. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Chen, S.; Huang, W.; Liu, D.; Ruan, G.; Huang, Q.; Gong, Q.; Liu, L.; Chen, H. Multiscale feature fusion network for 3D head MRI image registration. Med. Phys. 2023, 50, 5609–5620. [Google Scholar] [CrossRef]
- Shao, W.; Zuo, Y.; Shi, Y.; Wu, Y.; Tang, J.; Zhao, J.; Sun, L.; Lu, Z.; Sheng, J.; Zhu, Q.; et al. Characterizing the Survival-Associated Interactions between Tumor-infiltrating Lymphocytes and Tumors from Pathological Images and Multi-omics Data. IEEE Trans. Med. Imaging 2023, 42, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Sun, D.; Chang, C.; Zhou, S.; Huang, Q. An omics-to-omics joint knowledge association subtensor model for radiogenomics cross-modal modules from genomics and ultrasonic images of breast cancers. Comput. Biol. Med. 2023, 155, 106672. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, Y.; Wang, M.; Su, X. MRGCN: Cancer subtyping with multi-reconstruction graph convolutional network using full and partial multi-omics dataset. Bioinformatics 2023, 39, btad353. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Chen, X.M.; Zhai, J.; Chen, Y.D.; Liu, Q.X.; Tan, Z.X.; Chen, G.; Zhuang, K.L.; Zhang, J.Y.; Xu, X.; et al. Application of 3D modeling and fusion technology of medical image data in image teaching. BMC Med. Educ. 2021, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Abdullah, A.N.; Hashikin, N.A.; Ying, C.K.; Yeong, C.H.; Yoon, T.L.; Ng, K.H. Utilizing 3D Slicer to incorporate tomographic images into GATE Monte Carlo simulation for personalized dosimetry in yttrium-90 radioembolization. Med. Phys. 2022, 49, 7742–7753. [Google Scholar] [CrossRef] [PubMed]
- Bindschadler, M.; Buddhe, S.; Ferguson, M.R.; Jones, T.; Friedman, S.D.; Otto, R.K. HEARTBEAT4D: An Open-source Toolbox for Turning 4D Cardiac CT into VR/AR. J. Digit. Imaging 2022, 35, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.P.; Liu, M.; Wang, M.M.; Ding, J.; Mao, K.S.; Liu, K.F.; Li, S. 3D slicer-based calculation of hematoma irregularity index for predicting hematoma expansion in intracerebral hemorrhage. Bmc Neurol. 2022, 22, 452. [Google Scholar] [CrossRef]
- Levine, L.; Levine, M. DRRGenerator: A Three-dimensional Slicer Extension for the Rapid and Easy Development of Digitally Reconstructed Radiographs. J. Clin. Imaging Sci. 2020, 10, 69. [Google Scholar] [CrossRef]
- Yang, X.C.; Narasimhan, S.; Luo, M.; Thompson, R.C.; Chambless, L.B.; Morone, P.J.; He, L.; Dawant, B.M.; Miga, M.I. Development and evaluation of a “trackerless” surgical planning and guidance system based on 3D Slicer. J. Med. Imaging 2019, 6, 035002. [Google Scholar] [CrossRef]
