Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
Abstract
:1. Introduction
2. Materials and Methods
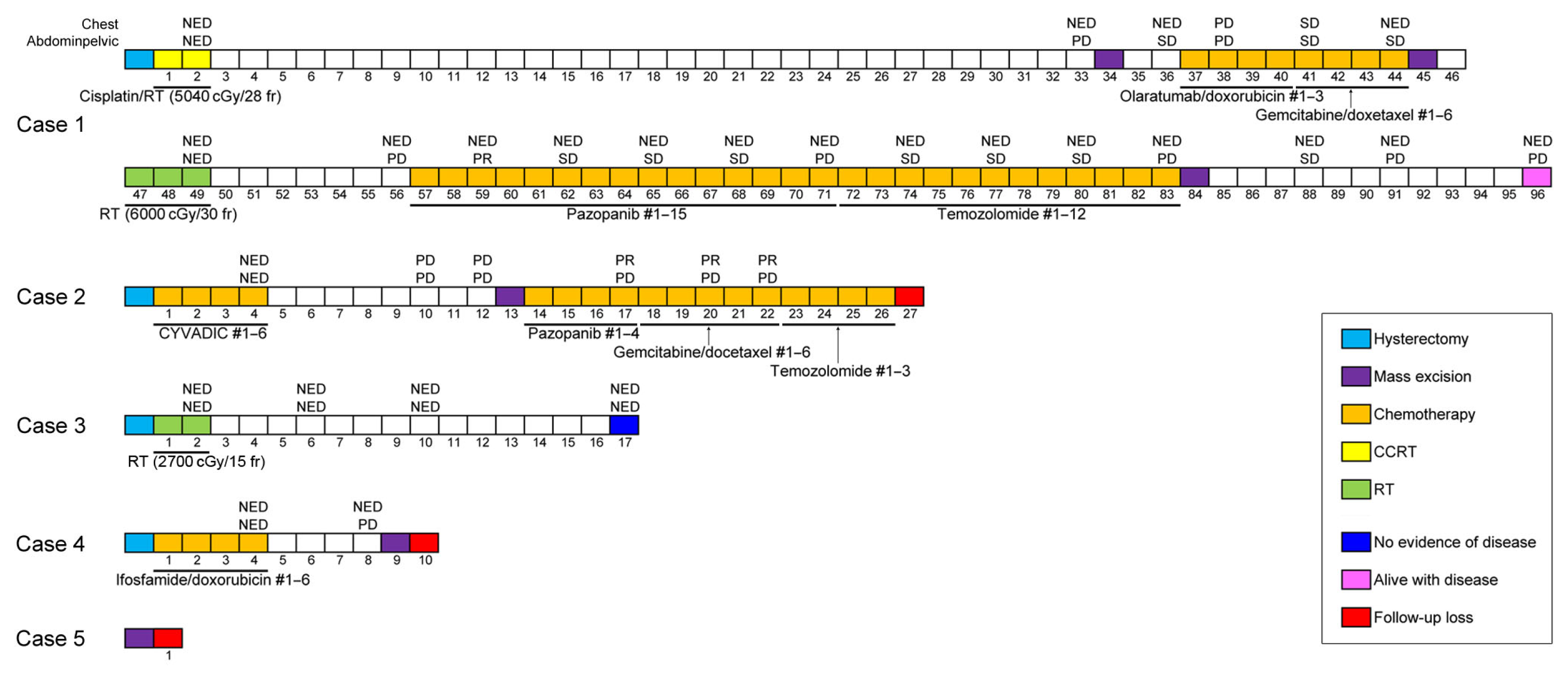
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asano, H.; Isoe, T.; Ito, Y.M.; Nishimoto, N.; Watanabe, Y.; Yokoshiki, S.; Watari, H. Status of the current treatment options and potential future targets in uterine leiomyosarcoma: A Review. Cancers 2022, 14, 1180. [Google Scholar] [CrossRef]
- Devaud, N.; Vornicova, O.; Abdul Razak, A.R.; Khalili, K.; Demicco, E.G.; Mitric, C.; Bernardini, M.Q.; Gladdy, R.A. Leiomyosarcoma: Current clinical management and future horizons. Surg. Oncol. Clin. N. Am. 2022, 31, 527–546. [Google Scholar] [CrossRef]
- Yu, S.; Hornick, J.L. Malignant mesenchymoma revisited: A clinicopathologic study of leiomyosarcomas with osteosarcomatous differentiation. Am. J. Surg. Pathol. 2022, 46, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Penel, N.; Coindre, J.M.; Giraud, A.; Terrier, P.; Ranchere-Vince, D.; Collin, F.; Guellec, S.L.E.; Bazille, C.; Lae, M.; de Pinieux, G.; et al. Presentation and outcome of frequent and rare sarcoma histologic subtypes: A study of 10,262 patients with localized visceral/soft tissue sarcoma managed in reference centers. Cancer 2018, 124, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): A report on 1007 patients from the multi-institutional collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Buzinskiene, D.; Mikenas, S.; Drasutiene, G.; Mongirdas, M. Uterine sarcoma: A clinical case and a literature review. Acta Med. Litu. 2018, 25, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Abeler, V.M.; Royne, O.; Thoresen, S.; Danielsen, H.E.; Nesland, J.M.; Kristensen, G.B. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009, 54, 355–364. [Google Scholar] [CrossRef]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hosh, M.; Antar, S.; Nazzal, A.; Warda, M.; Gibreel, A.; Refky, B. Uterine sarcoma: Analysis of 13,089 cases based on Surveillance, Epidemiology, and End Results Database. Int. J. Gynecol. Cancer 2016, 26, 1098–1104. [Google Scholar] [CrossRef]
- Toro, J.R.; Travis, L.B.; Wu, H.J.; Zhu, K.; Fletcher, C.D.; Devesa, S.S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results Program, 1978–2001: An analysis of 26,758 cases. Int. J. Cancer 2006, 119, 2922–2930. [Google Scholar] [CrossRef]
- Sparic, R.; Andjic, M.; Babovic, I.; Nejkovic, L.; Mitrovic, M.; Stulic, J.; Pupovac, M.; Tinelli, A. Molecular insights in uterine leiomyosarcoma: A systematic review. Int. J. Mol. Sci. 2022, 23, 9728. [Google Scholar] [CrossRef]
- Roberts, M.E.; Aynardi, J.T.; Chu, C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol. Oncol. 2018, 151, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Juhasz-Boss, I.; Gabriel, L.; Bohle, R.M.; Horn, L.C.; Solomayer, E.F.; Breitbach, G.P. Uterine leiomyosarcoma. Oncol. Res. Treat. 2018, 41, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Y.; Quan, Q.; Meng, Y.; Mu, X. Diagnostic value of preoperative CA125, LDH and HE4 for leiomyosarcoma of the female reproductive system. Cancer Manag. Res. 2021, 13, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Henricks, W.H.; Chu, Y.C.; Goldblum, J.R.; Weiss, S.W. Dedifferentiated liposarcoma: A clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am. J. Surg. Pathol. 1997, 21, 271–281. [Google Scholar] [CrossRef]
