From Data to Insights: How Is AI Revolutionizing Small-Bowel Endoscopy?
Abstract
:1. Introduction
2. Application in Small-Bowel Capsule Endoscopy
2.1. AI and Obscure Gastrointestinal Bleeding
2.2. AI and Vascular Lesions
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Vieira [25] | Angioectasia | 2019 | Retrospective | Detect SB angioectasia | - | Two different datasets | ML | S and SP over 96% | |
| Noya et al. [27] | Angioectasia | 2017 | Retrospective | Detect SB angioectasia | 799 lesion frames and 849 normal frames from 36 patients | 514 regions with lesion and 22,832 regions with no lesion | 514 regions with lesion and 22,832 regions with no lesion | CNN | S: 89.5%. Sp: 96.8% |
| Leenhardt et al. [28] | Angioectasia | 2019 | Retrospective | Detection of SB angioectasias | 4166 videos | 300 GI angioectasia images and 300 normal images | 300 GI angioectasia images and 300 normal images | CNN | S: 100%. Sp: 96% |
| Tsuboi et al. [29] | Angioectasia | 2020 | Retrospective | Detection of SB angioectasias | 189 patients | 2237 GI angioectasia from 141 patients | 488 images of angioectasia and 10,000 normal images from 48 patients | CNN | AUC 0.998 S: 98.8% Sp: 98.4% |
| Chu et al. [30] | Angioectasia | 2023 | Retrospective | Detect SB angioectasias (angioectasia, Dieulafoy’s lesion, and AV malformation) | 378 patients | 7393 lesion images | 1500 lesion images 1500 normal images | CNN | Acc: 99%. NPV: 98.7% PPV: 94.3% |
2.3. AI and Protruding Lesions
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Barbosa et al. [33] | Protruding lesions | 2008 | Retrospective | Detection of SB tumors | - | 104 tumor images 100 normal images | 92 tumor images 100 normal images | MLP | S: 98.7% Sp: 96.6% |
| Barbosa et al. [34] | Protruding lesions | 2012 | Retrospective | Detection of SB tumors | 700 tumoral frames and 2300 normal frames | - | - | MLP | S: 93.1% Sp: 93.9% |
| Li et al. [35] | Protruding lesions | 2009 | Retrospective | Detection of SB tumors | 150 abnormal images and 150 normal images from 2 patients | - | - | MLP | S: 89.8% Sp: 82.5% Acc: 86.1% |
| Li et al. [36] | Protruding lesions | 2011 | Retrospective | Detection of SB tumors | 600 images of tumors and 600 normal images from 10 patients | 540 normal images and 540 tumor images from 9 patients | 60 normal images and 60 tumor images from 1 patient | SVM | S: 82.3% Sp: 84.7% Acc: 83.5% |
| Li et al. [37] | Protruding lesions | 2012 | Retrospective | Detection of SB tumors | 600 images of tumors and 600 normal images from 10 patients | - | - | SVM | Acc: 92.4% |
| Yuan et al. [40] | Protruding lesions | 2017 | Retrospective | Polyp detection | 1000 polyp images and 3000 normal images | - | - | SSAEM | Acc: 98% |
| Saito et al. [41] | Protruding lesions | 2020 | Retrospective | Identify and classify protruding lesions | - | 30,584 WCE images of protruding lesions from 292 patients | 7507 images of protruding lesions from 93 patients and 10,000 normal images | CNN | S: 90.7% Sp: 79.8% |
| Saraiva et al. [32] | Protruding lesions | 2021 | Retrospective | Detect SB protruding lesions and evaluate the lesions’ bleeding potential | 1483 CE exams from 1229 patients. 18,625 images extracted | 14,900 images (2264 images of protruding lesions and 12,636 images of normal mucosa) | 3725 images of protruding lesions, and 3159 images with normal mucosa | CNN | S: 96.8% Sp: 96.5% Acc: 92.5% Reading time 70 frames per second |
2.4. AI and Pleomorphic Lesion Detection
2.5. AI and Small-Bowel Compartmentalization
2.6. AI and Celiac Disease
2.7. AI and Inflammatory Bowel Activity
2.8. AI and Small-Bowel Cleansing
2.9. Miscellaneous—AI and Hookworms/Functional Bowel disorders
3. Application in Device-Assisted Enteroscopy
3.1. AI and Vascular Lesions
3.2. AI and Ulcers and Erosions
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Device-Assisted Enteroscopy | |||||||||
