Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Pre-Procedure Baseline Data
2.2. Ultrasound Examinations
2.3. B-Mode
2.4. M-Mode
2.5. 2D Shear Wave Elastography (SWE)
2.6. Thoracentesis
2.7. Scoring of Non-Expandable Lung (NEL)—Reference Standard
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Test Modality | Cut-Point (cm) | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| M-mode Lung movement | ||||||
| Youden index | 0.75 | 0.75 | 0.78 | 0.71 | 0.52 | 0.89 |
| Min 0.80 specificity | 1.00 | 0.70 | 0.50 | 0.86 | 0.59 | 0.81 |
| Min 0.80 sensitivity | 0.70 | 0.72 | 0.81 | 0.64 | 0.47 | 0.89 |
| Min 0.75 specificity | 1.00 | 0.70 | 0.50 | 0.86 | 0.59 | 0.81 |
| Min 0.75 sensitivity | 0.76 | 0.71 | 0.78 | 0.71 | 0.52 | 0.89 |
| M-mode Diaphragm movement | ||||||
| Youden index | 0.82 | 0.74 | 0.76 | 0.71 | 0.51 | 0.88 |
| Min 0.80 specificity | 1.39 | 0.70 | 0.54 | 0.86 | 0.61 | 0.82 |
| Min 0.80 sensitivity | 0.56 | 0.66 | 0.88 | 0.43 | 0.38 | 0.90 |
| Min 0.75 specificity | 1.04 | 0.72 | 0.68 | 0.79 | 0.56 | 0.86 |
| Min 0.75 sensitivity | 0.83 | 0.72 | 0.76 | 0.71 | 0.51 | 0.88 |
| B-mode Diaphragm movement | ||||||
| Youden index | 0.79 | 0.67 | 0.63 | 0.71 | 0.46 | 0.83 |
| Min 0.80 specificity | 1.37 | 0.67 | 0.26 | 0.86 | 0.43 | 0.74 |
| Min 0.80 sensitivity | 0.43 | 0.74 | 0.80 | 0.50 | 0.39 | 0.86 |
| Min 0.75 specificity | 1.12 | 0.70 | 0.40 | 0.79 | 0.43 | 0.77 |
| Min 0.75 sensitivity | 0.47 | 0.71 | 0.77 | 0.50 | 0.38 | 0.84 |
| Test Modality | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| Test Modality | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| M-mode | ||||||
| Lung movement | 21.96 | 2.83–170.33 | 0.003 | 7.72 | 0.84–70.90 | 0.071 |
| Diaphragm movement | 2.99 | 1.13–7.93 | 0.027 | 1.56 | 0.55–4.45 | 0.399 |
| B-mode | ||||||
| Diaphragm movement | 2.31 | 0.71–7.48 | 0.163 | |||
| Lung sliding | 9.31 | 1.78–48.72 | 0.008 | 3.46 | 0.54–22.30 | 0.191 |
| ∆ Area | 1.02 | 0.94–1.09 | 0.661 | |||
| Shear Wave Elastography | ||||||
| Parietal pleura | 1.27 | 0.61–2.62 | 0.532 | |||
| Visceral pleura | 1.13 | 0.55–2.31 | 0.734 | |||
| Pleural effusion | 0.76 | 0.35–1.65 | 0.492 | |||
| The diagnosis is either | |
| Full Expansion (A) | |
| Probable Expansive Lung (B) | |
| Definitely NEL (C) | |
| probable NEL (D) | |
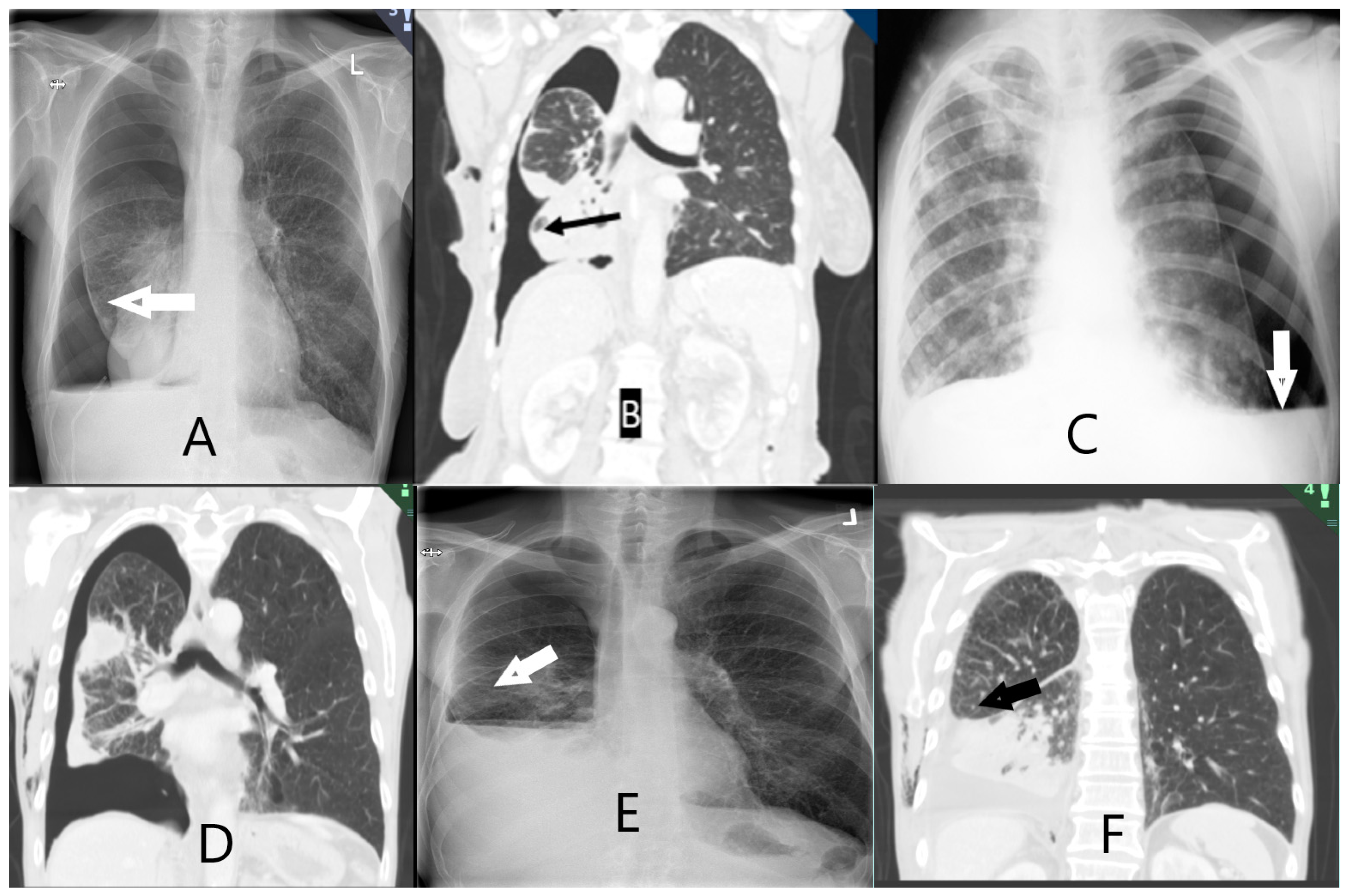