- Chapel, D.B.; Maccio, L.; Bragantini, E.; Zannoni, G.F.; Quade, B.J.; Parra-Herran, C.; Nucci, M.R. Dedifferentiated leiomyosarcoma of the uterus: A clinicopathologic and immunohistochemical analysis of 23 cases. Histopathology 2023, 82, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Shmookler, B.M.; Lauer, D.H. Retroperitoneal leiomyosarcoma. A clinicopathologic analysis of 36 cases. Am. J. Surg. Pathol. 1983, 7, 269–280. [Google Scholar] [PubMed]
- Dekanic, A.; Jonjic, N.; Savic Vukovic, A. Dedifferentiated leiomyosarcoma of the auricle with heterologous osteosarcoma component: Case report and literature review. Case Rep. Otolaryngol. 2022, 2022, 3684461. [Google Scholar] [CrossRef]
- Gaeta, R.; Matera, D.; Muratori, F.; Roselli, G.; Baldi, G.; Campanacci, D.A.; Franchi, A. Dedifferentiated soft tissue leiomyosarcoma with heterologous osteosarcoma component: Case report and review of the literature. Clin. Sarcoma Res. 2020, 10, 6. [Google Scholar] [CrossRef]
- Chen, E.; O’Connell, F.; Fletcher, C.D. Dedifferentiated leiomyosarcoma: Clinicopathological analysis of 18 cases. Histopathology 2011, 59, 1135–1143. [Google Scholar] [CrossRef]
- Nicolas, M.M.; Tamboli, P.; Gomez, J.A.; Czerniak, B.A. Pleomorphic and dedifferentiated leiomyosarcoma: Clinicopathologic and immunohistochemical study of 41 cases. Hum. Pathol. 2010, 41, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Akazawa, Y.; Yamaguchi, Y.; Mussazhanova, Z.; Kurohama, H.; Ueki, N.; Kohno, M.; Fukushima, A.; Kajimura, I.; Hiraki, H.; et al. Immunofluorescence analysis of DNA damage response protein p53-binding protein 1 in a case of uterine dedifferentiated leiomyosarcoma arising from leiomyoma. Pathol. Res. Pract. 2019, 215, 152640. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wu, W.L.; Shi, P.; Liu, T.M.; Yu, N.; Li, L. A case report of recurrent leiomyosarcoma with chondrosarcoma differentiation in the abdominal wall and a review of the literature. Pathol. Oncol. Res. 2023, 29, 1611109. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, E.; Kim, H.S. Mesonephric-like carcinosarcoma of the uterine corpus: Clinicopathological, molecular and prognostic characteristics in comparison with uterine mesonephric-like adenocarcinoma and conventional endometrial carcinosarcoma. Cancer Genom. Proteom. 2022, 19, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Yeo, M.K.; Lee, S.H.; Lee, M.Y.; Chae, S.W.; Kim, H.S.; Do, S.I. Clinicopathological and prognostic significance of programmed death ligand-1 SP142 expression in 132 patients with triple-negative breast cancer. In Vivo 2022, 36, 2890–2898. [Google Scholar] [CrossRef]
- Sohn, J.; Lee, Y.; Kim, H.S. Endometrioid carcinomas of the ovaries and endometrium involving endocervical polyps: Comprehensive clinicopathological analyses. Diagnostics 2022, 12, 2339. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Kim, S.; Kim, H.S.; Do, S.I. High receptor-interacting serine/threonine-protein kinase 3 (RIP3) expression serves as an independent poor prognostic factor for triple-negative breast carcinoma. Anticancer. Res. 2022, 42, 2753–2761. [Google Scholar] [CrossRef]
- Koh, H.H.; Park, E.; Kim, H.S. Mesonephric-like adenocarcinoma of the ovary: Clinicopathological and molecular characteristics. Diagnostics 2022, 12, 326. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, H.S. Clinicopathological and prognostic values of telomerase reverse transcriptase (TERT) promoter mutations in ovarian clear cell carcinoma for predicting tumor recurrence, platinum resistance and survival. Cancer Genom. Proteom. 2023, 20, 626–636. [Google Scholar] [CrossRef]
