Abstract
Intracranial chondroid tumors are a heterogeneous group of neoplasms characterized by the presence of a cartilage matrix. These tumors exhibit overlapping clinical and histological features. Mutations in IDH1/2 genes serve as important diagnostic markers of tumor type, particularly chondrosarcoma. To improve the accuracy of IDH1/2 diagnostics, we compared three methods: biochip assay, real-time PCR with DNA melting analysis using TaqMan probes and sequencing (qPCR-DMA-Sanger), and immunohistochemistry (IHC). Tumor samples from 96 patients were investigated. The IDH1 mutations were detected in 34/64 (53%) chondrosarcomas; IHC detected 27/56 (48.2%) mutations, the qPCR-DMA-Sanger method 27/59 (46%) mutations, and the biochip assay revealed 29/60 (48.3%) mutations. The detection of IDH1 mutations in chordoma (2/15) and osteosarcoma (2/7) suggested the need for a revised diagnosis. In benign tumors, IDH1 mutations were present in chondroma (4/6), but absent in chondromyxoid fibroma (0/4). The most frequent IDH1 mutations were R132C (60%), R132L, and R132G (13.5% each), R132H (8%), and R132S (5%). The concordance between the biochip assay and IHC was 90%, between IHC and PCR-DMA-Sanger 83%, and between biochip assay and qPCR-DMA-Sanger was 98%, respectively. No IDH2 mutations were found. The use of independent diagnostic methods may improve the detection of IDH-mutant specimens in chondroid tumors.
1. Introduction
Mutations in the genes of isocitrate dehydrogenases 1 and 2 (IDH1 and IDH2) play an essential role in the development of a number of tumors (gliomas, chondroid tumors, leukemia), and are important for the diagnosis and choice of therapy [1,2,3]. Isocitrate dehydrogenases are involved in the oxidative decarboxylation of isocitrate, converting it into α-ketoglutarate, an intermediate product of the Krebs cycle. Somatic mutations in the IDH1 and IDH2 genes are heterozygous nucleotide substitutions in the catalytic domain, leading to a loss of the normal functional activity of the enzyme, reduction of α-ketoglutarate levels, and formation of 2-hydroxyglutarate, which has oncogenic activity [3,4,5]. Mutations in the IDH1 gene occur in codon R132, with >90% of mutations in gliomas associated with arginine to histidine (p.R132H) substitution, while a wider range of amino acid changes have been observed in chondroid tumors [1,6,7,8]. In the IDH2 gene, mutations affect the R172 codon, with arginine to lysine (p.R172K) being the most frequent substitution [9]. Mutations in the IDH1 gene are much more common than mutations in the IDH2 gene and account for >95% of all cases of the IDH1/2 mutant genotype [3,6,8].
Intracranial chondroid tumors (ICHTs) are rare neoplasms, comprising, according to different data, from 0.15 to 2% of all head and neck tumors [10]. All ICHTs can be divided into two groups depending on the nature of the cartilage matrix. The first group is cartilaginous matrix tumors, which include chondromas and chondrosarcomas, as well as the chondroblastic variant of conventional osteosarcoma. The second group represents tumors containing mostly cartilage-like or myxoid matrix: chondroid chordoma, chondromesenchymal hamartoma, and chondromyxoid fibroma. Depending on the degree of malignancy, ICHTs can be divided into benign (chondroma, chondromesenchymal hamartoma, chondromyxoid fibroma), and malignant (chordoma, chondrosarcoma, chondroblastic osteosarcoma) tumors [11,12].
Intracranial chondrosarcomas represent a very heterogeneous group of ICHTs and are classified into several subtypes: conventional chondrosarcoma, clear cell chondrosarcoma, mesenchymal chondrosarcoma, and dedifferentiated chondrosarcoma. Conventional chondrosarcomas represent the most common pathological entity among all chondrosarcomas (80%) and, in turn, can be divided according to their bone location in central and peripheral chondrosarcomas [11,13]. In addition, the conventional chondrosarcomas are classified as primary or secondary if they arise de novo or from pre-existing lesions (osteochondroma, enchondroma) [11,12,14].
Regardless of the histological variant, ICHTs are most often localized in bones of the skull base, namely in the region of spheno-occipital synchondrosis [10]. The most common clinical manifestations of ICHTs are headache and oculomotor disorders associated with lesions of the third cranial nerves. The strategies of treatment and prognosis differ significantly between benign and different malignant types, while preoperative diagnostics is a difficult complex task [14]. The molecular analysis of clinically relevant diagnostic markers, exemplified by IDH1/2 mutations, can significantly improve decision-making in patient management. The hot-spot IDH1/2 mutations have been found to be hallmarks of several types of chondroid tumors and are mainly encountered in benign enchondromas (up to 87%) [7], central conventional chondrosarcomas (about 50%), and dedifferentiated chondrosarcomas [6,15].
The accuracy and reproducibility of the methods used to detect IDH1/2 mutations are important for implementing molecular findings into medical practice guidelines. Direct sequencing seems to be the “gold” standard in assessing the mutational status of IDH1/2 genes [16]. However, the sensitivity of this method depends on the quality of the sample; moreover, the threshold for the detection of mutant DNA against wild-type DNA is above 10% [16,17].
Another widely used method for detecting mutations in IDH1/2 genes is immunohistochemistry (IHC) using mono- or polyclonal antibodies, which is considered a fast, reliable, and cost-effective laboratory method [16,17]. At the same time, IHC is sensitive to the quality of the sample and requires the development of specific antibodies for the detection of mutation type. Also, the heterogeneity in staining of tumor specimens and cross-reactivity of antibodies may be observed [17,18,19].
Such methods of detecting IDH mutations as allele-specific PCR [20] or pyrosequencing [21] are used in clinical practice, but they have no significant advantages over traditional sequencing. The use of asymmetric real-time PCR in combination with DNA melting analysis using a TaqMan probe (qPCR-DMA) was proposed to detect somatic mutations with a sensitivity of 5%, but Sanger sequencing is additionally required to identify nucleotide substitutions [22].
The use of next-generation sequencing (NGS) allows identification of variant alleles at frequencies of 0.1% and lower [23], which may substantially increase the sensitivity of IDH1/2 genotyping. In addition to IDH1/2 hot-spot mutations, a complex mutational landscape including deleterious variants in TP53, EGFR, APC, and ATM genes has been revealed in chondrosarcomas [24]. However, this approach requires rather expensive equipment, sophisticated data analysis, highly qualified personnel, and a sufficient amount of tumor material; all of these things together probably make it not so cost-effective when examining a very limited number of targets.
Another diagnostic tool used in routine diagnostics is biological microarrays (biological microchips, biochips) [25]. It was shown previously that biochips can be used for the analysis of somatic mutations in different types of cancer (lung cancer, melanoma, colorectal cancer) [26]. The biochip-based approach was developed for the simultaneous analysis of mutations in the IDH1 and IDH2 genes and the ability to detect different IDH1/2 mutations was demonstrated in control samples [27].