| Entrapped lung scoring on chest X-ray or chest CT scan | |
| A. Definitely free. Complete apposition of parietal and visceral pleura | |
| B. Probably free. Apposition of parietal and visceral pleura in most places but some residual pleural fluid | |
| C. Definitely entrapped. Air separating the visceral and parietal pleura around the lower lobe | |
| D. Probably entrapped. Some air between visceral and parietal pleura in places around the lower lobe but residual pleural fluid obscuring some areas | |
| E. Unable to score. Insufficient drainage of pleural fluid to allow designation in one of the prior categories. |
| Correctly | |||||
|---|---|---|---|---|---|
| Cutpoint (mm) | Sensitivity | Specificity | Classified | LR+ | LR− |
| (≥0.07) | 100.00% | 0.00% | 69.57% | 1.0000 | |
| (≥0.14) | 100.00% | 14.29% | 73.91% | 1.1667 | 0.0000 |
| (≥0.19) | 96.88% | 28.57% | 76.09% | 1.3562 | 0.1094 |
| (≥0.21) | 96.88% | 35.71% | 78.26% | 1.5069 | 0.0875 |
| (≥0.28) | 96.88% | 42.86% | 80.43% | 1.6953 | 0.0729 |
| (≥0.4) | 96.88% | 50.00% | 82.61% | 1.9375 | 0.0625 |
| (≥0.42) | 93.75% | 50.00% | 80.43% | 1.8750 | 0.1250 |
| (≥0.44) | 90.63% | 57.14% | 80.43% | 2.1146 | 0.1641 |
| (≥0.46) | 87.50% | 57.14% | 78.26% | 2.0417 | 0.2188 |
| (≥0.56) | 84.38% | 57.14% | 76.09% | 1.9687 | 0.2734 |
| (≥0.63) | 84.38% | 64.29% | 78.26% | 2.3625 | 0.2431 |
| (≥0.7) | 81.25% | 64.29% | 76.09% | 2.2750 | 0.2917 |
| (≥0.74) | 78.13% | 64.29% | 73.91% | 2.1875 | 0.3403 |
| (≥0.76) | 78.13% | 71.43% | 76.09% | 2.7344 | 0.3062 |
| (≥0.83) | 68.75% | 71.43% | 69.57% | 2.4063 | 0.4375 |
| (≥0.9) | 62.50% | 71.43% | 65.22% | 2.1875 | 0.5250 |
| (≥0.97) | 59.38% | 71.43% | 63.04% | 2.0781 | 0.5687 |
| (≥1) | 50.00% | 85.71% | 60.87% | 3.5000 | 0.5833 |
| (≥1.01) | 46.88% | 85.71% | 58.70% | 3.2813 | 0.6198 |
| (≥1.02) | 43.75% | 85.71% | 56.52% | 3.0625 | 0.6562 |
| (≥1.04) | 43.75% | 92.86% | 58.70% | 6.1250 | 0.6058 |
| (≥1.1) | 40.63% | 100.00% | 58.70% | 0.5938 | |
| (≥1.11) | 37.50% | 100.00% | 56.52% | 0.6250 | |
| (≥1.12) | 34.38% | 100.00% | 54.35% | 0.6563 | |
| (≥1.46) | 28.13% | 100.00% | 50.00% | 0.7188 | |
| (≥1.53) | 25.00% | 100.00% | 47.83% | 0.7500 | |
| (≥1.6) | 21.88% | 100.00% | 45.65% | 0.7813 | |
| (≥1.74) | 15.63% | 100.00% | 41.30% | 0.8438 | |
| (≥1.94) | 12.50% | 100.00% | 39.13% | 0.8750 | |
| (≥2.08) | 9.38% | 100.00% | 36.96% | 0.9063 | |