- Park, S.; Cho, Y.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Clinicopathological and prognostic significance of L1 cell adhesion molecule (L1CAM) over-expression. Anticancer. Res. 2023, 43, 4559–4571. [Google Scholar] [CrossRef]
- Koh, H.H.; Park, E.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Genomic and immunohistochemical profiling with comprehensive clinicopathological analysis of 17 consecutive cases from a single institution. Biomedicines 2023, 11, 2269. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Comparison between mismatch repair protein immunostaining and microsatellite instability testing. Anticancer Res. 2023, 43, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oh, Y.L. Thyroid pathology, a clue to PTEN hamartoma tumor syndrome. J. Pathol. Transl. Med. 2023, 57, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Jang, W.; Kim, K.M.; Jang, K.T. Clinicopathologic significance of the delta-like ligand 4, vascular endothelial growth factor, and hypoxia-inducible factor-2alpha in gallbladder cancer. J. Pathol. Transl. Med. 2023, 57, 113–122. [Google Scholar] [CrossRef]
- Chang, S.; Choi, Y.L.; Shim, H.S.; Lee, G.K.; Ha, S.Y.; Korean Cardiopulmonary Pathology Study Group. Usefulness of BRAF VE1 immunohistochemistry in non-small cell lung cancers: A multi-institutional study by 15 pathologists in Korea. J. Pathol. Transl. Med. 2022, 56, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.H.; Oh, Y.L. Papillary and medullary thyroid carcinomas coexisting in the same lobe, first suspected based on fine-needle aspiration cytology: A case report. J. Pathol. Transl. Med. 2022, 56, 301–308. [Google Scholar] [CrossRef]
- Kim, H.; Na, K.; Bae, G.E.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Comprehensive immunohistochemical analyses using markers for mesonephric, endometrioid and serous tumors. Diagnostics 2021, 11, 2042. [Google Scholar] [CrossRef]
- Kobel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of p53 immunohistochemistry in endometrial carcinomas: Toward increased reproducibility. Int. J. Gynecol. Pathol. 2019, 38, S123–S131. [Google Scholar] [CrossRef]
- Oda, Y.; Miyajima, K.; Kawaguchi, K.; Tamiya, S.; Oshiro, Y.; Hachitanda, Y.; Oya, M.; Iwamoto, Y.; Tsuneyoshi, M. Pleomorphic leiomyosarcoma: Clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am. J. Surg. Pathol. 2001, 25, 1030–1038. [Google Scholar] [CrossRef]
- Demicco, E.G.; Boland, G.M.; Brewer Savannah, K.J.; Lusby, K.; Young, E.D.; Ingram, D.; Watson, K.L.; Bailey, M.; Guo, X.; Hornick, J.L.; et al. Progressive loss of myogenic differentiation in leiomyosarcoma has prognostic value. Histopathology 2015, 66, 627–638. [Google Scholar] [CrossRef]
- Nosaka, K.; Komatsu, H.; Oishi, T.; Horie, Y.; Harada, T.; Umekita, Y. A Case of dedifferentiated leiomyosarcoma of the uterus. Int. J. Pathol. Clin. Res. 2016, 2, 49. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours: Female Genital Tumours, 5th ed.; WHO Classification of Tumors Editorial Board, Ed.; WHO: Lyon, France, 2020. [Google Scholar]
- Sadiq, Q.; Khan, F. High grade sarcoma with chondrosarcomatous differentiation in primary uterine leiomyosarcoma; A rare case and review of literature. Gynecol. Oncol. Rep. 2022, 39, 100905. [Google Scholar] [CrossRef] [PubMed]