| Saraiva et al. [80] | Angioectasia | 2021 | Retrospective | Automatic detection of angioectasia | 72 patients | 5392 | 1348 | CNN | S: 88.5%, Sp: 97.1%, Acc: 95.3%, AUC: 0.98 |
| Martins et al. [85] | Ulcers and erosions | 2023 | Retrospective | Automatic detection of ulcers and erosions | 250 patients (6772 images) | 6094 | 678 | CNN | S: 89.7%, Sp: 99.5%, Acc: 98.6% |
| Cardoso et al. [86] | Protruding lesions | 2022 | Retrospective | Automatic detection of protruding lesions | 72 patients | 6340 | 1585 | CNN | S: 97.0% Sp: 97.4% Acc: 97.3% AUC 1.00 |
| Mendes et al. [87] | Multiple lesion detection | 2024 | Retrospective | Automatic detection of multiple clinically relevant lesions | 338 exams | 36,599 | 4066 | CNN | S: 88.9% Sp: 98.9% Acc: 96.8% |
3.3. AI and Protuberant Lesions
3.4. AI and Pleomorphic Multi-Lesion Detection
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.C.; Islam, S.U.; Noor, A.; Khan, S.; Afsar, W.; Nazir, S. Influential Usage of Big Data and Artificial Intelligence in Healthcare. Comput. Math. Methods Med. 2021, 2021, 5812499. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Afonso, J.; Andrade, P.; Cardoso, H.; Macedo, G. Artificial intelligence and capsule endoscopy: Unravelling the future. Ann. Gastroenterol. 2021, 34, 300–309. [Google Scholar] [CrossRef]
- Catlow, J.; Bray, B.; Morris, E.; Rutter, M. Power of big data to improve patient care in gastroenterology. Frontline Gastroenterol. 2022, 13, 237–244. [Google Scholar] [CrossRef]
- Pannala, R.; Krishnan, K.; Melson, J.; Parsi, M.A.; Schulman, A.R.; Sullivan, S.; Trikudanathan, G.; Trindade, A.J.; Watson, R.R.; Maple, J.T.; et al. Artificial intelligence in gastrointestinal endoscopy. VideoGIE 2020, 5, 598–613. [Google Scholar] [CrossRef]
- Okagawa, Y.; Abe, S.; Yamada, M.; Oda, I.; Saito, Y. Artificial Intelligence in Endoscopy. Dig. Dis. Sci. 2022, 67, 1553–1572. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, J.S.; Goong, H.J.; Lee, S.H.; Oh, E.H.; Park, J.; Kim, M.C.; Nam, K.; Yang, Y.J.; Kim, T.J.; et al. Use of device-assisted enteroscopy in small bowel disease: An expert consensus statement by the Korean Association for the Study of Intestinal Diseases. Intest. Res. 2023, 21, 3–19. [Google Scholar] [CrossRef]
- Cortegoso Valdivia, P.; Skonieczna-Zydecka, K.; Elosua, A.; Sciberras, M.; Piccirelli, S.; Rullan, M.; Tabone, T.; Gawel, K.; Stachowski, A.; Leminski, A.; et al. Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, E.J.; Tennyson, C.A.; Bhagat, G.; Lewis, S.K.; Green, P.H. Classification of videocapsule endoscopy image patterns: Comparative analysis between patients with celiac disease and normal individuals. Biomed. Eng. Online 2010, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Majtner, T.; Brodersen, J.B.; Herp, J.; Kjeldsen, J.; Halling, M.L.; Jensen, M.D. A deep learning framework for autonomous detection and classification of Crohn’s disease lesions in the small bowel and colon with capsule endoscopy. Endosc. Int. Open 2021, 9, E1361–E1370. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.; Cardoso, H.; Macedo, G. Artificial Intelligence in Capsule Endoscopy: A Gamechanger for a Groundbreaking Technique; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Awadie, H.; Zoabi, A.; Gralnek, I.M. Obscure-overt gastrointestinal bleeding: A review. Pol. Arch. Intern. Med. 2022, 132, 16253. [Google Scholar] [CrossRef]