In the present study, we applied wild-type blocking PCR with the use of locked nucleic acid (LNA) oligonucleotides to increase the sensitivity of the biochip assay for IDH1/2 mutations analysis. One aim of the study was to test this high-sensitive approach on a collection of chondroid tumor samples and to compare it with other methods: qPCR-DMA followed by Sanger sequencing and IHC. Another important aim was to determine the IDH mutational status in a series of 96 intracranial chondroid tumors, including chondrosarcomas, chordomas, osteosarcomas, chondromas, and chondromyxoid fibromas in order to evaluate the diagnostic utility of IDH1/2 mutations for these types of neoplasms.
2. Materials and Methods
2.1. Study Cohort
The retrospective study included ICHTs that were diagnosed between 2012 and 2019 in different Russian medical centers, mainly in the N.N. Burdenko National Medical Research Center of Neurosurgery (Moscow). The slides and paraffin blocks were centralized and reviewed at the Pathology Department of the Russian Children’s Clinical Hospital (N.I. Pirogov Russian National Research Medical University, Moscow).
Finally, we collected samples from 96 patients with ICHTs (96 surgical resections) ranging in age from 2 to 74 years (mean age 39.6 years), 29 males and 67 females. The diagnostic criteria based on morphological, radiological, and histological data, were applied as described earlier [28]. The diagnoses between the 96 cases were categorized as follows: chondrosarcoma (n = 64), chordoma (n = 15), osteosarcoma (n = 7), chondroma (n = 6), and chondromyxoid fibroma (n = 4).
All tissue samples were formalin-fixed and paraffin-embedded (FFPE). Before embedding, the decalcification stage was performed with the use of special decalcifying solutions (10% formic acid or 5% hydrochloric acid). After preparation, the FFPE blocks were used for histological analysis, immunohistochemical staining, and molecular genetic analysis.
2.2. DNA Isolation
DNA was extracted from FFPE tissue using QIAamp DNA FFPE (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol. Concentration and purity (260/280 nm ratio) of DNA were determined by using the NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, USA).
2.3. Real-Time PCR and DNA Melting Analysis Using TaqMan Probes and Sanger Sequencing (qPCR-DMA-Sanger)
Mutations were analyzed by asymmetric real-time PCR followed by melting analysis of the PCR product in the presence of TaqMan probes, as described previously [27]. Detection of mutations in the IDH1 and IDH2 genes was performed in different tubes. The primer and probe sequences are in Table S1.
The 25µL of PCR mixture contained 50 mM Tris-HCl, pH 8.8, 50 mM KCl, 0.01% (v/v) Tween 20, 3 mM MgCl2, 0.25 mM dNTPs, 1.25 U Taq polymerase, a primer pair (0.04 µM: 0.4 µM), 0.2 µM TaqMan probe (DNA Synthesis, Ltd., Moscow, Russia), and 5 µL of DNA template.
Briefly, the reaction was performed in a LightCycler 96 amplifier (Roche, Diagnostics, Rotkreuz, Switzerland) for both primer pairs: 95 °C for 5 min, then (95 °C for 13 s, 57 °C for 40 s, 72 °C for 20 s) × 53 cycles; melting of PCR products: 95 °C for 1 min, 55 °C for 4 min, then from 55 to 90 °C, increasing the temperature by 0.2 °C for each step with a step duration of 12 s. Further, samples positive for IDH1/2 mutations were sequenced on an Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, Waltham, USA) using the standard protocol.
2.4. Biochip Manufacturing
The biochip for the detection of IDH1 and IDH2 mutations and the sequences of immobilized oligonucleotides were described earlier [27]. Briefly, oligonucleotide probes 14–20 bp in length were synthesized on a 394 DNA/RNA synthesizer (Applied Biosystems, USA) using standard phosphoramidite chemistry. The oligonucleotides carry an amino group at the 3’-terminus for immobilization in the polyacrylamide gel drops on a biochip using a copolymerization method [25].
2.5. Wild-Type Blocking PCR and Hybridization with Biochip
To obtain a single-stranded and fluorescently labeled DNA fragment for hybridization on the biochip, a two-step nested PCR method was used as previously described [26,27]. In the first step, the wild-type blocking PCR with LNA probes was used. The sequences of primers and LNA probes are in Table S2. The concentration of LNA oligonucleotide was 0.02–0.2 µM for 5–10 ng of genomic DNA in the reaction. During the first step, a double-stranded product was generated, predominantly from mutant DNA. In the second step, the product of the first step was used as a matrix and asymmetric PCR was performed with simultaneous labeling with fluorescent Cy5-dUTP of the single-strand PCR product. Hybridization of the fluorescently labeled PCR product on a biochip was performed under the conditions described previously [26,27]. Fluorescent signals were registered using a biochip analyzer, and image analysis and genotype assignment were performed using the ImaGeWare ver.3.5 software (Biochip-IMB, Ltd., Moscow, Russia) [25,26].
2.6. Immunohistochemistry (IHC)
The 4 mm thick tissue sections were cut, heated at 58 °C for 2 h, deparaffinized, and immunostained on a fully automated Roche Ventana Bench-Mark Ultra system (Roche Diagnostics, Rotkreuz, Switzerland) with subsequent hematoxylin staining following the manufacturer’s guidelines. The tissue samples underwent immunostaining using specific antibodies, including a rabbit polyclonal anti-IDH1 R132 mutation antibody, a mouse IgG2b/K monoclonal antibody for brachyury, monoclonal antibodies for S100 protein, epithelial membrane antigen (EMA), and Ki-67 (Invitrogen, Thermo Ficher Scientific, Waltham, MA, USA).
3. Results
3.1. Clinical Characteristics
The basic criteria for patient inclusion in the study involved the presence of true cartilage or cartilage-like (chondroid) matrix in the tumor tissue and anatomical localization of the pathological process in the cranial bones. The histologic features and the clinical data available are in Table 1.

Table 1.
Clinicopathological characteristics of patients with intracranial chondroid tumors.
Malignant tumors were represented by chondrosarcoma (n = 64), chordoma (n = 15), and osteosarcoma (n = 7). Chondrosarcomas (n = 64) constituted the largest group. The S100 protein expression was detected in the vast majority of cases (96%). The following histologic subtypes were identified: primary central chondrosarcoma/atypical cartilaginous tumour (ACT), grade 1 (29.7%); primary central chondrosarcoma, grades 2 (65.6%) and 3 (1.6%); and dedifferentiated chondrosarcoma (3.1%). The most frequent tumor process involved the cavernous sinus (43%), sphenoid bone (40%), clivus (34%), temporal bone (34%), and ethmoid bone (25%). More rarely, the tumor process spread to the facial skeleton (15%), dura mater (9%), and falx cerebri (4%).
Among the chordoma specimens (n = 15), the majority belonged to the classical variant (n = 8, 53%), and one third (n = 5, 33%) was represented by the chondroid variant of chordoma with the presence of cartilaginous matrix. In one case, a chordoma combined with an aneurysmal bone cyst (ABC) was observed. Most chordomas involved the clivus (77%), sphenoid bone (54%), and cavernous sinus (38%). In 85% of cases, an expression of a specific brachyury protein was detected in the tumor cells.
Osteosarcoma specimens (n = 7) were represented by osteosarcoma, not otherwise specified (NOS) (n = 3), chondroblastic variant osteosarcoma (n = 3), and undifferentiated pleomorphic osteosarcoma (n = 1). Osteosarcomas involved various bones of the skull: cavernous sinus, sphenoid bone, ethmoid bone, and also orbit, maxillary sinuses, and nasopharynx.