- Rawish, K.R.; Fadare, O. Dedifferentiated leiomyosarcoma of the uterus with heterologous elements: A potential diagnostic pitfall. Case Rep. Obstet. Gynecol. 2012, 2012, 534634. [Google Scholar] [CrossRef]
- Parikh, P.; Maheshwari, A.; Rekhi, B. Two uncommon cases of uterine leiomyosarcomas displaying heterologous osteosarcomatous de-differentiation. J. Cancer Res. Ther. 2015, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Iihara, K.; Hirano, K.; Fujioka, Y.; Sakamoto, A. Leiomyosarcoma with dedifferentiation in a premenopausal patient discovered after uterine artery embolization. Pathol. Int. 2007, 57, 681–687. [Google Scholar] [CrossRef]
- Kousar, A.; Wald, A.I.; Heayn, M.; Cardillo, N.D.; Elishaev, E.; Bhargava, R. Dedifferentiated leiomyosarcoma: Morphology, immunohistochemistry, and molecular findings of a case and review of literature. Int. J. Gynecol. Pathol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cope, B.M.; Traweek, R.S.; Lazcano, R.; Keung, E.Z.; Lazar, A.J.; Roland, C.L.; Nassif, E.F. Targeting the molecular and immunologic features of leiomyosarcoma. Cancers 2023, 15, 2099. [Google Scholar] [CrossRef]
- Astolfi, A.; Nannini, M.; Indio, V.; Schipani, A.; Rizzo, A.; Perrone, A.M.; De Iaco, P.; Pirini, M.G.; De Leo, A.; Urbini, M.; et al. Genomic database analysis of uterine leiomyosarcoma mutational profile. Cancers 2020, 12, 2126. [Google Scholar] [CrossRef]
- Ciccarone, F.; Bruno, M.; De Paolis, E.; Piermattei, A.; De Bonis, M.; Lorusso, D.; Zannoni, G.F.; Normanno, N.; Minucci, A.; Scambia, G.; et al. Role of homologous recombination repair (HRR) genes in uterine leiomyosarcomas: A retrospective analysis. Cancers 2022, 14, 1934. [Google Scholar] [CrossRef]
- Beck, A.H.; Lee, C.H.; Witten, D.M.; Gleason, B.C.; Edris, B.; Espinosa, I.; Zhu, S.; Li, R.; Montgomery, K.D.; Marinelli, R.J.; et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene 2010, 29, 845–854. [Google Scholar] [CrossRef]
- Hass, R.; von der Ohe, J.; Ungefroren, H. The intimate relationship among EMT, MET and TME: A T(ransdifferentiation) E(nhancing) M(ix) to be exploited for therapeutic purposes. Cancers 2020, 12, 3674. [Google Scholar] [CrossRef]
- Varga, J.; Greten, F.R. Cell plasticity in epithelial homeostasis and tumorigenesis. Nat. Cell Biol. 2017, 19, 1133–1141. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Hammerlindl, H.; Schaider, H. Tumor cell-intrinsic phenotypic plasticity facilitates adaptive cellular reprogramming driving acquired drug resistance. J. Cell Commun. Signal. 2018, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hass, R. Retrodifferentiation: A mechanism for cellular regeneration? Biol. Chem. 2009, 390, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hass, R. Retrodifferentiation and cell death. Crit. Rev. Oncog. 1994, 5, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Cabillic, F.; Corlu, A. Regulation of transdifferentiation and retrodifferentiation by inflammatory cytokines in hepatocellular carcinoma. Gastroenterology 2016, 151, 607–615. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Easwaran, H.; Tsai, H.C.; Baylin, S.B. Cancer epigenetics: Tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol. Cell 2014, 54, 716–727. [Google Scholar] [CrossRef]
- Yamada, Y.; Haga, H.; Yamada, Y. Concise review: Dedifferentiation meets cancer development: Proof of concept for epigenetic cancer. Stem Cells Transl. Med. 2014, 3, 1182–1187. [Google Scholar] [CrossRef]