| (≥2.22) | 6.25% | 100.00% | 34.78% | 0.9375 | |
| (≥3.89) | 3.13% | 100.00% | 32.61% | 0.9688 | |
| (>3.89) | 0.00% | 100.00% | 30.43% | 1.0000 |
References
- Huggins, J.T.; Maldonado, F.; Chopra, A.; Rahman, N.; Light, R. Unexpandable lung from pleural disease. Respirology 2018, 23, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D. Therapeutic thoracentesis: The role of ultrasound and pleural manometry. Curr. Opin. Pulm. Med. 2007, 13, 312–318. [Google Scholar] [PubMed]
- Pereyra, M.F.; Ferreiro, L.; Valdés, L. Unexpandable lung. Arch. Bronconeumol. 2013, 49, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Rusch, V.W.; Strange, C.; Ginsberg, R.J.; Sahn, S.A. Pleurodesis using talc slurry. Chest 1994, 106, 342–346. [Google Scholar] [PubMed]
- Roberts, M.E.; Neville, E.; Berrisford, R.G.; Antunes, G.; Ali, N.J.; on behalf of the BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. 2), ii32–ii40. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Mercer, R.M.; Rahman, N.M. Thoracic ultrasound in the modern management of pleural disease. Eur. Respir. Rev. 2020, 29, 190136. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.S.; Lo, S.K.; Chuang, M.L.; Yang, C.T.; Tsao, T.C.Y.; Lee, C.H. Elastance of the pleural space: A predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann. Intern. Med. 1997, 126, 768–774. [Google Scholar] [CrossRef]
- Muruganandan, S.; Azzopardi, M.; Fitzgerald, D.B.; Shrestha, R.; Kwan, B.C.H.; Lam, D.C.L.; De Chaneet, C.C.; Ali, M.R.S.R.; Yap, E.; Tobin, C.L.; et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): An open-label randomised trial. Lancet Respir. Med. 2018, 6, 671–680. [Google Scholar] [CrossRef]
- Davies, H.E.; Mishra, E.K.; Kahan, B.C.; Wrightson, J.M.; Stanton, A.E.; Guhan, A.; Davies, C.; Grayez, J.; Harrison, R.; Prasad, A.; et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA J. Am. Med. Assoc. 2012, 307, 2383–2389. [Google Scholar] [CrossRef]
- Halford, P.J.; Bhatnagar, R.; White, P.; Haris, M.; Harrison, R.N.; Holme, J.; Sivasothy, P.; West, A.; Bishop, L.J.; Stanton, A.E.; et al. Manometry performed at indwelling pleural catheter insertion to predict unexpandable lung. J. Thorac. Dis. 2020, 12, 1374–1384. [Google Scholar] [CrossRef]