Benign intracranial tumors were represented by chondroma (n = 6) and chondromyxoid fibroma (n= 4). Most cases of chondroma affected the superior sagittal sinus (n = 4) and falx cerebri (n = 3).
3.2. Detection of IDH1/2 Mutations Using qPCR-DMA Followed by Sanger Sequencing
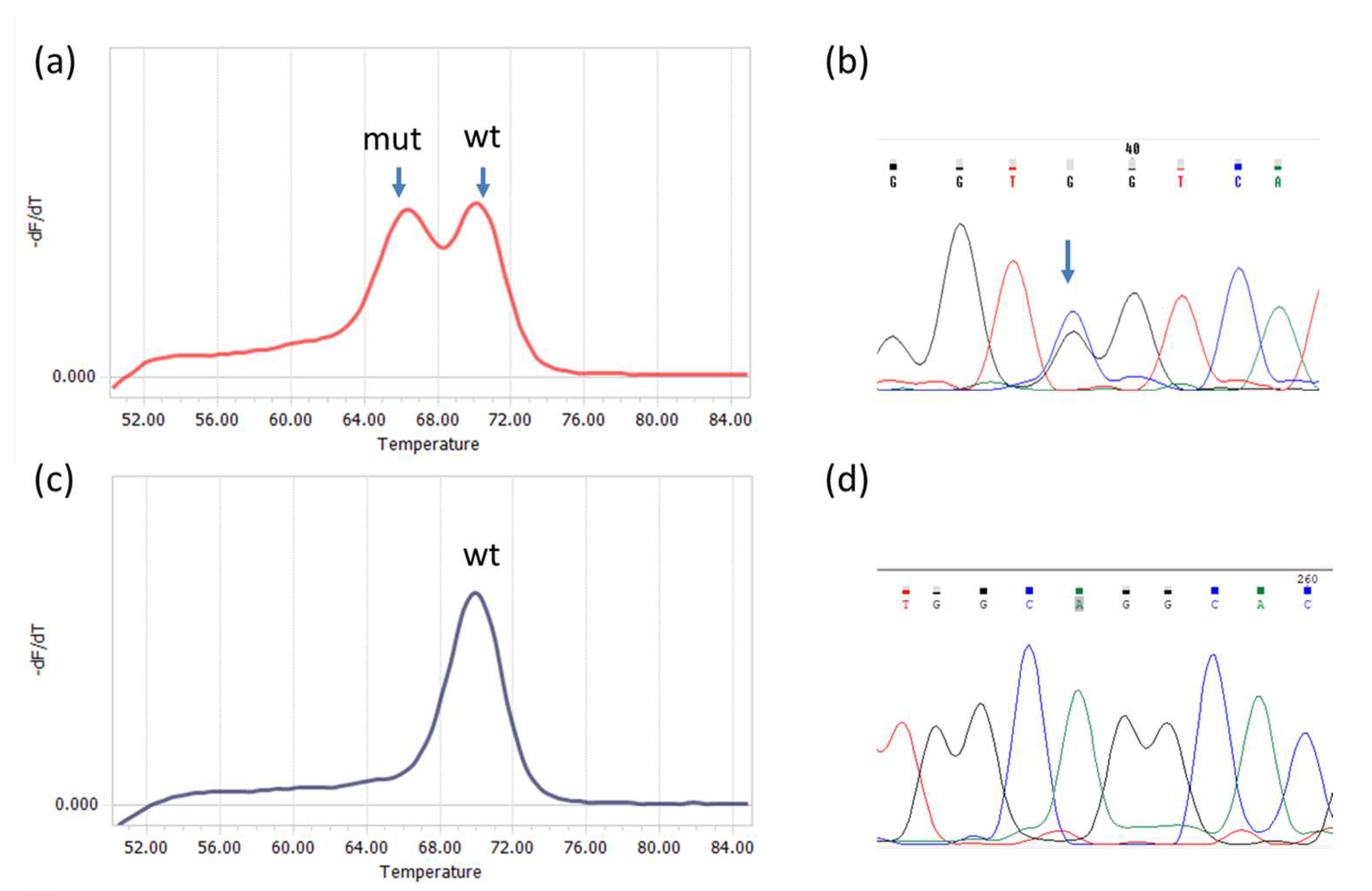
Analysis was performed for IDH1 and IDH2 mutations independently. All 96 tumor samples were screened for IDH1 mutations, but due to an insufficient amount of the material, only 79 were analyzed for IDH2 mutations. The sizes of IDH1 and IDH2 PCR products were 143 bp and 148 bp, respectively. Examples of melting curves and sequences obtained for the PCR products are shown in Figure 1.
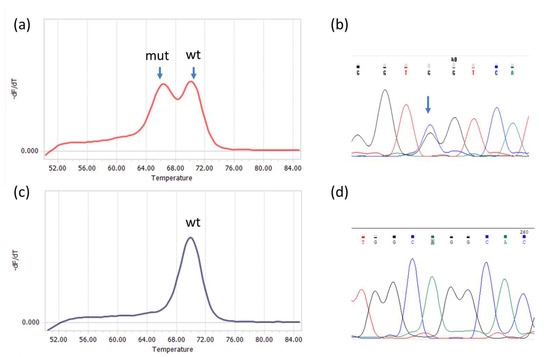
Figure 1.
Detection of R132 IDH1 and R172 IDH2 mutations by qPCR−DMA−Sanger; (a) melting curve for the IDH1 gene, one peak corresponds to a homoduplex of wild−type DNA with the TaqMan probe (wt), the other represents a heteroduplex of mutated DNA with the TaqMan probe; (b) sequencing of the PCR product shows the presence of CGT>GGT substitution in codon R132; (c) melting curve for the IDH2 gene with only one peak corresponding to wild−type DNA (wt); (d) the result was confirmed by wild-type sequence AGG in codon R172.
