The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Mitral Regurgitation
Abstract
:1. Introduction
2. How to Assess Mitral Regurgitation with CMR
3. Determination of the Severity of MR Using CMR Parameters
4. Application of CMR in Primary and Secondary Mitral Regurgitation
5. Limitations of CMR in the Assessment of Patients with MR
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- El Sabbagh, A.; Reddy, Y.N.; Nishimura, R.A. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC Cardiovasc. Imaging 2018, 11, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Suinesiaputra, A.; Bluemke, D.A.; Cowan, B.R.; Friedrich, M.G.; Kramer, C.M.; Kwong, R.; Plein, S.; Schulz-Menger, J.; Westenberg, J.J.M.; Young, A.A.; et al. Quantification of LV function and mass by cardiovascular magnetic resonance: Multi-center variability and consensus contours. J. Cardiovasc. Magn. Reson. 2015, 17, 63. [Google Scholar] [CrossRef]
- Uretsky, S.; Argulian, E.; Narula, J.; Wolff, S.D. Use of Cardiac Magnetic Resonance Imaging in Assessing Mitral Regurgitation: Current Evidence. J. Am. Coll. Cardiol. 2018, 71, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. College Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Lopez-Mattei, J.C.; Shah, D.J. The Role of Cardiac Magnetic Resonance in Valvular Heart Disease. Methodist DeBakey Cardiovasc. J. 2013, 9, 142–148. [Google Scholar] [CrossRef]
- Garg, P.; Swift, A.J.; Zhong, L.; Carlhäll, C.-J.; Ebbers, T.; Westenberg, J.; Hope, M.D.; Bucciarelli-Ducci, C.; Bax, J.J.; Myerson, S.G. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat. Rev. Cardiol. 2020, 17, 298–312. [Google Scholar] [CrossRef]
- Han, Y.; Peters, D.C.; Salton, C.J.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular Magnetic Resonance Characterization of Mitral Valve Prolapse. JACC Cardiovasc. Imaging 2008, 1, 294–303. [Google Scholar] [CrossRef]
- Sturla, F.; Onorati, F.; Puppini, G.; Pappalardo, O.A.; Selmi, M.; Votta, E.; Faggian, G.; Redaelli, A. Dynamic and quantitative evaluation of degenerative mitral valve disease: A dedicated framework based on cardiac magnetic resonance imaging. J. Thorac. Dis. 2017, 9, S225–S238. [Google Scholar] [CrossRef]
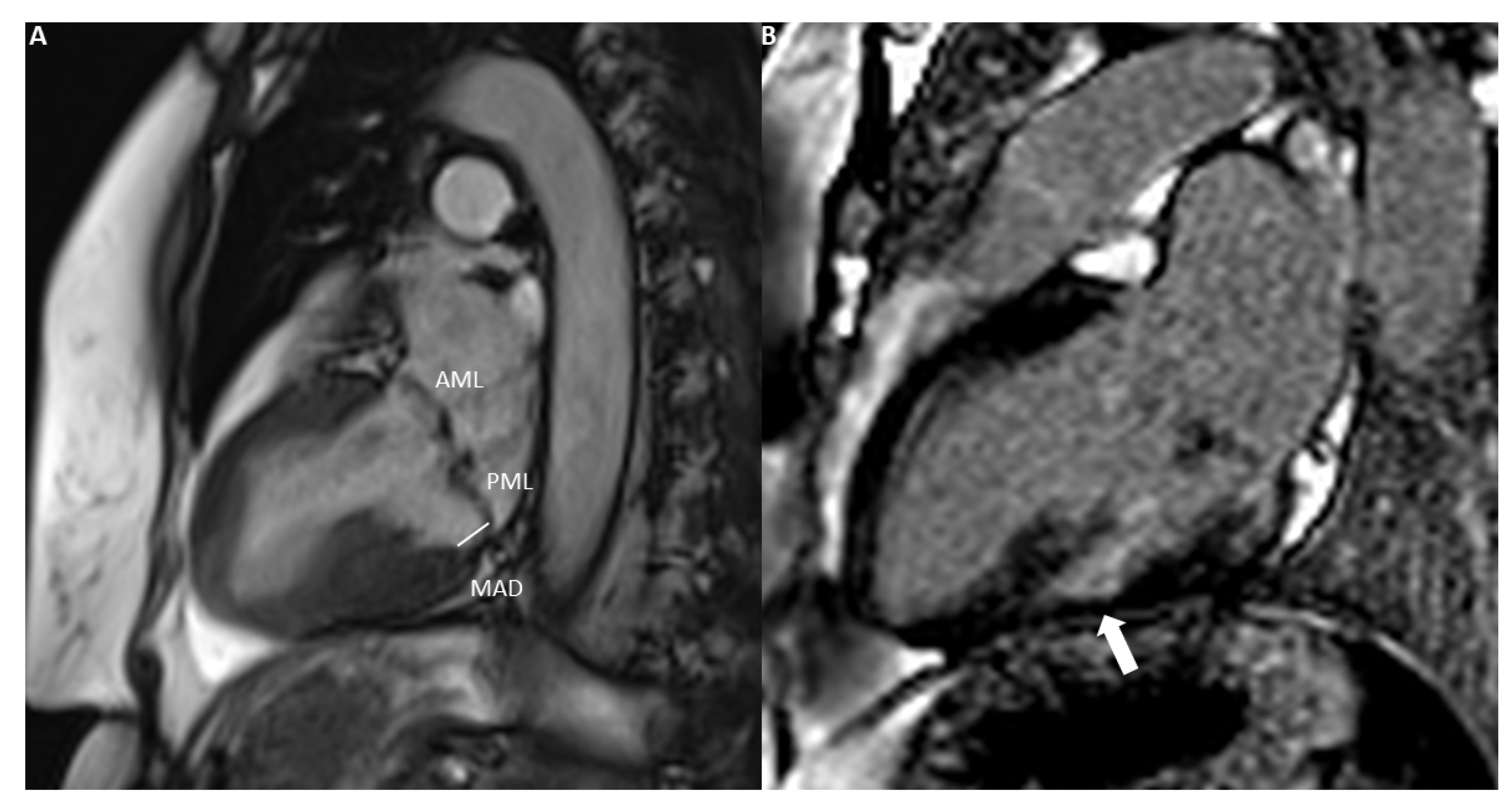
- Marra, M.P.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Essayagh, B.; Iacuzio, L.; Civaia, F.; Avierinos, J.-F.; Tribouilloy, C.; Levy, F. Usefulness of 3-Tesla Cardiac Magnetic Resonance to Detect Mitral Annular Disjunction in Patients With Mitral Valve Prolapse. Am. J. Cardiol. 2019, 124, 1725–1730. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ali, S.G.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Vermes, E.; Iacuzio, L.; Levy, F.; Bohbot, Y.; Renard, C.; Gerber, B.; Maréchaux, S.; Tribouilloy, C. Role of Cardiovascular Magnetic Resonance in Native Valvular Regurgitation: A Comprehensive Review of Protocols, Grading of Severity, and Prediction of Valve Surgery. Front. Cardiovasc. Med. 2022, 9, 881141. [Google Scholar] [CrossRef] [PubMed]