- Dubois-Pot-Schneider, H.; Fekir, K.; Coulouarn, C.; Glaise, D.; Aninat, C.; Jarnouen, K.; Le Guevel, R.; Kubo, T.; Ishida, S.; Morel, F.; et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology 2014, 60, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Watts, F.; Stewart, P.; Gill, A.J.; Krishnaswamy, M. SDHA deficient dedifferentiated gastrointestinal stromal tumour with a smooth-muscle immunophenotype. Pathology 2023. online ahead of print. [Google Scholar] [CrossRef]
- Shah, V.I.; Morgan, S.E.; Kobel, M.; Lee, C.H.; McCluggage, W.G. Dedifferentiation in breast metastasis of endometrial carcinoma: A diagnostic dilemma. Int. J. Gynecol. Pathol. 2022, 41, 35–39. [Google Scholar] [CrossRef]
- Tan, N.J.H.; Sun, I.S.Y.; Low, S.W.; Kuick, C.H.; Chang, K.T.E.; Tan, C.L. A rapidly fatal intracranial anaplastic hemangiopericytoma with de-novo dedifferentiation: Emphasis on diagnostic recognition, molecular confirmation and discussion on treatment dilemma. Brain Tumor Pathol. 2019, 36, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, P.; Debiec-Rychter, M.; Stul, M.; De Wever, I.; Van Oosterom, A.T.; Sciot, R. Changing phenotype of gastrointestinal stromal tumours under imatinib mesylate treatment: A potential diagnostic pitfall. Histopathology 2005, 47, 41–47. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Dilution | Clone | Company | Positive Control |
|---|---|---|---|---|
| Desmin | 1:200 | D33 | Agilent Technologies, Santa Clara, CA, USA | Normal myometrium |
| S100 protein | 1:5000 | polyclonal | Agilent Technologies, Santa Clara, CA, USA | Vulvar malignant melanoma |
| Myogenin | 1:50 | F5D | Cell marque, Rocklin, CA, USA | Uterine rhabdomyosarcoma |
| MyoD1 | 1:50 | EP212 | Cell marque, Rocklin, CA, USA | Uterine rhabdomyosarcoma |
| SATB2 | 1:2000 | EPNCIR130A | Abcam, Cambridge, UK | Colonic adenocarcinoma |
| p53 | 1:800 | DO-7 | Leica Biosystems, Deer Park, IL, USA | Ovarian HGSC |
| Retinoblastoma protein | 1:400 | 4H1 | Cell Signaling Technology, Beverly, MA, USA | Ocular retinoblastoma |
| Case No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (years) | 60 | 55 | 51 | 51 | 63 |
| Presenting symptoms | Abdominal discomfort, constipation | Uterine mass detected on routine examination | Uterine masses detected on routine examination | Uterine mass detected on routine examination | Abdominal discomfort and pain, palpable pelvic mass |
| Previous medical history | None | None | Thyroid cancer, umbilical hernia | Hyperthyroidism | None |
| Imaging findings of uterine tumor | NA | A large solid cystic mass | Multiple solid uterine masses measuring up to 8.9 cm | An 8.3 cm lobulated, heterogeneous mass with hypodense necrotic area | A 23.0 cm large heterogeneous mass with adnexal extension |
| Clinical impression | Uterine leiomyoma | Uterine leiomyoma | Uterine leiomyoma | Uterine leiomyoma | Uterine sarcoma |
| Surgical procedure for uterine tumor | TH | TH, BSO, PLND, PALND, OMT, APP | TH, bilateral salpingectomy | TH, BSO, OMT | Uterine mass excision, LO, LHC, left nephrectomy |
| Pathological diagnosis of uterine tumor | LMS | DDLMS | DDLMS-H | LMS | DDLMS-H |
| Greatest dimension of uterine tumor (cm) | NA | 27.8 | 8.9 | 8.3 | 23.0 |
| Adnexal extension | Absent | Absent | Absent | Absent | Present |
| Pelvic extension | Absent | Absent | Present | Absent | Present |
