Relationship of Serum Uric Acid with Kidney Function Decline Mediated by Systemic Arterial Stiffness: A Retrospective Cohort Study in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Individuals and Design
2.2. Data Collection
2.3. Measurement of Arterial Stiffness Parameters and Blood Pressure
2.4. Definition of Linkage between SUA and CAVI
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Participants with or without KFD
3.2. Correlation of Baseline CAVI with Clinical Variables
3.3. Relationship of Adjusted Hazard Ratio for KFD with Stratified CAVI and SUA
3.4. Cutoff Values of CAVI and SUA for Predicting KFD Obtained from ROC Curve Analysis
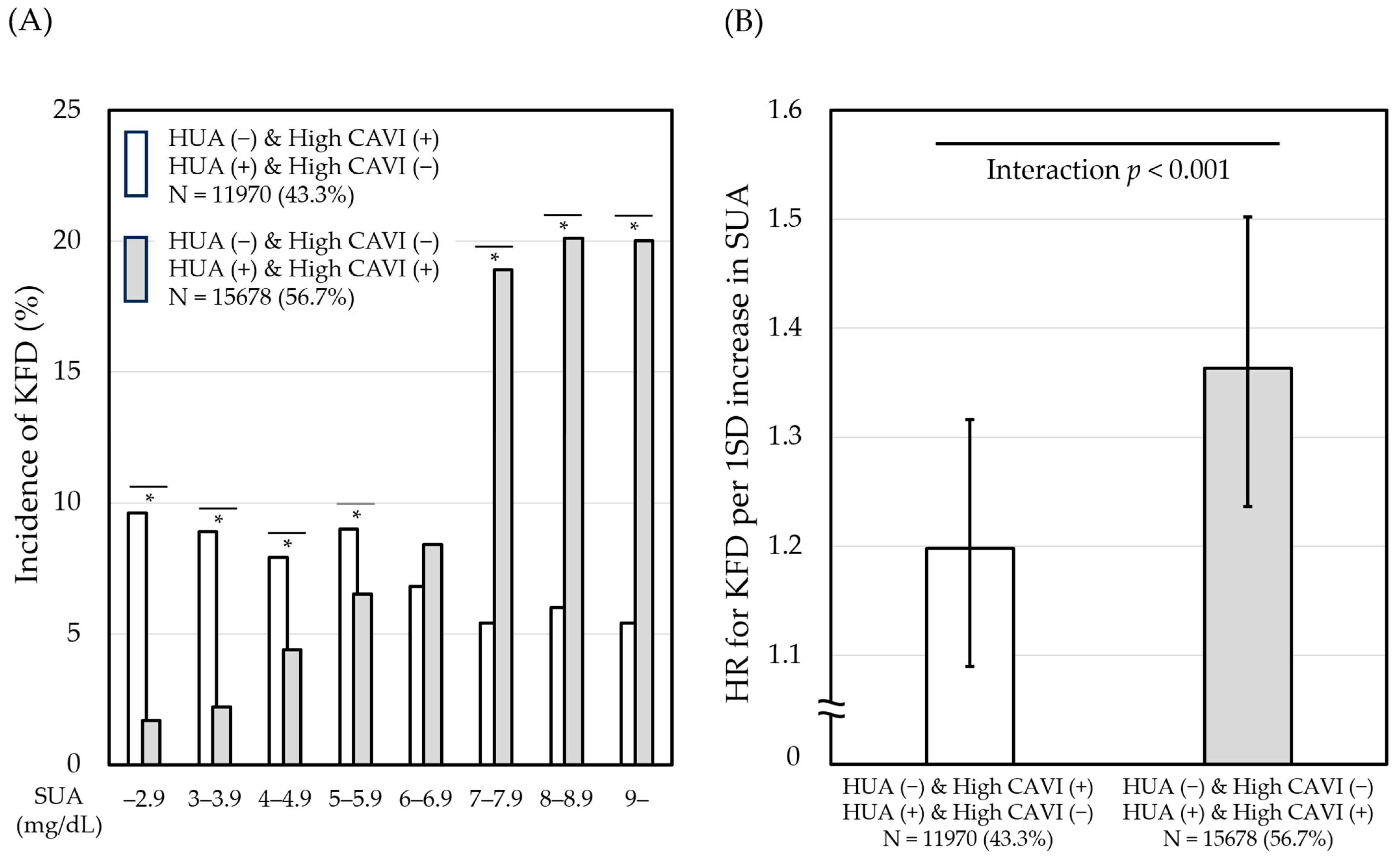
3.5. Effects of the Linkage between SUA and CAVI on the Relationship of SUA with KFD
3.6. Mediation Analysis of CAVI as Potential Mediator of the Association between SUA and KFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Kaze, A.D.; McMullan, C.J.; Isakova, T.; Waikar, S.S. Uric Acid and the Risks of Kidney Failure and Death in Individuals With CKD. Am. J. Kidney Dis. 2018, 71, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Nashar, K.; Fried, L.F. Hyperuricemia and the progression of chronic kidney disease: Is uric acid a marker or an independent risk factor? Adv. Chronic. Kidney Dis. 2012, 19, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.C.; Chen, Y.T.; Lin, Y.P.; Ou, S.M.; Yang, C.Y.; Lin, C.H.; Tarng, D.C.; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Hyperuricemia Predicts an Early Decline in Renal Function among Older People: A Community-Based Cohort Study. Sci. Rep. 2019, 9, 980. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective. Front. Pharmacol. 2020, 11, 582680. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham heart study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Havlik, R.; Lakatta, E.G.; Spurgeon, H.; Kritchevsky, S.; et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well functioning older adults. Circulation 2005, 111, 3384–3390. [Google Scholar] [CrossRef]
- Saiki, A.; Ohira, M.; Yamaguchi, T.; Nagayama, D.; Shimizu, N.; Shirai, K.; Tatsuno, I. New Horizons of Arterial Stiffness Developed Using Cardio-Ankle Vascular Index (CAVI). J. Atheroscler. Thromb. 2020, 27, 732–748. [Google Scholar] [CrossRef]
- Nakamura, K.; Iizuka, T.; Takahashi, M.; Shimizu, K.; Mikamo, H.; Nakagami, T.; Suzuki, M.; Hirano, K.; Sugiyama, Y.; Tomaru, T.; et al. Association between cardio-ankle vascular index and serum cystatin C levels in patients with cardiovascular risk factor. J. Atheroscler. Thromb. 2009, 16, 371–379. [Google Scholar] [CrossRef]
- Hitsumoto, T. Correlation Between the Cardio-Ankle Vascular Index and Renal Resistive Index in Patients With Essential Hypertension. Cardiol. Res. 2020, 11, 106–112. [Google Scholar] [CrossRef]