- Patel, A.; Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N. Obscure Gastrointestinal Bleeding and Capsule Endoscopy: A Win-Win Situation or Not? Cureus 2022, 14, e27137. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.S.; Strong, R. Gastrointestinal Angiodysplasia: Diagnosis and Management. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Saraiva, M.M.; Ferreira, J.P.S.; Ribeiro, T.; Cardoso, H.; Macedo, G. Performance of a convolutional neural network for automatic detection of blood and hematic residues in small bowel lumen. Dig. Liver Dis. 2021, 53, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Yan, G.; Qiu, X.; Cui, J. Bleeding detection in Wireless Capsule Endoscopy based on Probabilistic Neural Network. J. Med. Syst. 2011, 35, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, W.; Mandal, M.; Meng, M.Q. Computer-aided bleeding detection in WCE video. IEEE J. Biomed. Health Inf. 2014, 18, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Meng, M.Q. A deep convolutional neural network for bleeding detection in Wireless Capsule Endoscopy images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 639–642. [Google Scholar] [CrossRef]
- Fan, S.; Xu, L.; Fan, Y.; Wei, K.; Li, L. Computer-aided detection of small intestinal ulcer and erosion in wireless capsule endoscopy images. Phys. Med. Biol. 2018, 63, 165001. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Aoyama, K.; Saito, H.; Tsuboi, A.; Nakada, A.; Niikura, R.; Fujishiro, M.; Oka, S.; Ishihara, S.; et al. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest. Endosc. 2019, 89, 357–363.e352. [Google Scholar] [CrossRef]
- Wang, S.; Xing, Y.; Zhang, L.; Gao, H.; Zhang, H. A systematic evaluation and optimization of automatic detection of ulcers in wireless capsule endoscopy on a large dataset using deep convolutional neural networks. Phys. Med. Biol. 2019, 64, 235014. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Aoyama, K.; Saito, H.; Fujisawa, G.; Odawara, N.; Kondo, R.; Tsuboi, A.; Ishibashi, R.; Nakada, A.; et al. Clinical usefulness of a deep learning-based system as the first screening on small-bowel capsule endoscopy reading. Dig. Endosc. 2020, 32, 585–591. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Kato, Y.; Saito, H.; Tsuboi, A.; Nakada, A.; Niikura, R.; Fujishiro, M.; Oka, S.; Ishihara, S.; et al. Automatic detection of blood content in capsule endoscopy images based on a deep convolutional neural network. J. Gastroenterol. Hepatol. 2020, 35, 1196–1200. [Google Scholar] [CrossRef]
- Ghosh, T.; Chakareski, J. Deep Transfer Learning for Automated Intestinal Bleeding Detection in Capsule Endoscopy Imaging. J. Digit. Imaging 2021, 34, 404–417. [Google Scholar] [CrossRef]
- Mascarenhas Saraiva, M.J.; Afonso, J.; Ribeiro, T.; Ferreira, J.; Cardoso, H.; Andrade, A.P.; Parente, M.; Natal, R.; Mascarenhas Saraiva, M.; Macedo, G. Deep learning and capsule endoscopy: Automatic identification and differentiation of small bowel lesions with distinct haemorrhagic potential using a convolutional neural network. BMJ Open Gastroenterol. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.M.; Silva, C.P.; Costa, D.; Vaz, I.F.; Rolanda, C.; Lima, C.S. Automatic Segmentation and Detection of Small Bowel Angioectasias in WCE Images. Ann. Biomed. Eng. 2019, 47, 1446–1462. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.M.; Goncalves, B.; Goncalves, C.R.; Lima, C.S. Segmentation of angiodysplasia lesions in WCE images using a MAP approach with Markov Random Fields. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Noya, F.; Alvarez-Gonzalez, M.A.; Benitez, R. Automated angiodysplasia detection from wireless capsule endoscopy. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 2017, 3158–3161. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, R.; Vasseur, P.; Li, C.; Saurin, J.C.; Rahmi, G.; Cholet, F.; Becq, A.; Marteau, P.; Histace, A.; Dray, X. A neural network algorithm for detection of GI angiectasia during small-bowel capsule endoscopy. Gastrointest. Endosc. 2019, 89, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Oka, S.; Aoyama, K.; Saito, H.; Aoki, T.; Yamada, A.; Matsuda, T.; Fujishiro, M.; Ishihara, S.; Nakahori, M.; et al. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig. Endosc. 2020, 32, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Huang, F.; Gao, M.; Zou, D.W.; Zhong, J.; Wu, W.; Wang, Q.; Shen, X.N.; Gong, T.T.; Li, Y.Y.; et al. Convolutional neural network-based segmentation network applied to image recognition of angiodysplasias lesion under capsule endoscopy. World J. Gastroenterol. 2023, 29, 879–889. [Google Scholar] [CrossRef]