| Abdominal extension | Absent | Absent | Absent | Absent | Present |
| LN metastasis | Absent | Absent | Present (para-aortic) | Present (left pelvic) | Absent |
| Initial stage | IB | IB | IIIC | IIIC | IVC |
| Post-operative treatment | CCRT | Chemotherapy | RTx (interrupted) | Chemotherapy | NA (lost to follow-up) |
| Post-treatment recurrence | Present (ovary, colon, bladder, peritoneum, mesentery, omentum) | Present (vagina, bladder, peritoneum, lungs) | Absent | Present (left paracolic gutter) | NA (lost to follow-up) |
| Pathological diagnosis of recurrent tumor | Metastatic DDLMS-H | Metastatic DDLMS-H | None | Metastatic DDLMS-H | NA (lost to follow-up) |
| Treatment for recurrence | Excision, RTx, chemotherapy | Excision, chemotherapy | None | Excision | NA (lost to follow-up) |
| RFS (months) | 30 | 6 | 15 | 4 | NA (lost to follow-up) |
| Survival status | Alive with disease | NA (lost to follow-up) | No evidence of disease | NA (lost to follow-up) | NA (lost to follow-up) |
| OS (months) | 93 | NA (lost to follow-up) | 15 | NA (lost to follow-up) | NA (lost to follow-up) |
| Case No | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Dominant morphology of differentiated component | Spindle cell and epithelioid LMS | Epithelioid LMS | Spindle cell and epithelioid LMS | Spindle cell LMS | Spindle cell and epithelioid LMS | |
| Nuclear pleomorphism | Severe | Severe | Severe | Severe | Severe | |
| Mitotic count per 10 HPFs | 23 | 40 | 46 | 22 | 21 | |
| Tumor cell necrosis | Present | Present | Present | Present | Present | |
| Tumor border | Infiltrative | Infiltrative | Infiltrative | Infiltrative | Infiltrative | |
| Lymphocytic infiltrate | Absent | Present | Present | Present | Absent | |
| Histological type and proportion of heterologous component | CSA (80%) | RMS (60%) | RMS (20%) | CSA (60%) | CSA (30%) | |
| Desmin | Differentiated | DMP | DSP | DSP | Negative | NA |
| Dedifferentiated | FWP | Negative | Negative | Negative | NA | |
| S100 protein | Differentiated | FWP | Negative | NA | Negative | NA |
| Dedifferentiated | DSP | Negative | NA | FSP | NA | |
| Myogenin | Differentiated | Negative | Negative | Negative | NA | NA |
| Dedifferentiated | Negative | Negative | FWP | NA | NA | |
| MyoD1 | Differentiated | Negative | Negative | Negative | NA | NA |
| Dedifferentiated | Negative | Negative | Negative | NA | NA | |
| SATB2 | Differentiated | FWP | Negative | NA | NA | NA |
| Dedifferentiated | Negative | DSP | NA | NA | NA | |
| p53 | Differentiated | WT | WT | WT | NA | NA |
| Dedifferentiated | WT | OE | WT | NA | NA | |
| RB protein | Differentiated | No loss | No loss | NA | NA | NA |
| Dedifferentiated | No loss | No loss | NA | NA | NA | |
| Authors (Year Published) | Case No | Age (Years) | Presenting Symptom | Initial Stage | Treatment | Recurrence | Distant Metastasis | DFS (Months) | Outcome | OS or DSS (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Iihara et al., (2007) [46] | 1 | 48 | Hypermenorrhea for 4 years | IB | UAE, TH, CTx, GKRS | NA | Present (lungs, brain) | NA | DOD | 20 a |
| Chen et al., (2011) [20] | 2 | 59 | Pelvic pain | NA | TH, BSO | Absent | Absent | 28 | NED | 28 a |
| 3 | 80 | PMB | NA | Excision | NA | NA | NA | DOC | 12 a | |
| Rawish and Fadare (2012) [44] | 4 | 48 | NA | IA | SH, BSO, CTx | Present | Absent | 6 | AWD | 8 a |