- Polte, C.L.; Bech-Hanssen, O.; Johnsson, A.; Gao, S.A.; Lagerstrand, K.M. Mitral regurgitation quantification by cardiovascular magnetic resonance: A comparison of indirect quantification methods. Int. J. Cardiovasc. Imaging 2015, 31, 1223–1231. [Google Scholar] [CrossRef]
- Hundley, W.G.; Li, H.F.; Hillis, L.D.; Meshack, B.M.; Lange, R.A.; Willard, J.E.; Landau, C.; Peshock, R.M. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am. J. Cardiol. 1995, 75, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Kon, M.W.; Myerson, S.G.; Moat, N.E.; Pennell, D.J. Quantification of regurgitant fraction in mitral regurgitation by cardio-vascular magnetic resonance: Comparison of techniques. J. Heart Valve Dis. 2004, 13, 600–607. [Google Scholar]
- Gatehouse, P.D.; Rolf, M.P.; Graves, M.J.; Hofman, M.B.; Totman, J.; Werner, B.; Quest, R.A.; Liu, Y.; von Spiczak, J.; Dieringer, M.; et al. Flow measurement by cardiovascular magnetic resonance: A multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J. Cardiovasc. Magn. Reson. 2010, 12, 5. [Google Scholar] [CrossRef]
- Kilner, P.J.; Gatehouse, P.D.; Firmin, D.N. Flow measurement by magnetic resonance: A unique asset worth optimising. J. Cardiovasc. Magn. Reson. 2007, 9, 723–728. [Google Scholar] [CrossRef]
- Myerson, S.G.; Francis, J.M.; Neubauer, S. Direct and indirect quantification of mitral regurgitation with cardiovascular magnetic resonance, and the effect of heart rate variability. Magn. Reson. Mater. Phys. Biol. Med. 2010, 23, 243–249. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.-J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reson. 2015, 17, 72. [Google Scholar] [CrossRef]
- Fidock, B.; Archer, G.; Barker, N.; Elhawaz, A.; Al-Mohammad, A.; Rothman, A.; Hose, R.; Hall, I.R.; Grech, E.; Briffa, N.; et al. Standard and emerging CMR methods for mitral regurgitation quantification. Int. J. Cardiol. 2021, 331, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gupta, A.N.; Ma, L.E.; Scott, M.B.; Mason, O.R.; Wu, E.; Thomas, J.D.; Markl, M. Valvular regurgitation flow jet assessment using in vitro 4D flow MRI: Implication for mitral regurgitation. Magn. Reson. Med. 2022, 87, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Feneis, J.F.; Kyubwa, E.; Atianzar, K.; Cheng, J.Y.; Alley, M.T.; Vasanawala, S.S.; Demaria, A.N.; Hsiao, A. 4D flow MRI quantification of mitral and tricuspid regurgitation: Reproducibility and consistency relative to conventional MRI. J. Magn. Reson. Imaging 2018, 48, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Westenberg, J.J.; Bsc, P.J.V.D.B.; Swoboda, P.P.; Aziz, R.; Foley, J.R.; Fent, G.J.; Tyl, F.; Coratella, L.; ElBaz, M.S.; et al. Comparison of fast acquisition strategies in whole-heart four-dimensional flow cardiac MR: Two-center, 1.5 Tesla, phantom and in vivo validation study. J. Magn. Reson. Imaging 2018, 47, 272–281. [Google Scholar] [CrossRef]