- Satoh-Asahara, N.; Kotani, K.; Yamakage, H.; Yamada, T.; Araki, R.; Okajima, T.; Adachi, M.; Oishi, M.; Shimatsu, A.; Japan Obesity and Metabolic Syndrome Study (JOMS) Group. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: A multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis 2015, 242, 461–468. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, H.; Shirai, K.; Horinaka, S.; Higaki, J.; Yamamura, S.; Saiki, A.; Takahashi, M.; Masaki, M.; Okura, T.; et al. Predictive value of the cardio-ankle vascular index for cardiovascular events in patients at cardiovascular risk. J. Am. Heart Assoc. 2021, 10, e020103. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, D.; Fujishiro, K.; Miyoshi, T.; Horinaka, S.; Suzuki, K.; Shimizu, K.; Saiki, A.; Shirai, K. Predictive ability of arterial stiffness parameters for renal function decline: A retrospective cohort study comparing cardio-ankle vascular index, pulse wave velocity and cardio-ankle vascular index 0. J. Hypertens. 2022, 40, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, D.; Yamaguchi, T.; Saiki, A.; Imamura, H.; Sato, Y.; Ban, N.; Kawana, H.; Nagumo, A.; Shirai, K.; Tatsuno, I. High serum uric acid is associated with increased cardio-ankle vascular index (CAVI) in healthy Japanese individuals: A cross-sectional study. Atherosclerosis 2015, 239, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Mei, Y.; Dong, B.; Geng, Z.; Xu, L. Excess Uric Acid Induces Gouty Nephropathy Through Crystal Formation: A Review of Recent Insights. Front. Endocrinol. 2022, 13, 911968. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.J.; Wu, C.Y.; Yang, C.W.; Yang, H.Y. Hyperuricemia and Progression of Chronic Kidney Disease: A Review from Physiology and Pathogenesis to the Role of Urate-Lowering Therapy. Diagnostics 2021, 11, 1674. [Google Scholar] [CrossRef]
- Leyva, F.; Anker, S.D.; Godsland, I.F.; Teixeira, M.; Hellewell, P.G.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Uric acid in chronic heart failure: A marker of chronic inflammation. Eur. Heart J. 1998, 19, 1814–1822. [Google Scholar] [CrossRef]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Sarafidis, P.A.; Liakopoulos, V. Arterial stiffness: A novel risk factor for kidney injury progression? Am. J. Hypetens 2015, 28, 958–965. [Google Scholar] [CrossRef]
- McKelvey, T.G.; H€ollwarth, M.E.; Granger, D.N.; Engerson, T.D.; Landler, U.; Jones, H.P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am. J. Physiol. 1988, 254 Pt 1, G753–G760. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, C.A.; Butler, R.; Hill, A.; Belch, J.J.; Struthers, A.D. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002, 106, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Nishizawa, M.; Kiuchi, M.; Kiyosue, A.; Tomita, F.; Ohtani, H.; Abe, Y.; Kuga, H.; Miyazaki, S.; Kasai, T.; et al. Comparative effects of topiroxostat and febuxostat on arterial properties in hypertensive patients with hyperuricemia. J. Clin. Hypertens. 2021, 23, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Nishizawa, H.; Kawachi, Y.; Nakamura, T.; Akari, S.; Ono, Y.; Fukuda, S.; Kita, S.; Maeda, N.; Hoshide, S.; et al. The Effects of Topiroxostat, a Selective Xanthine Oxidoreductase Inhibitor, on Arterial Stiffness in Hyperuricemic Patients with Liver Dysfunction: A Sub-Analysis of the BEYOND-UA Study. Biomedicines 2023, 11, 674. [Google Scholar] [CrossRef]