3.3. Detection of IDH1/2 Mutations Using a Biochip Assay
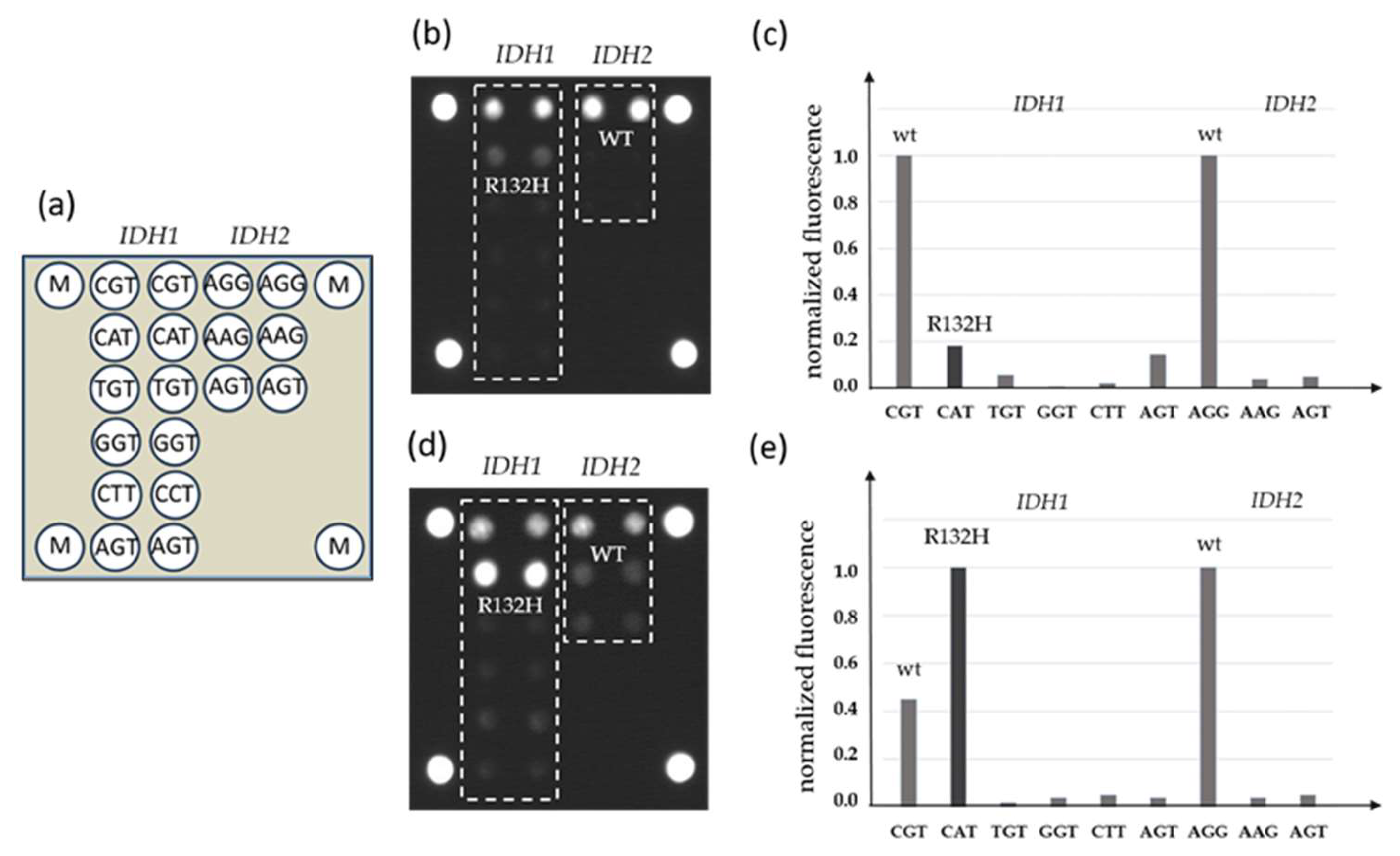
DNA samples from FFPE blocks were used in parallel for qPCR-DMA-Sanger and biochip assay. A combination of two-step nested PCR and hybridization analysis with immobilized oligonucleotide probes was used in the biochip assay (Figure 2).
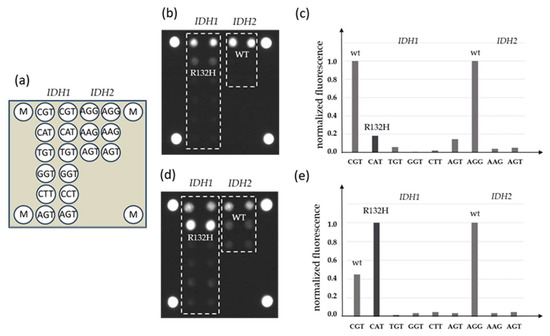
Figure 2.
Detection of IDH1 and IDH2 mutations by a biochip assay; (a) scheme of the biochip with immobilized probes; (b) the hybridization pattern without inhibition by LNA oligonucleotides and (c) levels of normalized fluorescent signals from biochip cells; (d) hybridization pattern after inhibition of wild-type DNA amplification by LNA oligonucleotides and (e) levels of normalized fluorescent signals from biochip cells, the IDH1 mutation R132H is revealed (M—fluorescent marker).
To increase the sensitivity of the assay, LNA oligonucleotides complementary to the IDH1 or IDH2 wild-type sequence in the corresponding position were added to the PCR mixture during the first step of the reaction.
It was shown previously that the wild-type DNA blocking during amplification could significantly increase the sensitivity of the assay up to 1% of mutated DNA against the 99% wild-type DNA background [26].
3.4. Detection of IDH1 mutations using IHC
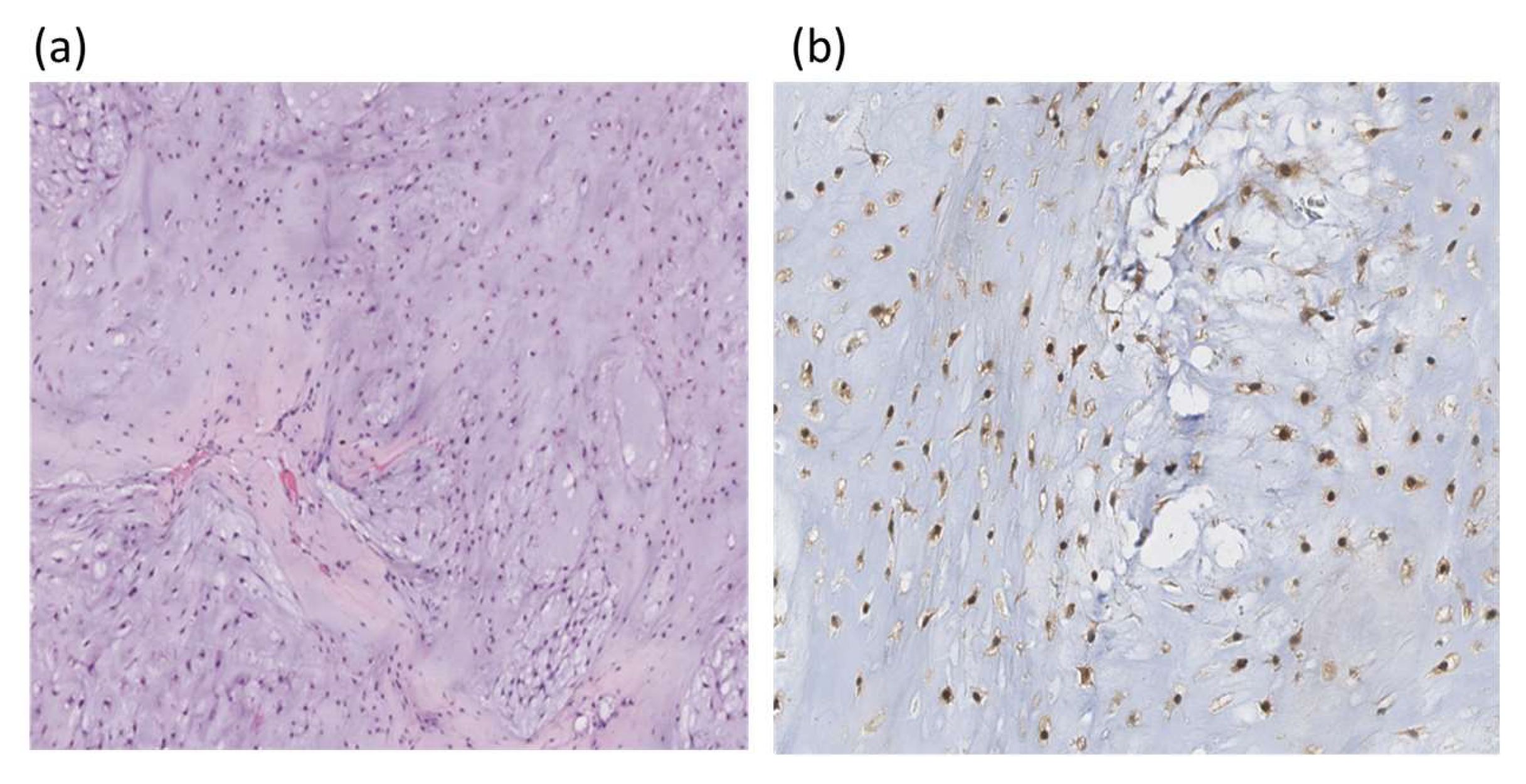
Using IHC, only IDH1 mutations were tested. An example of IHC staining of chondrosarcoma histological section with an IDH1 polyclonal antibody is in Figure 3.