- Heidecker, J.; Huggins, J.T.; Sahn, S.A.; Doelken, P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006, 130, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D.; Walkey, A.; Berkowitz, D.; Ernst, A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest 2006, 129, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D.J.; Reddy, C.B.; DeCamp, M.M.; Diekemper, R.L.; Gould, M.K.; Henry, T.; Iyer, N.P.; Lee, Y.C.G.; Lewis, S.Z.; Maskell, N.A.; et al. Management of malignant pleural effusions: An official ATS/STS/STR clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 839–849. [Google Scholar]
- Thomas, R.; Fysh, E.T.H.; Smith, N.A.; Lee, P.; Kwan, B.C.H.; Yap, E.; Horwood, F.; Piccolo, F.; Lam, D.; Garske, L.; et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: The AMPLE randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Lester, M.; Maldonado, F.; Rickman, O.B.; Roller, L.J.; Avasarala, S.K.; Katsis, J.M.; Lentz, R.J. Association between terminal pleural elastance and radiographic lung re-expansion after therapeutic thoracentesis in patients with symptomatic pleural effusion: A post-hoc analysis of a randomised trial. BMJ Open 2022, 12, e053606. [Google Scholar] [CrossRef]
- Chopra, A.; Judson, M.A.; Doelken, P.; Maldonado, F.; Rahman, N.M.; Huggins, J.T. The Relationship of Pleural Manometry with Postthoracentesis Chest Radiographic Findings in Malignant Pleural Effusion. Chest 2020, 157, 421–426. [Google Scholar]
- Havelock, T.; Teoh, R.; Laws, D.; Gleeson, F. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. 2), ii61–ii76. [Google Scholar] [CrossRef]
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.; et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. J. Cardio-Thoracic. Surg. 2019, 55, 116–132. [Google Scholar] [CrossRef]
- Flora, B.; Ahmad, S. Ultrasound as a noninvasive tool to diagnose trapped lung. Am. J. Respir. Crit. Care Med. 2017, 195, A6518. [Google Scholar]
- Salamonsen, M.R.; Lo, A.K.C.; Ng, A.C.T.; Bashirzadeh, F.; Wang, W.Y.S.; Fielding, D.I.K. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest 2014, 146, 1286–1293. [Google Scholar] [CrossRef]
- Aguilera Garcia, Y.; Palkar, A.; Koenig, S.J.; Narasimhan, M.; Mayo, P.H. Assessment of Diaphragm Function and Pleural Pressures During Thoracentesis. Chest 2020, 157, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Soldati, G.; Giannuzzi, R.; Berardi, S.; Portale, G.; Gentiloni Silveri, N. Ultrasound M-Mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med. Biol. 2011, 37, 44–52. [Google Scholar] [PubMed]