- Van de Bruaene, C.; De Looze, D.; Hindryckx, P. Small bowel capsule endoscopy: Where are we after almost 15 years of use? World J. Gastrointest. Endosc. 2015, 7, 13–36. [Google Scholar] [CrossRef]
- Mascarenhas Saraiva, M.; Afonso, J.; Ribeiro, T.; Ferreira, J.; Cardoso, H.; Andrade, P.; Gonçalves, R.; Cardoso, P.; Parente, M.; Jorge, R.; et al. Artificial intelligence and capsule endoscopy: Automatic detection of enteric protruding lesions using a convolutional neural network. Rev. Esp. Enferm. Dig. 2023, 115, 75–79. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Ramos, J.; Lima, C.S. Detection of small bowel tumors in capsule endoscopy frames using texture analysis based on the discrete wavelet transform. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 3012–3015. [Google Scholar] [CrossRef]
- Barbosa, D.C.; Roupar, D.B.; Ramos, J.C.; Tavares, A.C.; Lima, C.S. Automatic small bowel tumor diagnosis by using multi-scale wavelet-based analysis in wireless capsule endoscopy images. Biomed. Eng. Online 2012, 11, 3. [Google Scholar] [CrossRef]
- Li, B.; Meng, M.Q.; Xu, L. A comparative study of shape features for polyp detection in wireless capsule endoscopy images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 3731–3734. [Google Scholar] [CrossRef]
- Li, B.P.; Meng, M.Q. Comparison of several texture features for tumor detection in CE images. J. Med. Syst. 2012, 36, 2463–2469. [Google Scholar] [CrossRef]
- Li, B.; Meng, M.Q. Tumor recognition in wireless capsule endoscopy images using textural features and SVM-based feature selection. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 323–329. [Google Scholar] [CrossRef]
- Vieira, P.M.; Freitas, N.R.; Valente, J.; Vaz, I.F.; Rolanda, C.; Lima, C.S. Automatic detection of small bowel tumors in wireless capsule endoscopy images using ensemble learning. Med. Phys. 2020, 47, 52–63. [Google Scholar] [CrossRef]
- Vieira, P.M.; Ramos, J.; Lima, C.S. Automatic detection of small bowel tumors in endoscopic capsule images by ROI selection based on discarded lightness information. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 3025–3028. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Meng, M.Q. Deep learning for polyp recognition in wireless capsule endoscopy images. Med. Phys. 2017, 44, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Aoki, T.; Aoyama, K.; Kato, Y.; Tsuboi, A.; Yamada, A.; Fujishiro, M.; Oka, S.; Ishihara, S.; Matsuda, T.; et al. Automatic detection and classification of protruding lesions in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest. Endosc. 2020, 92, 144–151.e141. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Lee, H.H.; Park, C.; Tama, B.A.; Kim, J.S.; Cheung, D.Y.; Chung, W.C.; Cho, Y.S.; Lee, K.M.; Choi, M.G.; et al. Improved classification and localization approach to small bowel capsule endoscopy using convolutional neural network. Dig. Endosc. 2021, 33, 598–607. [Google Scholar] [CrossRef]
- Ding, Z.; Shi, H.; Zhang, H.; Meng, L.; Fan, M.; Han, C.; Zhang, K.; Ming, F.; Xie, X.; Liu, H.; et al. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology 2019, 157, 1044–1054.e1045. [Google Scholar] [CrossRef]