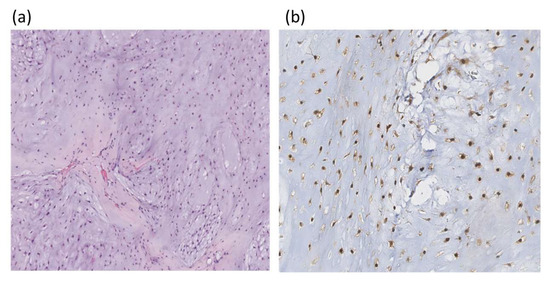
Figure 3.
Histologic section of chondrosarcoma; (a) hematoxylin and eosin staining ×100; (b) IHC with polyclonal IDH1 antibodies, the nuclear reaction is observed (×200).
3.5. IDH1/2 Mutational Status of Intracranial Chondroid Tumors
The 96 archived FFPE samples of ICHTs (chondrosarcoma, chordoma, osteosarcoma, chondroma, and chondromyxoid fibroma) were tested for the presence of IDH1 mutations using three methods: biochip assay, qPCR-DMA with the TaqMan probe followed by sequencing of IDH1-positive samples, and IHC with IDH1-specific polyclonal antibodies.
In chondrosarcomas, mutations in the IDH1 gene were detected at least by one method in 34/64 (53%) samples. The biochip assay identified 29/60 (48.3%) mutations, the qPCR-DMA with sequencing detected 27/59 (46%), and the IHC method could reveal 27/56 (48.2%) mutations. The diagnostic yield of all three methods together was 21/47 (45%) mutations (Table 2).

Table 2.
Chondrosarcoma cases positive for IDH1 mutations and determined by any of three methods (NOS—not otherwise specified, ND—not determined, GSR—chiasmal-sellar region; discrepancies in results between methods marked in bold).
The IDH1 mutations were also found in 2/15 chordomas; both samples positive for the IDH1 mutation histologically were diagnosed as chondroid chordoma. In one case (37 CRD), the IDH1 mutation was detected by two methods: qPCR-DMA-Sanger and IHC; no brachyury expression was found in the sample. In the case of 38 CRD, the IDH1 mutation was revealed by both molecular methods, while the IHC method was not applicable to this sample due to the small quantity of tumor material (Table 3).

Table 3.
The IDH1 mutations found in other tumor types (NOS—not otherwise specified, ND—not determined, GSR—chiasmal-sellar region; discrepancies in results between methods marked in bold, CHOS—chondroblastic osteosarcoma, CRD—chordoma, CHND—chondroma).
In osteosarcoma patients (n = 7), two samples were found to be positive for the IDH1 mutation; both cases were diagnosed as a chondroblastic variant of osteosarcoma. In one case, 35 CHOS, the IDH1 mutation was detected only by qPCR-DMA-Sanger and in the other case, 36 CHOS, the mutation was revealed only by IHC.
The IDH1 mutations were also detected in 4/6 (66%) chondroma samples (two mutations found by IHC and one of the molecular methods, while two mutations were revealed by one of the molecular methods). No IDH1 mutations were found in chondromyxoid fibroma (n = 4).
The summary of IDH1 mutation findings using different approaches is in Table 4.

Table 4.
Summary of IDH1 mutation discovery using different approaches.
Among IDH1 mutations in the total sample, TGT was the most frequently detected codon at 59.5%, CTT and GGT at 13.5% each, CAT at 8%, and AGT at 5%. In chondrosarcomas, this proportion between different mutations was practically the same: TGT at 60%, CTT and GGT at 17% each, CAT at 3%, and AGT at 3% (Table 5).

Table 5.
Frequency of different nucleotide changes in codon 132 of the IDH1 gene revealed by molecular methods (nucleotide change marked in bold).
The concordance in mutation analysis between the different methods was calculated (Table 6). Between the biochip assay and IHC, the percentage of matched cases was 90%; between qPCR-DMA-Sanger and IHC, it was 83%; and between the biochip assay and qPCR-DMA-Sanger, it was 98%, respectively.

Table 6.
Comparison of different approaches to diagnostics of the IDH1 mutations in chondrosarcomas: biochip assay, qPCR- DMA-Sanger, and IHC.
The mutations in the IDH2 gene were analyzed only by two molecular methods: qPCR-DMA-Sanger and biochip assay. In summary, the testing for IDH2 mutations was applicable to 79 samples of ICHTs and no IDH2 mutations were found.
4. Discussion
Our study is primarily aimed at validation of a novel biochip-based approach to diagnose IDH1 and IDH2 mutations by comparing with known methods such as IHC and real-time PCR and DNA melting analysis using TaqMan probes combined with Sanger sequencing (Figure 1, Figure 2 and Figure 3). The methods were applied to study a sample of 96 ICHTs localized predominantly at the base of the skull (Table 1). There are few available studies on the frequency of IDH1/2 mutations in cartilage tumors of this rare localization, and they mainly concern chondrosarcomas [8,29,30,31,32]. The IDH1/2 mutation rate in different histological tumor types provides us with important insights into the clinical utility of IDH1/2 diagnostic markers and pathways of malignant neoplasms.
The main problem in mutation analysis of cartilaginous tumors is the aggressive processing of the investigated material during the mandatory decalcification procedure; the pre-treatment prior to embedding into paraffin may be the most common cause of failed results [33]. In our study, the IHC method was the most sensitive to aggressive decalcification and was successful in only 84/96 (88%) samples, whereas the biochip assay was able to analyze 89/96 (93%) tumor samples, and qPCR-DMA-Sanger 88/96 (92%), respectively. Nevertheless, the detection rate of the IDH1 mutation was almost similar for all three methods in the whole sample of 96 tumors (36%, 36%, and 37%, see Table 4), but in chondrosarcomas, qPCR-DMA-Sanger showed a slightly lower sensitivity (46%) compared to biochip (48.3%) or IHC (48.2%) methods.