- Ma, L.E.; Yerly, J.; Piccini, D.; Di Sopra, L.; Roy, C.W.; Carr, J.C.; Rigsby, C.K.; Kim, D.; Stuber, M.; Markl, M. 5d flow mri: A fully self-gated, free-running framework for cardiac and respiratory motion–resolved 3d hemodynamics. Radiol. Cardiothorac. Imaging 2020, 2, e200219. [Google Scholar] [CrossRef]
- Blanken, C.P.S.; Westenberg, J.J.M.; Aben, J.-P.; Bijvoet, G.P.; Chamuleau, S.A.J.; Boekholdt, S.M.; Nederveen, A.J.; Leiner, T.; Van Ooij, P.; Planken, R.N. Quantification of mitral valve regurgitation from 4D flow MRI using semiautomated flow tracking. Radiol. Cardiothorac. Imaging 2020, 2, e200004. [Google Scholar] [CrossRef]
- Safarkhanlo, Y.; Jung, B.; Bernhard, B.; Peper, E.S.; Kwong, R.Y.; Bastiaansen, J.A.M.; Gräni, C. Mitral valve regurgitation assessed by intraventricular CMR 4D-flow: A systematic review on the technological aspects and potential clinical applications. Int. J. Cardiovasc. Imaging 2023, 39, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Botis, I.; Efstathiadou, A.; Papanastasiou, C.A.; Kokkinidis, D.G.; Zegkos, T.; Efthimiadis, G.; Kamperidis, V.; Khalique, O.K.; Kampaktsis, P.N.; Karamitsos, T.D. Evaluation of mitral regurgitation by cardiac magnetic resonance and transthoracic echocardiography: A systematic review and meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1513–1521. [Google Scholar] [CrossRef]
- Heitner, J.; Bhumireddy, G.P.; Crowley, A.L.; Weinsaft, J.; Haq, S.A.; Klem, I.; Kim, R.J.; Jollis, J.G. Clinical application of cine-MRI in the visual assessment of mitral regurgitation compared to echocardiography and cardiac catheterization. PLoS ONE 2012, 7, e40491. [Google Scholar] [CrossRef] [PubMed]
- Penicka, M.; Vecera, J.; Mirica, D.C.; Kotrc, M.; Kockova, R.; Van Camp, G. Prognostic Implications of Magnetic Resonance–Derived Quantification in Asymptomatic Patients With Organic Mitral Regurgitation: Comparison with Doppler Echocardiography-Derived Integrative Approach. Circulation 2018, 137, 1349–1360. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kang, J.-W.; Yang, D.H.; Lee, S.; Sun, B.J.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Song, J.-K. Impact of a Geometric Correction for Proximal Flow Constraint on the Assessment of Mitral Regurgitation Severity Using the Proximal Flow Convergence Method. J. Cardiovasc. Ultrasound 2018, 26, 33–39. [Google Scholar] [CrossRef]
- Uretsky, S.; Argulian, E.; Marcoff, L.; Koulogiannis, K.; Supariwala, A.; Rosenthal, M.; Brown, M.J.; Jagarlamudi, A.; Chaudhry, F.; Awan, H.; et al. A Comparative Assessment of Echocardiographic Parameters for Determining Mitral Re-gurgitation Severity. Circulation 2015, 132 (Suppl. S3), A12680. [Google Scholar] [CrossRef]