| Parikh et al., (2015) [45] | 5 | 60 | Abdominal pain for 4 months | NA | TH, BS | NA | NA | NA | NA | NA |
| 6 | 38 | Infertility | NA | Myomectomy | NA | NA | NA | NA | NA | |
| Nosaka et al., (2016) [41] | 7 | 63 | Bloody vaginal discharge for 2 months | IVB | TH, BSO, CTx | NA | Present (lungs) | NA | NA | NA |
| Yu and Hornick (2022) [3] | 8 | 63 | NA | NA | Surgery, CTx | Present | Present (lungs, diaphragm, heart, SVC, kidney) | NA | DOD | 7.5 a |
| 9 | 40 | NA | NA | Surgery, CTx | Present | Present (colon, retroperitoneum) | NA | DOD | 18 a | |
| 10 | 48 | NA | NA | Surgery, CTx, RTx | Present | Present (lungs, liver, peritoneum, colon, small bowel) | NA | DOD | 6.5 a | |
| 11 | 55 | NA | NA | CTx | Absent | Present (pancreas, bone, lungs) | NA | DOD | NA | |
| 12 | 52 | NA | NA | Surgery, CTx | Present | Present (lungs, abdomen) | NA | AWD | 36 a | |
| 13 | 61 | NA | NA | Surgery, CTx, RTx | Present | Present (lungs, pleura) | NA | DOD | 17 a | |
| 14 | 53 | NA | NA | Surgery | Absent | NA | NA | DOD | 3.5 a | |
| 15 | 62 | NA | NA | NA | NA | NA | NA | NA | NA | |
| 16 | 43 | NA | NA | Surgery, CTx | Present | Present (small bowel) | NA | AWD | 32 a | |
| 17 | 52 | NA | NA | Surgery, CTx | Present | Present (liver, small bowel) | NA | AWD | 28 a | |
| Sadiq and Khan (2022) [43] | 18 | 68 | Vaginal bleeding | NA | TH, BSO | NA | NA | NA | NA | NA |
| Chapel et al., (2023) [16] | 19 | 88 | NA | IB | Surgery | NA | NA | 2 | DOD | 2 b |
| 20 | 66 | NA | IB | Surgery | NA | NA | 7 | DOD | 11 b | |
| 21 | 68 | NA | IB | Surgery, CTx | Present | NA | 3 | DOD | 20 b | |
| 22 | 42 | NA | IB | Surgery | NA | NA | NA | DOD | 23 b | |
| 23 | 61 | NA | IB | Surgery | NA | NA | NA | DOD | 5 b | |
| 24 | 57 | NA | IIIB | Surgery | NA | NA | NA | NA | NA | |
| 25 | 46 | NA | II | Surgery | NA | NA | 23 | DOD | 31 b | |
| 26 | 54 | NA | IB | Surgery | NA | NA | NA | DOD | 73 b | |
| 27 | 59 | NA | IB | CTx | NA | NA | 114 | NED | 114 b | |
| 28 | 50 | NA | IIIB | Surgery | NA | NA | 12 | AWD | 12 b | |
| 29 | 53 | NA | IA | Surgery | NA | NA | 21 | AWD | 50 b | |
| 30 | 63 | NA | IV | CTx | NA | Present | 8 | DOD | 16 b | |
| 31 | 53 | NA | IV | CTx | NA | Present | NA | DOD | 15 b | |
| 32 | 54 | NA | IV | Surgery | NA | Present | NA | DOD | 3 b | |
| 33 | 52 | NA | IV | CTx | NA | Present | NA | DOD | 10 b | |
| 34 | 57 | NA | IB | CTx for recurrence | Present | Present | 4 | NED | 109 b | |
| 35 | 90 | NA | II | Surgery | NA | NA | NA | NA | NA | |
| 36 | 54 | NA | II | CTx and RTx for recurrence | Present | NA | 2 | NED | 56 b | |
| 37 | 70 | NA | IB | RTx | NA | NA | 15 | AWD | 44 b | |
| 38 | 75 | NA | IB | Surgery | NA | NA | NA | NA | NA | |
| 39 | 55 | NA | IB | CTx for recurrence | Present | NA | 4 | AWD | 4 b | |
| 40 | 60 | NA | IB | CTx for recurrence | Present | NA | 8 | DOD | 13 b | |
| 41 | 63 | NA | II | Surgery | NA | NA | NA | NA | NA | |
| Kousar et al., (2023) [47] | 42 | 42 | Acute abdominal pain and vaginal bleeding | IV | Surgery, CTx | Present | Present (liver, peritoneum) | NA | NA | NA |
| Authors (Year Published) | Case No | Tumor Size (cm) | Dominant Morphology of Differentiated Component | Histological Type of Heterologous Component (Proportion) |
|---|---|---|---|---|
| Iihara et al., (2007) [46] | 1 | 14.0 | Spindle cell LMS | Mixed proliferation of scattered bizarre cells and spindle cells without specific structures (NA) |