The higher efficiency of IHC compared to conventional Sanger sequencing has been previously demonstrated [19]. Insufficient sensitivity of Sanger sequencing in detecting mutations present in less than <30% of the sequenced PCR product may lead to false negative results in samples with a low frequency of IDH-mutant alleles. Incorrect assignment of genotype, in turn, can be a source of error in evaluating the prognostic significance of IDH mutations [13].
High-resolution melting analysis can be used to detect IDH1/2 mutations, which is less costly and time-consuming, yet more sensitive than Sanger sequencing [22,34]. In our study, we used a combination of asymmetric real-time PCR and TaqMan probe melting assay (qPCR-DMA) with Sanger sequencing of IDH-positive samples to increase the efficiency of analysis, validate the findings, and identify the nucleotide substitution. In one case, we discovered the IDH1 mutation by qPCR-DMA, but direct sequencing revealed an IDH wild-type genotype.
The concordance between biochip assay and IHC analysis was 90%, between IHC and qPCR-DMA-Sanger was 83%, and between biochip assay and qPCR-DMA-Sanger was 98%, respectively (Table 6). The frequency of IDH1 mutations in chondrosarcomas detected by all three methods was 45%, increasing to 53% when a positive result obtained by at least one method was considered. Discrepancies between the methods could be due to the rigorous pretreatment of chondroid tissue before analysis, which may affect the immunogenicity of samples in IHC or degrade DNA in molecular analysis, leading to false negatives in either case. Another reason could be the heterogeneity of the tumor and the use of tissue sections from various areas for different methods.
In our study, the frequency of IDH1 mutations differed significantly between tumor types (Table 2 and Table 3). In malignant tumors, IDH1-positive cases were predominantly found in 48% of chondrosarcomas, which is consistent with other studies [29,31]. In general, the frequency of IDH1 mutation in head chondrosarcomas can vary from 0% to 85% depending on the localization, histological type, and stage [8,16,29,30,31]. In our study, two cases of dedifferentiated chondrosarcoma had an IDH mutated genotype, defined at least by one method, and no significant difference in IDH1 mutation frequency between G1 and G2-G3 grades of central chondrosarcoma were found (52% and 51%, respectively). The distribution of IDH1 mutations by type in our study was close to that described previously: TGT (R132C) accounted for 60% (Table 5) [8].
Clinically, radiologically, and pathomorphologically, chondrosarcoma overlaps with another malignant neoplasm, chordoma, and the differential diagnosis between these tumors presents a major diagnostic challenge [28,35]. Chordomas arise from embryonic remnants of primitive notochord, while chondrosarcomas arise from primitive mesenchymal cells or from the embryonic remnant of the cartilaginous matrix of the skull [36]. Chondrosarcomas treated with similar comprehensive strategies have a significantly better prognosis than chordomas, with overall survival rates of approximately 65% for chordomas and 80% for chondrosarcomas at 5 years, 30% and 50% at 10 years, respectively [37].
The main difference between these two chondroid neoplasms is that IDH1 and IDH2 mutations, which are frequently found in chondrosarcomas, are not detected in chordomas [6,29,31]. In our study, two IDH1-positive cases were found among 15 chordoma specimens, which can be interpreted as misdiagnosed tumors. In addition, one of these cases was negative for brachyury, a transcription factor encoded by the T gene whose expression is highly specific for chordomas (Table 3). On the other hand, tissue decalcification can also lead to a loss of brachyury expression [38].
A similar situation was found in osteosarcoma samples, where among seven tumors, two chondroblastic osteosarcomas were also IDH1 mutation-positive (Table 3). Since IDH1/2 mutations are uncharacteristic for chordoma and osteosarcoma, these results may request a revision of the diagnosis. In addition, the possibility of false-positive IDH mutations should be tested, for example, using different diagnostic approaches.
In the analysis of six benign intracranial chondromas, IDH1 mutations were detected in 4/6 (66%) (Table 3). However, it should be noted that the sample of chondromas was small, and the results obtained by different methods were inconsistent due to the low quality of tumor material. Chondromas are uncommon intracranial tumors with an estimated incidence rate of 0.2–0.3% of all intracranial tumors [39]. Chondroma malignant degeneration has not been identified, recurrences are rare (5–18%), and so local excision is the treatment of choice. The literature describes that IDH1/2 mutations are frequently found in central and periosteal chondromas [6], often associated with Ollier’s disease and Maffucci syndrome [7]. These data suggest that chondromas and chondrosarcomas should be considered as being at opposite ends of the pathologic spectrum from benign to malignant tumors, and that somatic IDH mutations are suggested to be early events in malignant transformation [40]. Further studies are needed to elucidate this issue. It should be noted that no IDH1/2 mutations were found in another benign tumor, chondromyxoid fibroma (n = 4) (Table 3).
Despite their significant role in the early stages of tumor development, the prognostic value of IDH mutations in chondrosarcoma appears to be controversial, with different studies indicating a better [41] or worse [24] prognosis, or no association between IDH mutation and outcome [42]. These contradictions may be explained by additional molecular events, which occur during tumor development [13]. It was shown that besides IDH mutations; chondrosarcomas often carried mutations in TP53, CDKN2A/B, COL2A1, YEATS2, NRAS, and TERT genes [13,43,44]. In addition, the overall phenotype of DNA hypermethylation that is characteristic for IDH-mutant tumors may change during tumor progression [45]. It is postulated that these genetic and epigenetic events lead to the formation of different molecular subtypes in groups of patients with IDH-mutant or IDH wild-type chondrosarcomas and affect prognosis and response to treatment. Thus, the complexity of the mutational and epigenetic landscapes must be taken into account when developing new therapeutic strategies for chondroid tumors.
The limitations associated with this study should be mentioned. The first limitation is related to the peculiarities of FFPE block preparation from chondroid tissues. Aggressive decalcification could lead to deterioration in the quality of tissue samples for IHC staining and degradation of DNA for genetic testing which, in both cases, could affect the results of IDH1 and IDH2 mutation analysis. In a number of cases, the small amount of material did not allow for a proper examination by all three methods.
Second, the heterogeneity across tumor samples and the inability to use the same blocks of tumor tissue in different techniques due to the limited amount of biological material could lead to discrepancies in results between methods.
Third, the number of patients in the groups with different neoplasms varied significantly. The most representative group consisted of patients with chondrosarcoma, but the small number of chondroma cases did not allow for a definite conclusion about the IDH1/2 mutational landscape in this benign tumor with a rare intracranial localization.
Fourth, our study did not investigate the impact of other potential molecular biomarkers that may be associated with IDH1/2 mutations and influence their diagnostic and prognostic significance.
5. Conclusions
This paper presents a biochip assay for the diagnosis of IDH1/2 somatic mutations in tumor samples. The main advantages of the method are high sensitivity due to the blocking of wild-type PCR, the possibility of mutation type detection, and simultaneous analysis of IDH1 and IDH2 mutations. The method was validated on 96 chondroid tumor samples and showed its efficiency in IDH1/2 mutation analysis when compared with IHC and real-time PCR with DNA melting analysis using TaqMan probes followed by Sanger sequencing. At the same time, the use of several independent techniques can significantly increase the detection rate of IDH-positive samples.