- Gelfand, E.V.; Hughes, S.; Hauser, T.H.; Yeon, S.B.; Goepfert, L.; Kissinger, K.V.; Rofsky, N.M.; Manning, W.J. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: Optimizing correlation with Doppler echocardiography. J. Cardiovasc. Magn. Reson. 2006, 8, 503–507. [Google Scholar] [CrossRef]
- Le Goffic, C.; Toledano, M.; Ennezat, P.-V.; Binda, C.; Castel, A.-L.; Delelis, F.; Graux, P.; Tribouilloy, C.; Maréchaux, S. Quantitative Evaluation of Mitral Regurgitation Secondary to Mitral Valve Prolapse by Magnetic Resonance Imaging and Echocardiography. Am. J. Cardiol. 2015, 116, 1405–1410. [Google Scholar] [CrossRef]
- Uretsky, S.; Animashaun, I.B.; Sakul, S.; Aldaia, L.; Marcoff, L.; Koulogiannis, K.; Argulian, E.; Rosenthal, M.; Wolff, S.D.; Gillam, L.D. American Society of Echocardiography Algorithm for Degenerative Mitral Regurgitation: Comparison With CMR. JACC Cardiovasc. Imaging 2022, 15, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Uretsky, S.; Morales, D.C.V.; Aldaia, L.; Mediratta, A.; Koulogiannis, K.; Marcoff, L.; Sakul, S.; Wolff, S.D.; Gillam, L.D. Characterization of Primary Mitral Regurgitation With Flail Leaflet and/or Wall-Impinging Flow. J. Am. Coll. Cardiol. 2021, 78, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Capron, T.; Cautela, J.; Scemama, U.; Miola, C.; Bartoli, A.; Theron, A.; Pinto, J.; Porto, A.; Collart, F.; Lepidi, H.; et al. Cardiac magnetic resonance assessment of left ventricular dilatation in chronic severe left-sided regurgitations: Comparison with standard echocardiography. Diagn. Interv. Imaging 2020, 101, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Kusunose, K.; Obuchowski, N.A.; Jellis, C.; Griffin, B.P.; Flamm, S.D.; Kwon, D.H. Prognostic Impact of Ischemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients With Primary Mitral Regurgitation With and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Polte, C.L.; Gao, S.A.; Johnsson, Å.A.; Lagerstrand, K.M.; Bech-Hanssen, O. Characterization of Chronic Aortic and Mitral Regurgitation Undergoing Valve Surgery Using Cardiovascular Magnetic Resonance. Am. J. Cardiol. 2017, 119, 2061–2068. [Google Scholar] [CrossRef]
- Aplin, M.; Kyhl, K.; Bjerre, J.; Ihlemann, N.; Greenwood, J.P.; Plein, S.; Uddin, A.; Tønder, N.; Høst, N.B.; Ahlström, M.G.; et al. Cardiac remodelling and function with primary mitral valve insufficiency studied by magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 863–870. [Google Scholar] [CrossRef]
- Myerson, S.G.; d’Arcy, J.; Christiansen, J.P.; Dobson, L.E.; Mohiaddin, R.; Francis, J.M.; Prendergast, B.; Greenwood, J.P.; Karamitsos, T.D.; Neubauer, S. Determination of Clinical Outcome in Mitral Regurgitation With Cardiovascular Magnetic Resonance Quantification. Circulation 2016, 133, 2287–2296. [Google Scholar] [CrossRef]