- Boon, A.J.; Harper, C.J.; Ghahfarokhi, L.S.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar]
- Skaarup, S.H.; Løkke, A.; Laursen, C.B. The Area method: A new method for ultrasound assessment of diaphragmatic movement. Crit. Ultrasound J. 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Wen, X.; Yu, X.; Tian, Y.; Liu, Z.; Cheng, W.; Li, H.; Kang, J.; Wei, T.; Yuan, S.; Tian, J. Quantitative shear wave elastography in primary invasive breast cancers, based on collagen-S100A4 pathology, indicates axillary lymph node metastasis. Quant. Imaging Med. Surg. 2020, 10, 624–633. [Google Scholar] [CrossRef]
- Ozgokce, M.; Yavuz, A.; Akbudak, I.; Durmaz, F.; Uney, I.; Aydin, Y.; Yildiz, H.; Batur, A.; Arslan, H.; Dundar, I. Usability of Transthoracic Shear Wave Elastography in Differentiation of Subpleural Solid Masses. Ultrasound Q. 2018, 34, 233–237. [Google Scholar] [CrossRef]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018, 7, e1438111. [Google Scholar] [CrossRef]
- Jiang, B.; Li, X.-L.; Yin, Y.; Zhang, Q.; Zang, T.; Song, W.-S.; Wang, X.-M.; Kang, J.; Herth, F.J.; Hou, G. Ultrasound elastography: A novel tool for the differential diagnosis of pleural effusion. Eur. Respir. J. 2019, 54, 1802018. [Google Scholar] [CrossRef]
- Porcel, J.M. Ultrasound-based elastography: “hard” to implement in the pleural effusion work-up? Eur. Respir. J. 2019, 54, 1901587. [Google Scholar] [CrossRef]
- Kollmann, C.; Jenderka, K.V.; Moran, C.M.; Draghi, F.; Jimenez Diaz, J.F.; Sande, R. EFSUMB clinical safety statement for diagnostic ultrasound—(2019 revision). Ultraschall der Medizin 2020, 41, 387–389. [Google Scholar]
- Gogtay, N.J.; Thatte, U.M. Statistical evaluation of diagnostic tests—Part 2 Pre-test and post-test probability and odds, likelihood ratios, receiver operating characteristic curve, youden’s index and diagnostic test biases. J. Assoc. Physicians India 2017, 65, 86–91. [Google Scholar]
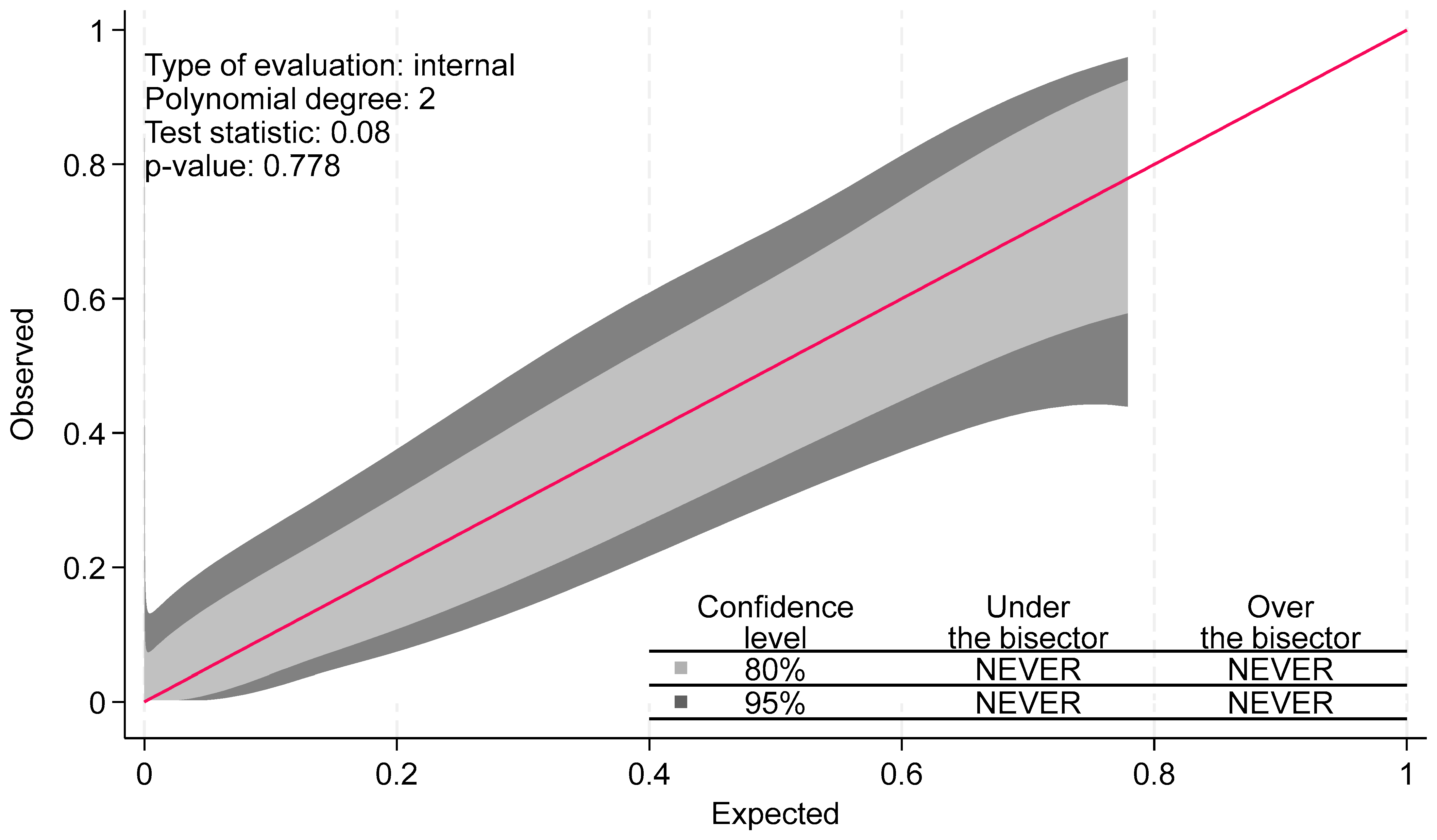
- Nattino, G.; Finazzi, S.; Bertolini, G. A new calibration test and a reappraisal of the calibration belt for the assessment of prediction models based on dichotomous outcomes. Stat. Med. 2014, 33, 2390–2407. [Google Scholar] [PubMed]