- Eskandari, A.; Nasseri, S.; Gholamhosseinian, H.; Hosseini, S.; Farzaneh, M.J.K.; Keramati, A.; Naji, M.; Rostami, A.; Momennezhad, M. Evaluation of the heart and lung dosimetric parameters in deep inspiration breath hold using 3D Slicer. Radiat. Oncol. J. 2020, 38, 68–76. [Google Scholar] [CrossRef]
- You, Y.J.; Niu, Y.L.; Sun, F.B.; Huang, S.; Ding, P.Y.; Wang, X.H.; Zhang, X.; Zhang, J. Three-dimensional printing and 3D slicer powerful tools in understanding and treating neurosurgical diseases. Front. Surg. 2022, 9, 1030081. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.G.; Li, X.H.; Wang, K.Z.; Li, Z.; Yang, P. An automatic measurement system of distal femur morphological parameters using 3D slicer software. Bone 2022, 156, 116300. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.L.; Li, Y.; Wang, Y.Z.; Guo, W.C.; Zhang, K.; Du, Y.H.; Shi, H.W.; Qian, T. The preoperative evaluation value of 3D-slicer program before microsurgical vascular decompression in patients with hemifacial spasm. Clin. Neurol. Neurosurg. 2022, 217, 107241. [Google Scholar] [CrossRef]
- Liao, R.F.; Liu, L.M.; Song, B.; Wan, X.H.; Wang, S.; Xu, J.H. 3D-Slicer Software-Assisted Neuroendoscopic Surgery in the Treatment of Hypertensive Cerebral Hemorrhage. Comput. Math. Methods Med. 2022, 2022, 7156598. [Google Scholar] [CrossRef] [PubMed]
- Huie, J.M.; Summers, A.P.; Kawano, S.M. SegmentGeometry: A Tool for Measuring Second Moment of Area in 3D Slicer. Integr. Org. Biol. 2022, 4, obac009. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, X.; Tian, M.; He, W.; Li, S.X.; Liang, Z.; Gao, Y. Open-source algorithm and software for computed tomography-based virtual pancreatoscopy and other applications. Vis. Comput. Ind. Biomed. Art 2022, 5, 20. [Google Scholar] [CrossRef]
- Briend, F.; Leroux, E.; Nathou, C.; Delcroix, N.; Dollfus, S.; Etard, O. GeodesicSlicer: A Slicer Toolbox for Targeting Brain Stimulation. Neuroinformatics 2020, 18, 509–516. [Google Scholar] [CrossRef]
- Zaffino, P.; Merola, A.; Leuzzi, D.; Sabatino, V.; Cosentino, C.; Spadea, M.F. SlicerArduino: A Bridge between Medical Imaging Platform and Microcontroller. Bioengineering 2020, 7, 109. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Wang, J.J.; Wang, C.Q.; Wei, F.; Zhang, C.C.; Wei, H.J.; Ye, X.L.; Xu, J.W. FreeSurfer and 3D Slicer-Assisted SEEG Implantation for Drug-Resistant Epilepsy. Front. Neurorobotics 2022, 16, 848746. [Google Scholar] [CrossRef]