- Tanaka, A.; Taguchi, I.; Hisauchi, I.; Yoshida, H.; Shimabukuro, M.; Hongo, H.; Ishikawa, T.; Kadokami, T.; Yagi, S.; Sata, M.; et al. DIANA study investigators., Clinical effects of a selective urate reabsorption inhibitor dotinurad in patients with hyperuricemia and treated hypertension: A multicenter, prospective, exploratory study (DIANA). Eur. J. Med. Res. 2023, 28, 238. [Google Scholar] [CrossRef]
- Kanda, E.; Muneyuki, T.; Kanno, Y.; Suwa, K.; Nakajima, K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: A prospective cohort study. PLoS ONE 2015, 10, e0118031. [Google Scholar] [CrossRef]
- Ueda, H.; Inoue, K.; Seki, R.; Nemoto, Y.; Terawaki, H. Lower range of serum uric acid level increases risk of rapid decline of kidney function in young and middle-aged adults: The Yuport Medical Checkup Center Study. Clin. Exp. Nephrol. 2023, 27, 435–444. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Liu, S.; Liang, X.; Song, Y.; Feng, L.; Zhong, L.; Guo, X. Idiopathic renal hypouricemia: A case report and literature review. Mol. Med. Rep. 2019, 20, 5118–5124. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical -caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kawakami, H.; Fukuda, M.; Shiigi, H. Recurrence of acute renal failure and renal hypouricaemia. Pediatr. Nephrol. 1993, 7, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Mazzierli, T.; Cirillo, L.; Palazzo, V.; Ravaglia, F.; Becherucci, F. Clinical features suggesting renal hypouricemia as the cause of acute kidney injury: A case report and review of the literature. J. Nephrol. 2023, 36, 651–657. [Google Scholar] [CrossRef]


| Variable | Individuals without KFD (N = 25,828) | Individuals with KFD (N = 1820) | p Value |
|---|---|---|---|
| Male gender (%) | 44.0 | 46.9 | 0.018 |
| Age (years) | 44 (36–56) | 61 (52–68) | <0.001 |
| Height (meter) | 1.62 (1.56–1.70) | 1.61 (1.55–1.68) | <0.001 |
| BMI (kg/m2) | 21.9 (19.9–24.2) | 22.9 (20.8–24.9) | <0.001 |
| SBP (mmHg) | 116 (107–127) | 126 (113–138) | <0.001 |
| DBP (mmHg) | 72 (65–80) | 78 (70–86) | <0.001 |
| CAVI | 7.4 (6.9–8.2) | 8.4 (7.7–9.1) | <0.001 |
| FPG (mg/dL) | 85 (80–91) | 89 (84–97) | <0.001 |
| TC (mg/dL) | 208 (185–235) | 217 (195–241) | <0.001 |
| LDL-C (mg/dL) | 121 (100–144) | 130 (109–150) | <0.001 |
| HDL-C (mg/dL) | 67 (56–81) | 63 (53–77) | <0.001 |
| TG (mg/dL) | 78 (55–117) | 96 (69–139) | <0.001 |
| ln(TG) | 4.36 (4.01–4.76) | 4.56 (4.23–4.93) | <0.001 |
| Creatinine (mg/dL) | 0.68 (0.59–0.81) | 0.77 (0.69–0.92) | <0.001 |
| eGFR (mL/min/1.73 m2) | 82.6 (74.6–92.0) | 65.6 (62.6–69.6) | <0.001 |
| % ΔeGFR/y | −1.34 (−3.33–0.69) | −3.26 (−5.21–−1.15) | <0.001 |
| SUA (mg/dL) | 4.9 (4.0, 5.9) | 5.3 (4.4, 6.3) | <0.001 |
| SBP ≥ 140 and/or DBP ≥ 90 mmHg (%) | 12.7 | 27.5 | <0.001 |