- Uretsky, S.; Gillam, L.; Lang, R.; Chaudhry, F.A.; Argulian, E.; Supariwala, A.; Gurram, S.; Jain, K.; Subero, M.; Jang, J.J.; et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: A prospective multicenter trial. J. Am. Coll. Cardiol. 2015, 65, 1078–1088. [Google Scholar] [CrossRef]
- Flynn, M.; Curtin, R.; Nowicki, E.R.; Rajeswaran, J.; Flamm, S.D.; Blackstone, E.H.; Mihaljevic, T. Regional wall motion abnormalities and scarring in severe functional ischemic mitral regurgitation: A pilot cardiovascular magnetic resonance imaging study. J. Thorac. Cardiovasc. Surg. 2009, 137, 1063–1070.e2. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Yang, E.Y.; El Tallawi, K.C.; Nagueh, S.F.; Nabi, F.; Khan, M.A.; Nguyen, D.T.; Graviss, E.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Prognostic Implications of Diffuse Interstitial Fibrosis in Asymptomatic Primary Mitral Regurgitation. Circulation 2019, 140, 2122–2124. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.K.; Kim, J.; Siden, J.Y.; Rodriguez-Diego, S.; Alakbarli, J.; Di Franco, A.; Weinsaft, J.W. Utility of cardiac magnetic resonance for evaluation of mitral regurgitation prior to mitral valve surgery. J. Thorac. Dis. 2017, 9, S246–S256. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Michelena, H.; Bichara, V.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Mitral valve prolapse with mid-late systolic mitral regurgitation: Pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation 2012, 125, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Pavon, A.G.; Guglielmo, M.; Mennilli, P.M.; Falcão, M.B.L.; Bergamaschi, L.; Costantin, D.F.; Vivaldo, M.; Leo, L.A.; Schlossbauer, S.; Roy, C.W.; et al. The Role of Cardiovascular Magnetic Resonance in Patients with Mitral Regurgitation. J. Cardiovasc. Dev. Dis. 2022, 9, 399. [Google Scholar] [CrossRef]
- Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features. Diagnostics 2021, 11, 683. [Google Scholar] [CrossRef]
- Basso, C.; Marra, M.P.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Beaufils, A.-L.C.D.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.-M.; et al. Replacement Myocardial Fibrosis in Patients With Mitral Valve Prolapse. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef]
- Auricchio, A.; Prinzen, F.W. Non-responders to cardiac resynchronization therapy: The magnitude of the problem and the issues. Circ. J. 2011, 75, 521–527. [Google Scholar] [CrossRef]
- Leyva, F.; Foley, P.W.; Chalil, S.; Ratib, K.; Smith, R.E.; Prinzen, F.; Auricchio, A. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2011, 13, 29. [Google Scholar] [CrossRef]
- van der Bijl, P.; Khidir, M.; Marsan, N.A.; Delgado, V.; Leon, M.B.; Stone, G.W.; Bax, J.J. Effect of Functional Mitral Regurgitation on Outcome in Patients Receiving Cardiac Resynchronization Therapy for Heart Failure. Am. J. Cardiol. 2019, 123, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef]