The IDH1 mutations have been found predominantly in intracranial chondrosarcomas, as well as in chondromas affecting mainly the base of the skull. A further study in a larger sample may be required to determine the frequency of IDH1/2 mutations in chondromas of the head.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14020200/s1, Table S1: Primers and TaqMan probes used in real-time PCR with DNA melting analysis title; Table S2: Primers and LNA probes used in biochip assay.
Author Contributions
Conceptualization, T.N. and D.R.; methodology (molecular methods), V.V. and D.G.; methodology (immunohistochemistry), A.S.; clinical data and analysis, A.S. and D.R.; experimental investigation, V.V. and D.G.; writing—original draft preparation, T.N., V.V. and A.S.; supervision, T.N. and D.R.; project administration, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (grant no. 22-15-00304).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee of the N.I. Pirogov Russian National Research Medical University (protocol code 167; 10 February 2019).
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Watanbe, T.; Nobusawa, S.; Kleihues, P.; Ohgaki, H. IDH1 mutations are early events in the development of astrocytomas and oligo dendrogliomas. Am. J. Pathol. 2009, 174, 1149–1153. [Google Scholar] [CrossRef]
- Liu, X.; Ling, Z.-Q. Role of isocitrate dehydrogenase 1/2 (IDH 1/2) gene mutations in human tumors. Histol. Histopathol. 2015, 30, 1155–1160. [Google Scholar] [PubMed]
- Pirozzi, C.J.; Yan, H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Carbonneau, M.; Gagne, L.M.; Lalonde, M.E.; Germain, M.A.; Motorina, A.; Guiot, M.C.; Secco, B.; Vincent, E.E.; Tumber, A.; Hulea, L.; et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat. Commun. 2016, 7, 12700. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Pollock, R.; O’Donnell, P.; Grigoriadis, A.; Diss, T.; et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Pansuriya, T.C.; van Eijk, R.; d’Adamo, P.; van Ruler, M.A.; Kuijjer, M.L.; Oosting, J.; Cleton-Jansen, A.M.; van Oosterwijk, J.G.; Verbeke, S.L.; Meijer, D.; et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat. Genet. 2011, 43, 1256–1261. [Google Scholar] [CrossRef]
- Tallegas, M.; Miquelestorena-Standley, É.; Labit-Bouvier, C.; Badoual, C.; Francois, A.; Gomez-Brouchet, A.; Aubert, S.; Collin, C.; Tallet, A.; de Pinieux, G. IDH mutation status in a series of 88 head and neck chondrosarcomas: Different profile between tumors of the skull base and tumors involving the facial skeleton and the laryngotracheal tract. Hum. Pathol. 2019, 84, 183–191. [Google Scholar] [CrossRef]
- Kats, L.M.; Reschke, M.; Taulli, R.; Pozdnyakova, O.; Burgess, K.; Bhargava, P.; Straley, K.; Karnik, R.; Meissner, A.; Small, D.; et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Bloch, O.G.; Jian, B.J.; Yang, I.; Han, S.J.; Aranda, D.; Ahn, B.J.; Parsa, A.T. A systematic review of intracranial chondrosarcoma and survival. J. Clin. Neurosci. 2009, 16, 1547–1551. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.W.; Silva, T.M.; Bovée, J.V.M.G. New molecular entities of soft tissue and bone tumors. Curr. Opin. Oncol. 2022, 34, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Venneker, S.; Bovée, J.V.M.G. IDH Mutations in Chondrosarcoma: Case Closed or Not? Cancers 2023, 15, 3603. [Google Scholar] [CrossRef] [PubMed]
- Gómez-León, N.; Galán-González, I.; Moreno-Casado, M.J.; Benavides-de-Quirós, C.; Muñoz-Hernández, P.; Fernández-Rico, P.; Rodríguez-Laval, V. Chondroid Tumors: Review of Salient Imaging Features and Update on the WHO Classification. Curr. Probl. Diagn. Radiol. 2023, 52, 197–211. [Google Scholar] [CrossRef]
- Chen, S.; Fritchie, K.; Wei, S.; Ali, N.; Curless, K.; Shen, T.; Brini, A.T.; Latif, F.; Sumathi, V.; Siegal, G.P.; et al. Diagnostic Utility of IDH1/2 Mutations to Distinguish Dedifferentiated Chondrosarcoma from Undifferentiated Pleomorphic Sarcoma of Bone. Hum. Pathol. 2017, 65, 239–246. [Google Scholar] [CrossRef]
- Setola, E.; Benini, S.; Righi, A.; Gamberi, G.; Carretta, E.; Ferrari, C.; Avnet, S.; Palmerini, E.; Magagnoli, G.; Gambarotti, M. IDH mutations in G2-3 conventional central bone chondrosarcoma: A mono institutional experience. BMC Cancer 2023, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Tian, W.; Matsuda, M.; Yamamoto, T.; Ishikawa, E.; Kaneko, M.K.; Yamazaki, K.; Kato, Y.; Matsumura, A. Detection of IDH1 mutation in human gliomas: Comparison of immunohistochemistry and sequencing. Brain Tumor Pathol. 2011, 28, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sharma, M.C.; Jha, P.; Pathak, P.; Suri, V.; Sarkar, C.; Chosdol, K.; Suri, A.; Kale, S.S.; Mahapatra, A.K.; et al. Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing. Neuro-Oncology 2013, 15, 718–726. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Hartmann, C.; Preusser, M.; Ströbel, T.; Dubbink, H.J.; Kros, J.M.; von Deimling, A.; Boisselier, B.; Sanson, M.; Halling, K.C.; et al. Interlaboratory comparison of IDH mutation detection. J. Neuro-Oncol. 2013, 112, 173–178. [Google Scholar] [CrossRef]
- Loussouarn, D.; Le Loupp, A.G.; Frenel, J.S.; Leclair, F.; Von Deimling, A.; Aumont, M.; Martin, S.; Campone, M.; Denis, M.G. Comparison of immunohistochemistry, DNA sequencing and allele-specific PCR for the detection of IDH1 mutations in gliomas. Int. J. Oncol. 2012, 40, 2058–2062. [Google Scholar]
- Arita, H.; Narita, Y.; Matsushita, Y.; Fukushima, S.; Yoshida, A.; Takami, H.; Miyakita, Y.; Ohno, M.; Shibui, S.; Ichimura, K. Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Brain Tumor Pathol. 2015, 32, 22–30. [Google Scholar] [CrossRef]
- Botezatu, I.V.; Panchuk, I.O.; Stroganova, A.M.; Senderovich, A.I.; Kondratova, V.N.; Shelepov, V.P.; Lichtenstein, A.V. TaqMan probes as blocking agents for enriched PCR amplification and DNA melting analysis of mutant genes. Biotechniques 2017, 62, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Stoneking, M. A new approach for detecting low-level mutations in next-generation sequence data. Genome Biol. 2012, 13, R34. [Google Scholar] [CrossRef]