| Chen et al., (2011) [20] | 2 | 6.8 | Spindle cell LMS | MFH-like morphology (NA) |
| 3 | 18.0 | Spindle cell LMS | MFH-like morphology (NA) | |
| Rawish and Fadare (2012) [44] | 4 | 18.0 | Spindle cell LMS | OSA (10%) |
| Parikh et al., (2015) [45] | 5 | 30.0 | Spindle cell LMS | Osteochondroid differentiation and MNGCs (NA) |
| 6 | 12.5 | Spindle cell LMS | OSA (NA) | |
| Nosaka et al., (2016) [41] | 7 | 14.0 | Spindle cell LMS | Undifferentiated pleomorphic sarcoma (NA) |
| Yu and Hornick (2022) [3] | 8 | 11.7 | Spindle cell LMS | OSA (NA) |
| 9 | NA | Spindle cell LMS | OSA (NA) | |
| 10 | 12.0 | Spindle cell LMS | OSA (NA) | |
| 11 | 9.0 | Spindle cell LMS | OSA (NA) | |
| 12 | 20.0 | Spindle cell LMS | OSA (NA) | |
| 13 | 18.0 | Spindle cell LMS | OSA (NA) | |
| 14 | 16.0 | Spindle cell LMS | OSA (NA) | |
| 15 | 18.0 | Spindle cell LMS | OSA (NA) | |
| 16 | 17.0 | Spindle cell LMS | OSA (NA) | |
| 17 | 18.8 | Spindle cell LMS | OSA (NA) | |
| Sadiq and Khan (2022) [43] | 18 | Up to 4.0 | Spindle cell LMS | CSA (NA) |
| Chapel et al., (2023) [16] | 19 | NA | Spindle cell LMS | MFH-like morphology (30%) |
| 20 | 6.5 | Spindle cell LMS | MFH-like morphology (20%) | |
| 21 | 8.5 | Spindle cell LMS and CLM | MFH-like morphology (40%) | |
| 22 | 10.0 | CLM | MFH-like morphology (50%) | |
| 23 | 7.0 | Epithelioid LMS | MFH-like morphology (5%) | |
| 24 | 12.0 | Spindle cell LMS | MFH-like morphology (15%) | |
| 25 | 12.1 | Spindle cell LMS | MFH-like morphology and OSA (20%) | |
| 26 | 8.2 | Spindle cell LMS | MFH-like morphology (10%) | |
| 27 | 6.8 | Spindle cell LMS | MFH-like morphology (40%) | |
| 28 | 3.0 | Spindle cell LMS | MFH-like morphology (50%) | |
| 29 | 4.5 | STUMP | MFH-like morphology (30%) | |
| 30 | 11.7 | Spindle cell LMS | MFH-like morphology and OSA (10%) | |
| 31 | 8.0 | Spindle cell LMS | MFH-like morphology (25%) | |
| 32 | 25.0 | Spindle cell LMS | MFH-like morphology (20%) | |
| 33 | 20.0 | Spindle cell LMS | MFH-like morphology, OSA, and CSA (70%) | |
| 34 | 9.0 | CLM | MFH-like morphology (50%) | |
| 35 | 20.0 | LBN | MFH-like morphology (50%) | |
| 36 | 14.5 | CLM | MFH-like morphology (20%) | |
| 37 | 11.0 | STUMP | MFH-like morphology (40%) | |
| 38 | 6.1 | Spindle cell LMS | MFH-like morphology (70%) | |
| 39 | 10.0 | Spindle cell LMS | MFH-like morphology (40%) | |
| 40 | 14.3 | LM, LBM, and spindle cell LMS | MFH-like morphology (10%) | |
| 41 | 7.7 | Spindle cell LMS | MFH-like morphology (70%) | |
| Kousar et al., (2023) [47] | 42 | 10.5, 6.7 (fundus); 1.7 (body) | LM-like areas, STUMP, and frank LMS | Areas showing extreme hypercellularity and large polygonal cells with significant cytological atypia and brisk mitotic activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Bae, H.; Kim, H.-S. Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review. Diagnostics 2024, 14, 160. https://doi.org/10.3390/diagnostics14020160
Kim S, Bae H, Kim H-S. Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review. Diagnostics. 2024; 14(2):160. https://doi.org/10.3390/diagnostics14020160
Chicago/Turabian StyleKim, Suyeon, Hyunsik Bae, and Hyun-Soo Kim. 2024. "Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review" Diagnostics 14, no. 2: 160. https://doi.org/10.3390/diagnostics14020160
APA StyleKim, S., Bae, H., & Kim, H.-S. (2024). Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review. Diagnostics, 14(2), 160. https://doi.org/10.3390/diagnostics14020160