- Sebro, R.; Mongan, J. TotalSegmentator: A Gift to the Biomedical Imaging Community. Radiol. Artif. Intell. 2023, 5, e230235. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhang, X. Evaluation of Multimedia Courseware-Assisted Teaching Effect of Medical Images Based on the Deep Learning Algorithm. J. Environ. Public Health 2022, 2022, 5991087. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.C.; Yu, J.; Larkin, M.B.; Graves, E.K.; Mears, D. A Multimedia Educational Module for Teaching Early Medical Neuroanatomy. MedEdPORTAL 2020, 16, 10885. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Engineering, J.O. Retracted: VR/AR Technology in Human Anatomy Teaching and Operation Training. J. Healthc. Eng. 2023, 2023, 9780813. [Google Scholar] [PubMed]
- Agbafe, V.; Jazayeri, H.E.; Baker, N.; Cederna, P.S. Augmenting Medical and Surgical Education with Virtual Reality (VR). Plast. Reconstr. Surg. 2023, 152, 556e–558e. [Google Scholar] [CrossRef]
- Vergara, D.; Fernández-Arias, P.; Extremera, J.; Dávila, L.P.; Rubio, M.P. Educational trends post COVID-19 in engineering: Virtual laboratories. Mater. Today Proc. 2022, 49, 155–160. [Google Scholar] [CrossRef]
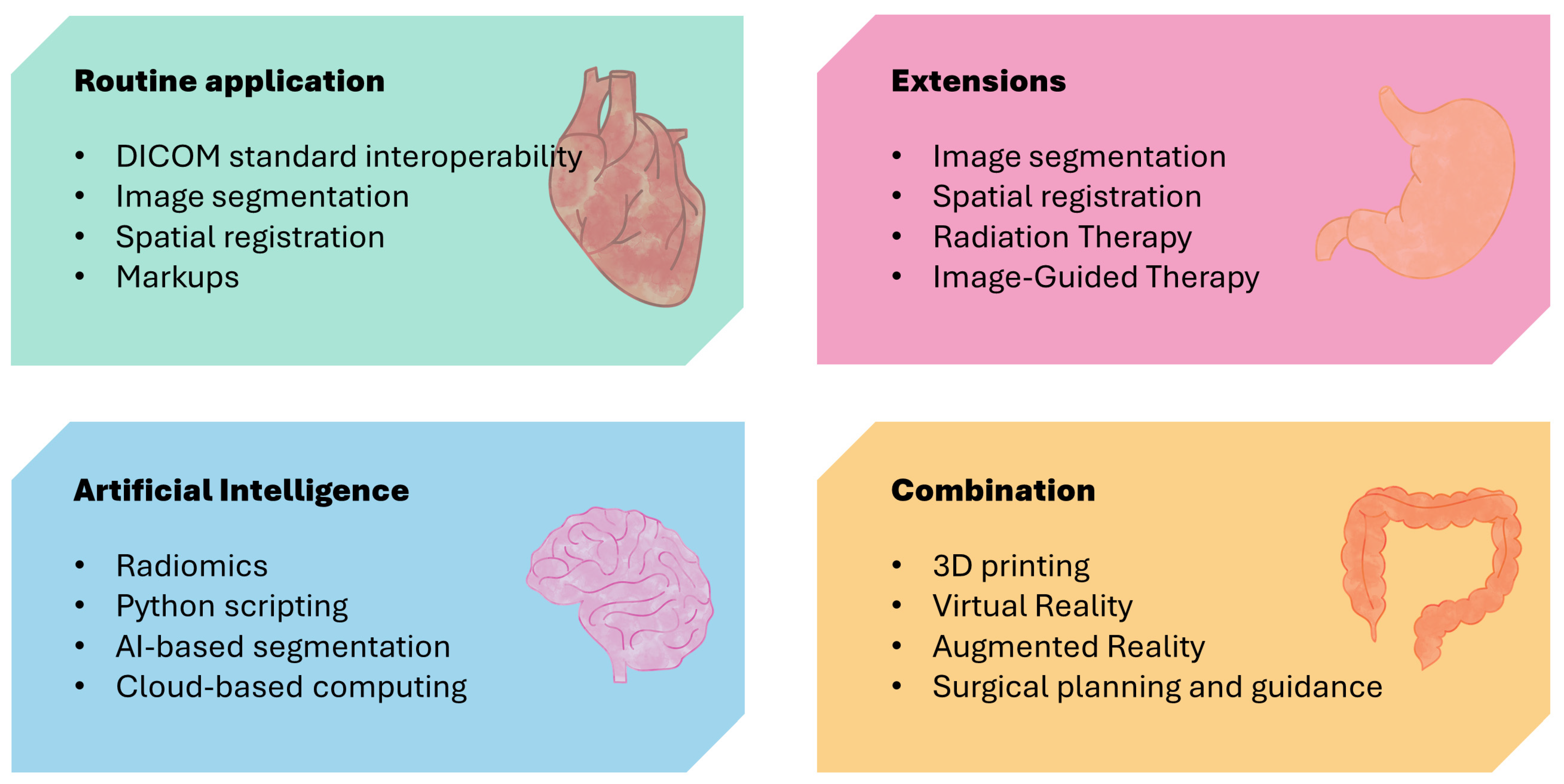
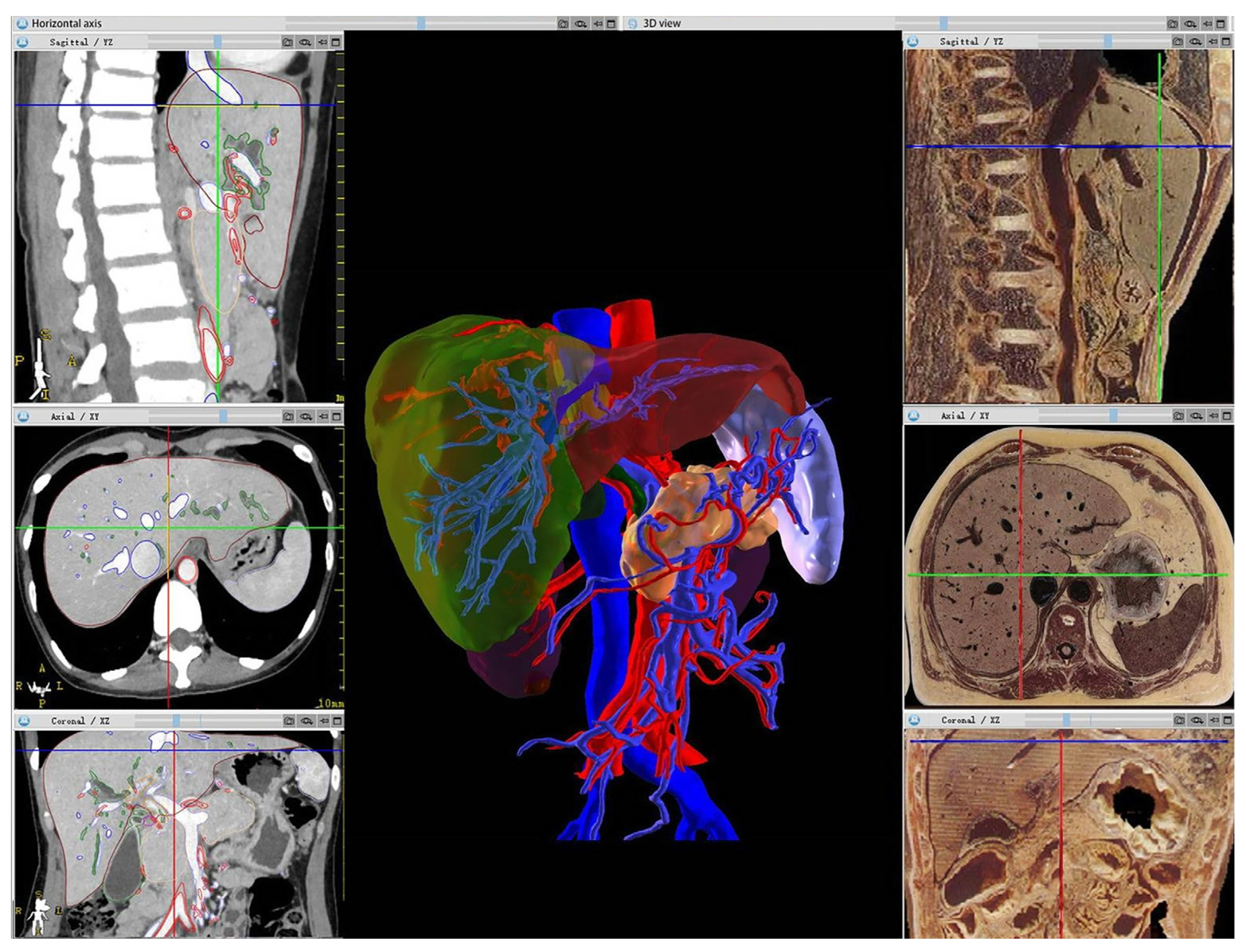
| Features | Introduction |
|---|---|
| Open source | Its source code is freely available and modifiable. This allows users to access, learn, and improve the software freely, catering to the needs of different users. |
| Multi-modal image processing | Handling various types of medical image data, including CT, MRI, PET, etc., it provides a range of powerful image-processing algorithms for tasks such as image segmentation, registration, reconstruction, etc. |
| Scalability | It offers a flexible plugin architecture that allows users to add new functionalities and algorithms as needed. This enables users to customize and extend the software’s capabilities according to their research requirements. |
| Visualization capabilities | It possesses rich visualization capabilities, such as volume rendering, surface rendering, slice display, etc., allowing medical image data to be presented in different ways. These capabilities enable users to better understand and analyze image data. |
| Research support | Providing a range of tools and functionalities for medical imaging research, it assists users in measurement, quantitative analysis, statistical modeling, etc., supporting the progress of medical research. |
| AI imaging analysis platform | It is a platform for a large number of AI imaging analysis technologies, not only reducing the threshold for application but also lowering the developmental difficulty, enhancing the practicality of AI technology. |
| Inclusion | Exclusion |
|---|---|