- Lugowska, I.; Teterycz, P.; Mikula, M.; Kulecka, M.; Kluska, A.; Balabas, A.; Piatkowska, M.; Wagrodzki, M.; Pienkowski, A.; Rutkowski, P.; et al. IDH1/2 Mutations Predict Shorter Survival in Chondrosarcoma. J. Cancer 2018, 9, 998–1005. [Google Scholar] [CrossRef]
- Gryadunov, D.; Dementieva, E.; Mikhailovich, V.; Nasedkina, T.; Rubina, A.; Savvateeva, E.; Fesenko, E.; Chudinov, A.; Zimenkov, D.; Kolchinsky, A.; et al. Gel-based microarrays in clinical diagnostics in Russia. Expert Rev. Mol. Diagn. 2011, 11, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, M.; Ghukasyan, L.; Abramov, I.; Ryabaya, O.; Stepanova, E.; Kudryavtseva, A.; Sadritdinova, A.; Dzhumakova, C.; Belysheva, T.; Surzhikov, S.; et al. Detection of BRAF, NRAS, KIT, GNAQ, GNA11 and MAP2K1/2 mutations in Russian melanoma patients using LNA PCR clamp and biochip analysis. Oncotarget 2017, 8, 52304–52320.23. [Google Scholar] [CrossRef] [PubMed]
- Varachev, V.O.; Guskov, D.A.; Shekhtman, A.P.; Rogozhin, D.V.; Polyakov, S.A.; Zasedatelev, A.S.; Chudinov, A.V.; Nasedkina, T.V. Biological Microarray for Detection of Somatic Mutations in the Genes of Isocitrate Dehydrogenase 1 and 2. Russ. J. Bioorganic Chem. 2023, 49, 1137–1142. (In Russian) [Google Scholar] [CrossRef]
- Shekhtman, A.P.; Rogozhin, D.V. Morphological diagnosis of chondroid tumors of skull bones: Actual situation. Bone Soft Tissue Sarcomas Tumors Ski. 2021, 13, 32–40. (In Russian) [Google Scholar] [CrossRef]
- Arai, M.; Nobusawa, S.; Ikota, H.; Takemura, S.; Nakazato, Y. Frequent IDH1/2 mutations in intracranial chondrosarcoma: A possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol. 2012, 29, 201–206. [Google Scholar] [CrossRef]
- Kanamori, H.; Kitamura, Y.; Kimura, T.; Yoshida, K.; Sasaki, H. Genetic characterization of skull base chondrosarcomas. J. Neurosurg. 2015, 123, 1036–1041. [Google Scholar] [CrossRef]
- Kitamura, Y.; Sasaki, H.; Yoshida, K. Genetic aberrations and molecular biology of skull base chordoma and chondrosarcoma. Brain Tumor Pathol. 2017, 34, 78–90. [Google Scholar] [CrossRef]
- Asioli, S.; Ruengwanichayakun, P.; Zoli, M.; Guaraldi, F.; Sollini, G.; Greco, P.; Facco, C.; Gibertoni, D.; Jiménez, B.V.; Benini, S.; et al. Association of Clinicopathological Features With Outcome in Chondrosarcomas of the Head and Neck. Otolaryngol. Head Neck Surg. 2021, 164, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Miquelestorena-Standley, E.; Jourdan, M.L.; Collin, C.; Bouvier, C.; Larousserie, F.; Aubert, S.; Gomez-Brouchet, A.; Guinebretière, J.M.; Tallegas, M.; Brulin, B.; et al. Effect of decalcification protocols on immunohistochemistry and molecular analyses of bone samples. Mod. Pathol. 2020, 33, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Barkoh, B.A.; Chen, Z.; Ma, D.; Reddy, N.; Medeiros, L.J.; Luthra, R. Diagnostic testing for IDH1 and IDH2 variants in acute myeloid leukemia an algorithmic approach using high-resolution melting curve analysis. J. Mol. Diagn. 2011, 13, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Almefty, K.; Pravdenkova, S.; Colli, B.O.; Al-Mefty, O.; Gokden, M. Chordoma and chondrosarcoma: Similar, but quite different, skull base tumors. Cancer 2007, 110, 2457–2467. [Google Scholar] [CrossRef]
- Kremenevski, N.; Schlaffer, S.M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef]
- Bohman, L.E.; Koch, M.; Bailey, R.L.; Alonso-Basanta, M.; Lee, J.Y. Skull base chordoma and chondrosarcoma: Influence of clinical and demographic factors on prognosis; a SEER analysis. World Neurosurg. 2014, 85, 806–814. [Google Scholar] [CrossRef]
- Presneau, N.; Shalaby, A.; Ye, H.; Pillay, N.; Halai, D.; Idowu, B.; Tirabosco, R.; Whitwell, D.; Jacques, T.S.; Kindblom, L.G.; et al. Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: A genetic and functional-based study. J. Pathol. 2011, 223, 327–335. [Google Scholar] [CrossRef]
- Elhakeem, A.A.S.; Essa, A.A.; Soliman, R.K. Chondroma of the falx cerebri: A case report and review of literature. Neuropathology 2019, 39, 461–466. [Google Scholar] [CrossRef]
- Pathmanapan, S.; Poon, R.; De Renshaw, T.B.; Nadesan, P.; Nakagawa, M.; Seesankar, G.A.; Ho Loe, A.K.; Zhang, H.H.; Guinovart, J.J.; Duran, J.; et al. Mutant IDH regulates glycogen metabolism from early cartilage development to malignant chondrosarcoma formation. Cell Rep. 2023, 42, 112578. [Google Scholar] [CrossRef]
- Zhu, G.G.; Nafa, K.; Agaram, N.; Zehir, A.; Benayed, R.; Sadowska, J.; Borsu, L.; Kelly, C.; Tap, W.D.; Fabbri, N.; et al. Genomic Profiling Identifies Association of IDH1/IDH2 Mutation with Longer Relapse-Free and Metastasis-Free Survival in High-Grade Chondrosarcoma. Clin. Cancer Res. 2020, 26, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Cleven, A.H.G.; Suijker, J.; Agrogiannis, G.; Briaire-de Bruijn, I.H.; Frizzell, N.; Hoekstra, A.S.; Wijers-Koster, P.M.; Cleton-Jansen, A.M.; Bovée, J.V.M.G. IDH1 or -2 mutations do not predict outcome and do not cause loss of 5-hydroxymethylcytosine or altered histone modifications in central chondrosarcomas. Clin. Sarcoma Res. 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Totoki, Y.; Yoshida, A.; Hosoda, F.; Nakamura, H.; Hama, N.; Ogura, K.; Yoshida, A.; Fujiwara, T.; Arai, Y.; Toguchida, J.; et al. Unique Mutation Portraits and Frequent COL2A1 Gene Alteration in Chondrosarcoma. Genome Res. 2014, 24, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Yang, C.; Seger, N.; Hesla, A.C.; Tsagkozis, P.; Larsson, O.; Lin, Y.; Haglund, F. TERT Promoter Mutation Is an Objective Clinical Marker for Disease Progression in Chondrosarcoma. Mod. Pathol. 2021, 34, 2020–2027. [Google Scholar] [CrossRef]
- Venneker, S.; Kruisselbrink, A.B.; Baranski, Z.; Palubeckaite, I.; Briaire-de Bruijn, I.H.; Oosting, J.; French, P.J.; Danen, E.H.J.; Bovée, J.V.M.G. Beyond the Influence of IDH Mutations: Exploring Epigenetic Vulnerabilities in Chondrosarcoma. Cancers 2020, 12, 3589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).