- Nattino, G.; Lemeshow, S.; Phillips, G.; Finazzi, S.; Bertolini, G. Assessing the calibration of dichotomous outcome models with the calibration belt. Stata J. 2017, 17, 1003–1014. [Google Scholar] [CrossRef]
- Dresler, C.M.; Olak, J.; Herndon, J.E.; Richards, W.G.; Scalzetti, E.; Fleishman, S.B.; Kernstine, K.H.; Demmy, T.; Jablons, D.M.; Kohman, L.; et al. Phase III Intergroup Study of Talc Poudrage vs Talc Slurry Sclerosis for Malignant Pleural Effusion. Chest 2005, 127, 909–915. [Google Scholar] [PubMed]
- Psallidas, I.; Kalomenidis, I.; Porcel, J.M.; Robinson, B.W.; Stathopoulos, G.T. Malignant pleural effusion: From bench to bedside. Eur. Respir. Rev. 2016, 25, 189–198. [Google Scholar] [CrossRef]
- Mummadi, S.R.; Stoller, J.K.; Lopez, R.; Kailasam, K.; Gillespie, C.T.; Hahn, P.Y. Epidemiology of Adult Pleural Disease in the United States. Chest 2021, 160, 1534–1551. [Google Scholar] [CrossRef]
- Walker, S.; Bibby, A.C.; Maskell, N.A. Current best practice in the evaluation and management of malignant pleural effusions. Ther. Adv. Respir. Dis. 2017, 11, 105–114. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Corcoran, J.P.; Maldonado, F.; Feller-Kopman, D.; Janssen, J.; Astoul, P.; Rahman, N.M. Advanced medical interventions in pleural disease. Eur. Respir. Rev. 2016, 25, 199–213. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar]
- Huggins, J.T.; Sahn, S.A.; Heidecker, J.; Ravenel, J.G.; Doelken, P. Characteristics of trapped lung: Pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007, 131, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Doelken, P. Pleural Manometry. In Principles and Practice of Interventional Pulmonology [Internet]; Springer: New York, NY, USA, 2013; pp. 571–575. [Google Scholar] [CrossRef]
- Lentz, R.J.; Lerner, A.D.; Pannu, J.K.; Merrick, C.M.; Roller, L.; Walston, C.; Valenti, S.; Goddard, T.; Chen, H.; Huggins, J.T.; et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: A multicentre, single-blind randomised controlled trial. Lancet Respir. Med. 2019, 7, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Doelken, P.; Hu, K.; Huggins, J.T.; Judson, M.A. Pressure-Dependent Pneumothorax and Air Leak: Physiology and Clinical Implications. Chest 2023, 164, 796–805. [Google Scholar] [CrossRef] [PubMed]


| Variable | Expandable Lung | Non-Expandable Lung | p-Value |
|---|---|---|---|
| n = 35 | n = 14 | ||