| Peer-reviewed articles. | Conference poster papers. |
| Research published in 2019 or later. | Studies not written in English. |
| Resources on higher education. | Redundant studies (i.e., studies with a similar focus conducted by the same author(s) and published in different venues, demonstrating little or no discernible difference; in our analysis, we specifically examined the most recent study conducted by the primary author). |
| Traditional Teaching Drawbacks | Educational Platform Benefits |
|---|---|
| Limited methods, lacks variety | Boosts autonomy with diverse learning resources |
| Neglects student subjectivity | Enhances practical skills via virtual labs and scenarios |
| Confined to textbook content | Increases interest through interactive and immersive technologies |
| Monotonous methods hinder skill development | Reduces costs and is less reliant on physical resources |
| Function | 3D Slicer Application |
|---|---|
| Visualization and Analysis | Its various visualization methods, such as 3D reconstruction, slice display, and voxel rendering, enhance medical students’ understanding and analysis of imaging data for accurate report writing. |
| Measurement and Labeling | AI-driven measurement and labeling tools in 3D Slicer enable precise description and localization of lesions, structures, and anatomical features in reports, facilitating effective communication of observations and findings. |
| Interactivity and Usability | Its user-friendly interface and interactive operations make it easy for medical students to navigate and manipulate imaging data, improving efficiency and reducing report writing difficulty. |
| Data Integration and Sharing | Supporting various medical imaging data formats, including DICOM, NIfTI, and STL, 3D Slicer allows convenient integration and sharing of imaging data from different sources for comprehensive report writing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Feng, H.; Zhao, Y.; Zhang, S. Exploring the Application of the Artificial-Intelligence-Integrated Platform 3D Slicer in Medical Imaging Education. Diagnostics 2024, 14, 146. https://doi.org/10.3390/diagnostics14020146
Zhang Y, Feng H, Zhao Y, Zhang S. Exploring the Application of the Artificial-Intelligence-Integrated Platform 3D Slicer in Medical Imaging Education. Diagnostics. 2024; 14(2):146. https://doi.org/10.3390/diagnostics14020146
Chicago/Turabian StyleZhang, Ying, Hongbo Feng, Yan Zhao, and Shuo Zhang. 2024. "Exploring the Application of the Artificial-Intelligence-Integrated Platform 3D Slicer in Medical Imaging Education" Diagnostics 14, no. 2: 146. https://doi.org/10.3390/diagnostics14020146