| FPG ≥ 126 mg/dL (%) | 1.3 | 3.1 | <0.001 |
| LDL-C ≥ 140, HDL-C < 40 and/or TG ≥ 150 mg/dL (%) | 37.8 | 49.1 | <0.001 |
| Proteinuria (%) | 5.0 | 7.4 | <0.001 |
| Current smoking (%) | 35.1 | 32.9 | 0.061 |
| Habitual alcohol drinking (%) | 34.2 | 33.1 | 0.331 |
| Receiving treatment for | |||
| Hypertension (%) | 7.1 | 22.8 | <0.001 |
| Diabetes mellitus (%) | 3.9 | 6.8 | <0.001 |
| Dyslipidemia (%) | 6.0 | 15.1 | <0.001 |
| Gout/Hyperuricemia (%) | 0.9 | 3.2 | <0.001 |
| CAVI vs. | Univariate | Multivariate * | ||
|---|---|---|---|---|
| ρ | p Value | Standardized β | p Value | |
| Age (years) | 0.796 | <0.001 | 0.766 | <0.001 |
| Sex (male, 1; female, 0) | 0.020 | 0.001 | 0.063 | <0.001 |
| Current smoking | −0.072 | <0.001 | −0.013 | 0.002 |
| Habitual alcohol consumption | −0.049 | <0.001 | 0.009 | 0.028 |
| Height (meter) | −0.172 | <0.001 | ||
| BMI (kg/m2) | −0.055 | <0.001 | −0.160 | <0.001 |
| SBP (mmHg) | 0.402 | <0.001 | 0.116 | <0.001 |
| DBP (mmHg) | 0.417 | <0.001 | ||
| FPG (mg/dL) | 0.394 | <0.001 | 0.052 | <0.001 |
| LDL-C (mg/dL) | 0.246 | <0.001 | <0.001 | 0.965 |
| HDL-C (mg/dL) | 0.008 | 0.168 | ||
| TG (mg/dL) | 0.230 | <0.001 | ||
| ln(TG) | 0.230 | <0.001 | 0.037 | <0.001 |
| eGFR (mL/min/1.73 m2) | −0.386 | <0.001 | −0.018 | <0.001 |
| SUA (mg/dL) | 0.067 | <0.001 | 0.039 | <0.001 |
| Proteinuria | −0.003 | 0.572 | ||
| Parameter | Sex | Cutoff | Sensitivity | Specificity | AUC (95% CI) | p Value |
|---|---|---|---|---|---|---|
| CAVI | Male | 8.0 | 0.708 | 0.687 | 0.757 (0.741–0.772) | <0.001 |
| Female | 7.9 | 0.669 | 0.663 | 0.726 (0.711–0.742) | <0.001 | |
| SUA | Male | 6.3 | 0.500 | 0.614 | 0.564 (0.544–0.584) | <0.001 |
| Female | 4.5 | 0.572 | 0.594 | 0.614 (0.595–0.632) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagayama, D.; Watanabe, Y.; Fujishiro, K.; Suzuki, K.; Shirai, K.; Saiki, A. Relationship of Serum Uric Acid with Kidney Function Decline Mediated by Systemic Arterial Stiffness: A Retrospective Cohort Study in Japan. Diagnostics 2024, 14, 195. https://doi.org/10.3390/diagnostics14020195
Nagayama D, Watanabe Y, Fujishiro K, Suzuki K, Shirai K, Saiki A. Relationship of Serum Uric Acid with Kidney Function Decline Mediated by Systemic Arterial Stiffness: A Retrospective Cohort Study in Japan. Diagnostics. 2024; 14(2):195. https://doi.org/10.3390/diagnostics14020195
Chicago/Turabian StyleNagayama, Daiji, Yasuhiro Watanabe, Kentaro Fujishiro, Kenji Suzuki, Kohji Shirai, and Atsuhito Saiki. 2024. "Relationship of Serum Uric Acid with Kidney Function Decline Mediated by Systemic Arterial Stiffness: A Retrospective Cohort Study in Japan" Diagnostics 14, no. 2: 195. https://doi.org/10.3390/diagnostics14020195
APA StyleNagayama, D., Watanabe, Y., Fujishiro, K., Suzuki, K., Shirai, K., & Saiki, A. (2024). Relationship of Serum Uric Acid with Kidney Function Decline Mediated by Systemic Arterial Stiffness: A Retrospective Cohort Study in Japan. Diagnostics, 14(2), 195. https://doi.org/10.3390/diagnostics14020195