| Age | 71 (63–76) | 76 (68–80) | 0.071 # |
| Sex (female) | 18 (51%) | 6 (43%) | 0.59 ¤ |
| Smoking status | 0.40 ¤ | ||
| Current/Former | 8 (23%)/19 (54%) | 3 (21%)/10 (71%) | |
| Never | 8 (23%) | 1 (7%) | |
| Accumulated smoking pack years | 34 (15–43) | 28 (18–40) | 0.81 # |
| Known malignancy | 31 (89%) | 13 (93%) | 0.65 ¤ |
| Pleural fluid volume drained (mL) | 1290 (604) | 888 (620) | 0.049 ∩ |
| Test Modality | Median (IQR) | AUC | 95% CI |
|---|---|---|---|
| M-mode | |||
| Lung movement (cm) | 0.94 (0.44–1.11) | 0.81 | 0.68–0.95 |
| Diaphragm movement (cm) | 1.21 (0.56–2.01) | 0.77 | 0.61–0.93 |
| B-mode | |||
| Diaphragm movement (cm) | 0.86 (0.41–1.27) | 0.65 | 0.46–0.84 |
| Area method—delta (cm2) | 11.50 (6.69–15.95) | 0.60 | 0.40–0.79 |
| Lung sliding * | 24 (50%) | 0.74 | 0.61–0.87 |
| Shear Wave Elastography | |||
| Visceral pleura (m/s) | 1.64 (1.47–2.08) | 0.59 | 0.40–0.79 |
| Pleural effusion (m/s) | 1.64 (1.34–2.06) | 0.53 | 0.34–0.71 |
| Parietal pleura (m/s) | 2.54 (2.03–3.09) | 0.57 | 0.39–0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, J.K.; Fjaellegaard, K.; Rasmussen, D.B.; Alstrup, G.; Høegholm, A.; Sidhu, J.S.; Sivapalan, P.; Gerke, O.; Bhatnagar, R.; Clementsen, P.F.; et al. Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography. Diagnostics 2024, 14, 204. https://doi.org/10.3390/diagnostics14020204
Petersen JK, Fjaellegaard K, Rasmussen DB, Alstrup G, Høegholm A, Sidhu JS, Sivapalan P, Gerke O, Bhatnagar R, Clementsen PF, et al. Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography. Diagnostics. 2024; 14(2):204. https://doi.org/10.3390/diagnostics14020204
Chicago/Turabian StylePetersen, Jesper Koefod, Katrine Fjaellegaard, Daniel B. Rasmussen, Gitte Alstrup, Asbjørn Høegholm, Jatinder Singh Sidhu, Pradeesh Sivapalan, Oke Gerke, Rahul Bhatnagar, Paul Frost Clementsen, and et al. 2024. "Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography" Diagnostics 14, no. 2: 204. https://doi.org/10.3390/diagnostics14020204
APA StylePetersen, J. K., Fjaellegaard, K., Rasmussen, D. B., Alstrup, G., Høegholm, A., Sidhu, J. S., Sivapalan, P., Gerke, O., Bhatnagar, R., Clementsen, P. F., Laursen, C. B., & Bodtger, U. (2024). Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography. Diagnostics, 14(2), 204. https://doi.org/10.3390/diagnostics14020204