- Podlesnikar, T.; Delgado, V.; Bax, J.J. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int. J. Cardiovasc. Imaging 2018, 34, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Ray, S.G.; Yonan, N.; Williams, S.G.; Flett, A.S.; et al. Schmitt, Comprehensive validation of cardiovascular magnetic resonance techniques for the as-sessment of myocardial extracellular volume. Circ. Cardiovasc. Imaging 2013, 6, 373–383. [Google Scholar] [CrossRef]
- Badau, C.I.R.; Coldea, L.A. The value of T1 mapping in patients with chronic mitral regurgitation. Eur. Heart J. 2022, 43, ehac544.238. [Google Scholar] [CrossRef]
- Agbor-Etang, B.B.; Lim, L.J.; Ordovas, K.G.; Delling, F.N. Evidence of Abnormal T1 Mapping in Arrhythmic Mitral Valve Prolapse Without Significant Mitral Regurgitation. Circulation 2020, 142 (Suppl. S3), A16798. [Google Scholar] [CrossRef]
- Hor, K.N.; Baumann, R.; Pedrizzetti, G.; Tonti, G.; Gottliebson, W.M.; Taylor, M.; Benson, W.; Mazur, W. Magnetic Resonance Derived Myocardial Strain Assessment Using Feature Tracking. J. Vis. Exp. 2011, 48, e2356. [Google Scholar] [CrossRef]
- Zerhouni, E.A.; Parish, D.M.; Rogers, W.J.; Yang, A.; Shapiro, E.P. Human heart: Tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology 1988, 169, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Hor, K.N.; Kowallick, J.T.; Beerbaum, P.; Kutty, S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking. Circ. Cardiovasc. Imaging 2016, 9, e004077. [Google Scholar] [CrossRef]
- Guglielmo, M.; Fusini, L.; Muscogiuri, G.; Baessato, F.; Loffreno, A.; Cavaliere, A.; Rizzon, G.; Baggiano, A.; Rabbat, M.G.; Muratori, M.; et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur. Radiol. 2021, 31, 1100–1109. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European So-ciety of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Kusunose, K.; Obuchowski, N.A.; Cavalcante, J.L.; Popovic, Z.B.; Thomas, J.D.; Desai, M.Y.; Flamm, S.D.; Griffin, B.P. Predictors and Prognostic Impact of Progressive Ischemic Mitral Regurgitation in Patients With Advanced Ischemic Cardiomyopathy: A Multimodality Study. Circ. Cardiovasc. Imaging 2016, 9, e004577. [Google Scholar] [CrossRef] [PubMed]
- Kaji, S.; Nasu, M.; Yamamuro, A.; Tanabe, K.; Nagai, K.; Tani, T.; Tamita, K.; Shiratori, K.; Kinoshita, M.; Senda, M.; et al. Annular geometry in patients with chronic ischemic mitral regurgitation: Three-dimensional magnetic resonance imaging study. Circulation 2005, 112, I409–I414. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Craig, C.; Strugnell, W.; Gaikwad, N.; Ischenko, M.; Speranza, V.; Chan, J.; Neill, J.; Platts, D.; Scalia, G.M.; Burstow, D.J.; et al. Quantitation of mitral regurgitation after percutaneous MitraClip repair: Comparison of Doppler echocardiography and cardiac magnetic resonance imaging. Ann. Cardiothorac. Surg. 2015, 4, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.; Bhumireddy, G.; Dabiesingh, D.; Khan, S.; Ho, J.; Krishna, N.; Dontineni, N.; Socolow, J.; Briggs, W.; Klem, I.; et al. Importance of papillary muscle infarction detected by cardiac magnetic resonance imaging in predicting cardiovascular events. Int. J. Cardiol. 2016, 220, 558–563. [Google Scholar] [CrossRef] [PubMed]
- von Stumm, M.; Petersen, J.; Sinn, M.; Holst, T.; Sequeira-Gross, T.M.; Müller, L.; Pausch, J.; Bannas, P.; Adam, G.; Reichenspurner, H.; et al. Correlation of Myocardial Native T1 and Left Ventricular Reverse Remodeling after Valvular Surgery. J. Clin. Med. 2023, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.C.; Löffler, A.I.; Salerno, M. Role of Cardiac Magnetic Resonance Imaging in Valvular Heart Disease: Diagnosis, Assessment, and Management. Curr. Cardiol. Rep. 2018, 20, 119. [Google Scholar] [CrossRef]
- Myerson, S.G. Heart valve disease: Investigation by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012, 14, 7. [Google Scholar] [CrossRef]
- Gabriel, R.S.; Kerr, A.J.; Raffel, O.C.; Stewart, R.A.; Cowan, B.R.; Occleshaw, C.J. Mapping of mitral regurgitant defects by cardiovascular magnetic resonance in moderate or severe mitral regurgitation secondary to mitral valve prolapse. J. Cardiovasc. Magn. Reson. 2008, 10, 16. [Google Scholar] [CrossRef]
- Søgaard, S.B.; Gustavsen, P.; Dalsgaard, M.; Vejlstrup, N.G.; Madsen, P.L. Cardiac magnetic resonance imaging with standard imaging planes for mitral valve scallop pathology: Interrater agreement and comparison with echocardiography. Int. J. Cardiovasc. Imaging 2021, 37, 605–611. [Google Scholar] [CrossRef]
- Chuang, M.L.; Gona, P.; Hautvast, G.L.; Salton, C.J.; Blease, S.J.; Yeon, S.B.; Breeuwer, M.; O’Donnell, C.J.; Manning, W.J. Correlation of Trabeculae and Papillary Muscles With Clinical and Cardiac Characteristics and Impact on CMR Measures of LV Anatomy and Function. JACC Cardiovasc. Imaging 2012, 5, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Papavassiliu, T.; Kühl, H.P.; Schröder, M.; Süselbeck, T.; Bondarenko, O.; Böhm, C.K.; Beek, A.; Hofman, M.M.B.; van Rossum, A.C. Effect of Endocardial Trabeculae on Left Ventricular Measurements and Measurement Reproducibility at Cardiovascular MR Imaging. Radiology 2005, 236, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.T.; Götte, M.J.W.; Dewaal, L.K.; Stam, M.R.; Van der Geest, R.J.; Heethaar, R.M.; Van Rossum, A.C. The influence of through-plane motion on left ventricular volumes measured by magnetic resonance imaging: Implications for image acquisition and analysis. J. Cardiovasc. Magn. Reson. 1999, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
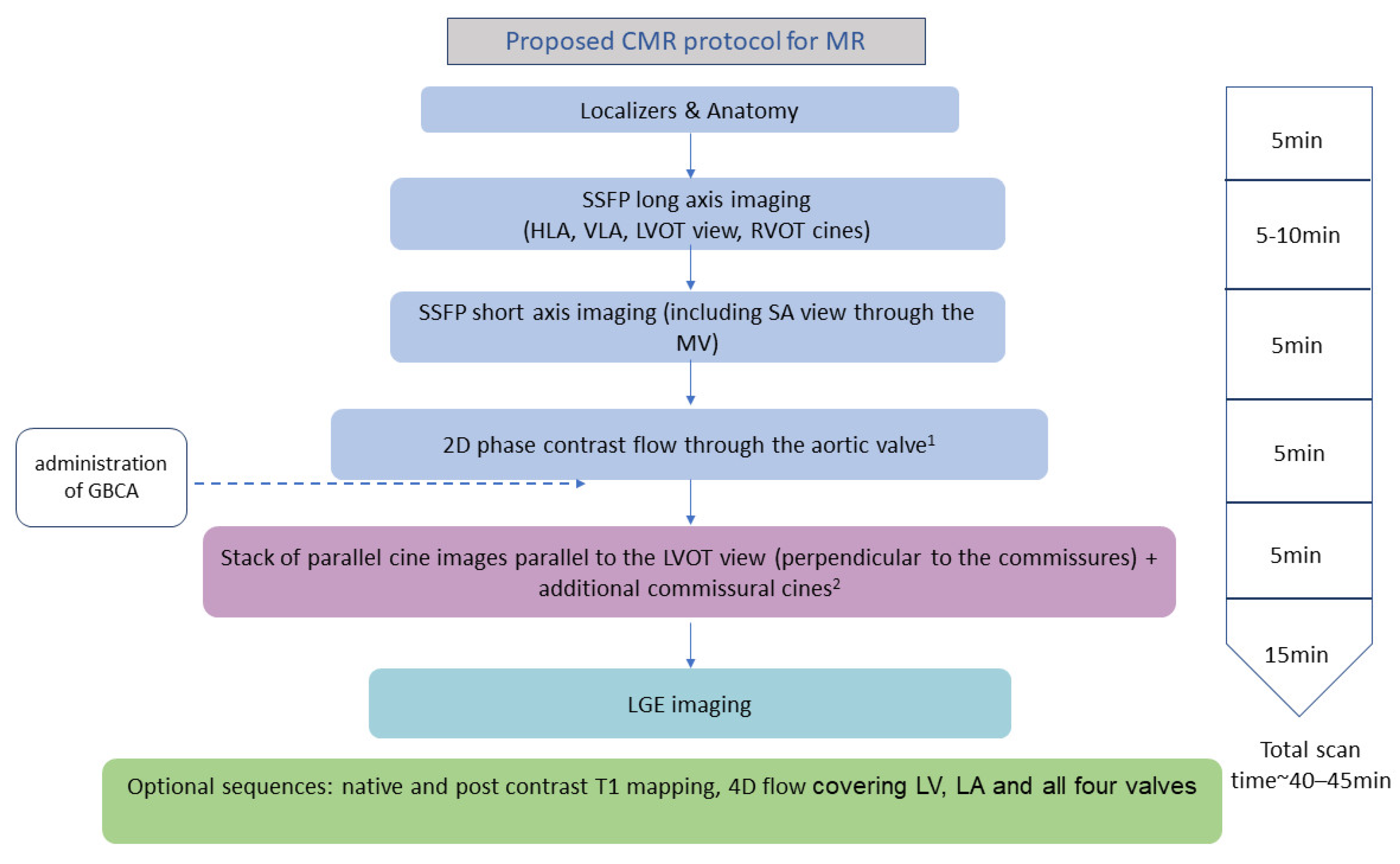

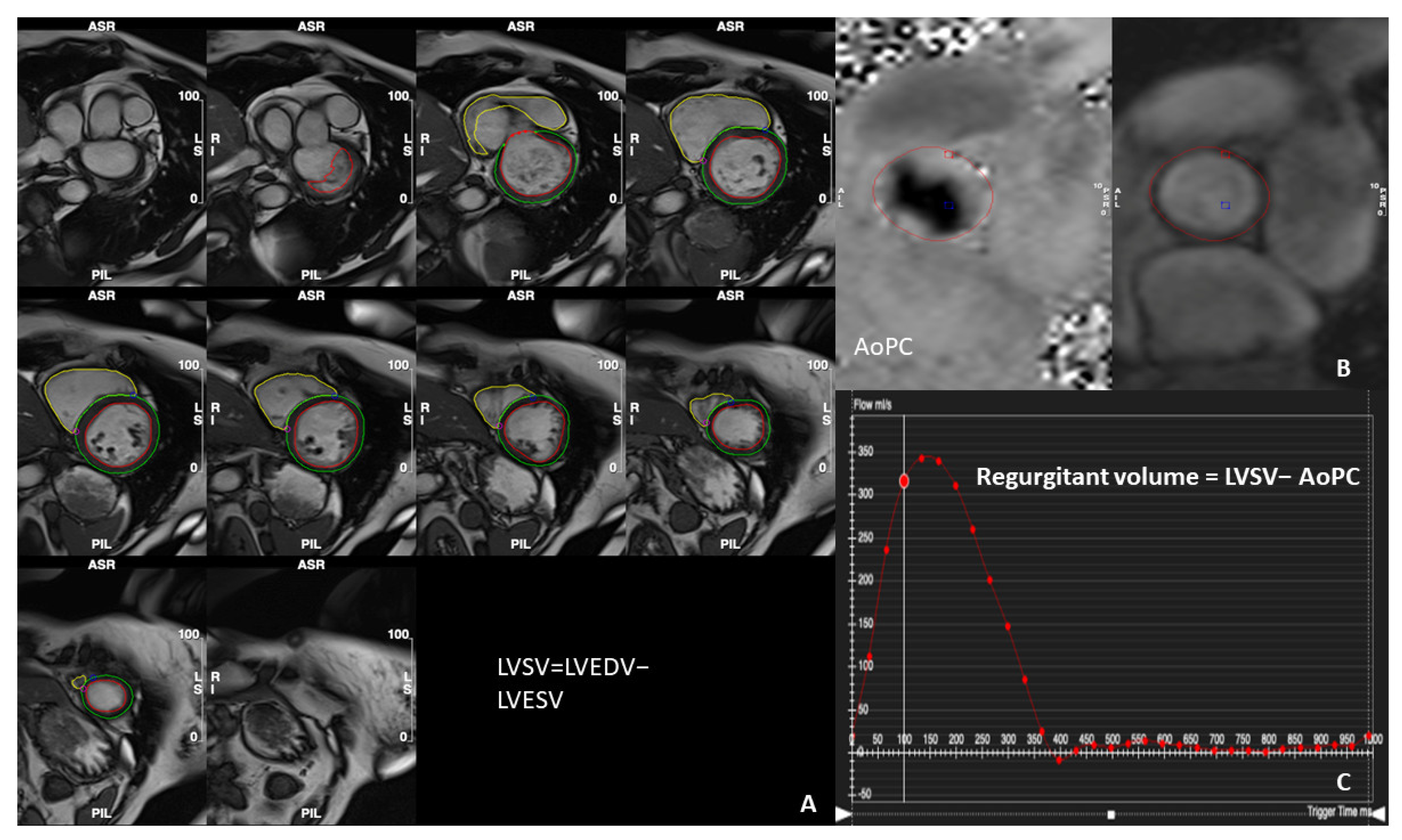
| Method | Strengths | Pitfalls | |
|---|---|---|---|
| Standard method | Regurgitant volume = LVSV- AoPC (mL/cardiac cycle) Regurgitant fraction = (Regurgitant fraction/LV stroke volume) × 100% |
|
|
| Alternative method (Cine) | Regurgitant volume = LVSV − RVSV |
|
|
| 4D flow direct tracking of mitral regurgitant jet |
|
|
| Thresholds for Severe Mitral Regurgitation | ||||||
|---|---|---|---|---|---|---|
| Study | Population | Regurgitant Volume | Regurgitant Fraction | LVEDVi | LVESVi | LAVi |
| Uretsky et al., 2022 Ref. [41] | 152 patients with degenerative MR | ≥60 mL | ≥50% | N/A | N/A | N/A |
| Uretsky et al., 2021 Ref. [42] | 158 patients with primary MR and Presence of a flail leaflet or Coanda on echo | ≥60 mL | ≥50% | N/A | N/A | N/A |
| Capron et al., 2020 Ref. [43] | 44 patients with moderate to severe chronic primary MR | ≥60 mL | N/A | ≥92 mL/m2 | N/A | N/A |
| Cavalcante et al., 2020 Ref. [44] | 578 patients with ICM and ischemic MR | N/A | ≥35% (significant MR) | N/A | N/A | N/A |
| Kitkungvan et al., 2018 Ref. [45] | 356 primary MR patients | N/A | ≥50% | ≥95 mL/m2 | N/A | N/A |
| Penicka et al., 2018 Ref. [36] | 258 asymptomatic patients with moderate/severe primary MR | ≥60 mL | N/A | N/A | N/A | N/A |
| Polte et al., 2017 Ref. [46] | 40 patients with moderate/severe MR on echo | >64 mL >32 mL/m2 (i) | >41% | 120 mL/m2 | N/A | N/A |
| Aplin et al., 2016 Ref. [47] | 72 patients, primary MR on echocardiography | >39 mL >21 mL/m2 (i) | >27% | >108 mL/m2 | >72 mL/m2 | >83 mL/m2 |
| Myerson et al., 2016 Ref. [48] | 109, asymptomatic patients with moderate/severe MR on echo | >55 mL >29 mL/m2 (i) | >40% | ≥95 mL/m2 | >36 mL/m2 | N/A |
| Uretsky et al., 2015 Ref. [49] | 103 patients with MR on echocardiography | ≥60 mL | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botis, I.; Bazmpani, M.-A.; Daios, S.; Ziakas, A.; Kamperidis, V.; Karamitsos, T.D. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Mitral Regurgitation. Diagnostics 2024, 14, 644. https://doi.org/10.3390/diagnostics14060644
Botis I, Bazmpani M-A, Daios S, Ziakas A, Kamperidis V, Karamitsos TD. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Mitral Regurgitation. Diagnostics. 2024; 14(6):644. https://doi.org/10.3390/diagnostics14060644
Chicago/Turabian StyleBotis, Ioannis, Maria-Anna Bazmpani, Stylianos Daios, Antonios Ziakas, Vasileios Kamperidis, and Theodoros D. Karamitsos. 2024. "The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Mitral Regurgitation" Diagnostics 14, no. 6: 644. https://doi.org/10.3390/diagnostics14060644
APA StyleBotis, I., Bazmpani, M.-A., Daios, S., Ziakas, A., Kamperidis, V., & Karamitsos, T. D. (2024). The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Mitral Regurgitation. Diagnostics, 14(6), 644. https://doi.org/10.3390/diagnostics14060644