- Otani, K.; Nakada, A.; Kurose, Y.; Niikura, R.; Yamada, A.; Aoki, T.; Nakanishi, H.; Doyama, H.; Hasatani, K.; Sumiyoshi, T.; et al. Automatic detection of different types of small-bowel lesions on capsule endoscopy images using a newly developed deep convolutional neural network. Endoscopy 2020, 52, 786–791. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Kato, Y.; Saito, H.; Tsuboi, A.; Nakada, A.; Niikura, R.; Fujishiro, M.; Oka, S.; Ishihara, S.; et al. Automatic detection of various abnormalities in capsule endoscopy videos by a deep learning-based system: A multicenter study. Gastrointest. Endosc. 2021, 93, 165–173.e161. [Google Scholar] [CrossRef]
- Vieira, P.M.; Freitas, N.R.; Lima, V.B.; Costa, D.; Rolanda, C.; Lima, C.S. Multi-pathology detection and lesion localization in WCE videos by using the instance segmentation approach. Artif. Intell. Med. 2021, 119, 102141. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Z.; Liu, X.; Bai, J.; Liao, G. Organic Boundary Location Based on Color-Texture of Visual Perception in Wireless Capsule Endoscopy Video. J. Healthc. Eng. 2018, 2018, 3090341. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J. Gastroenterol. 2022, 28, 154–175. [Google Scholar] [CrossRef]
- Zhou, T.; Han, G.; Li, B.N.; Lin, Z.; Ciaccio, E.J.; Green, P.H.; Qin, J. Quantitative analysis of patients with celiac disease by video capsule endoscopy: A deep learning method. Comput. Biol. Med. 2017, 85, 1–6. [Google Scholar] [CrossRef]
- Koh, J.E.W.; Hagiwara, Y.; Oh, S.L.; Tan, J.H.; Ciaccio, E.J.; Green, P.H.; Lewis, S.K.; Acharya, U.R. Automated diagnosis of celiac disease using DWT and nonlinear features with video capsule endoscopy images. Future Gener. Comput. Syst. 2018, 90, 86–93. [Google Scholar] [CrossRef]
- Wang, X.; Qian, H.; Ciaccio, E.J.; Lewis, S.K.; Bhagat, G.; Green, P.H.; Xu, S.; Huang, L.; Gao, R.; Liu, Y. Celiac disease diagnosis from videocapsule endoscopy images with residual learning and deep feature extraction. Comput. Methods Programs Biomed. 2020, 187, 105236. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, C.A.; Dulf, E.H.; Ciobanu, L. Automated detection of celiac disease using Machine Learning Algorithms. Sci. Rep. 2022, 12, 4071. [Google Scholar] [CrossRef]
- Chetcuti Zammit, S.; McAlindon, M.E.; Greenblatt, E.; Maker, M.; Siegelman, J.; Leffler, D.A.; Yardibi, O.; Raunig, D.; Brown, T.; Sidhu, R. Quantification of Celiac Disease Severity Using Video Capsule Endoscopy: A Comparison of Human Experts and Machine Learning Algorithms. Curr. Med. Imaging 2023, 19, 1455–1662. [Google Scholar] [CrossRef]
- Goran, L.; Negreanu, A.M.; Stemate, A.; Negreanu, L. Capsule endoscopy: Current status and role in Crohn’s disease. World J. Gastrointest. Endosc. 2018, 10, 184–192. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Klang, E.; Barash, Y.; Margalit, R.Y.; Soffer, S.; Shimon, O.; Albshesh, A.; Ben-Horin, S.; Amitai, M.M.; Eliakim, R.; Kopylov, U. Deep learning algorithms for automated detection of Crohn’s disease ulcers by video capsule endoscopy. Gastrointest. Endosc. 2020, 91, 606–613.e602. [Google Scholar] [CrossRef]
- Takenaka, K.; Kawamoto, A.; Okamoto, R.; Watanabe, M.; Ohtsuka, K. Artificial intelligence for endoscopy in inflammatory bowel disease. Intest. Res. 2022, 20, 165–170. [Google Scholar] [CrossRef]
- Barash, Y.; Azaria, L.; Soffer, S.; Margalit Yehuda, R.; Shlomi, O.; Ben-Horin, S.; Eliakim, R.; Klang, E.; Kopylov, U. Ulcer severity grading in video capsule images of patients with Crohn’s disease: An ordinal neural network solution. Gastrointest. Endosc. 2021, 93, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Klang, E.; Grinman, A.; Soffer, S.; Margalit Yehuda, R.; Barzilay, O.; Amitai, M.M.; Konen, E.; Ben-Horin, S.; Eliakim, R.; Barash, Y.; et al. Automated Detection of Crohn’s Disease Intestinal Strictures on Capsule Endoscopy Images Using Deep Neural Networks. J. Crohns Colitis 2021, 15, 749–756. [Google Scholar] [CrossRef]
- Noorda, R.; Nevárez, A.; Colomer, A.; Pons Beltrán, V.; Naranjo, V. Automatic evaluation of degree of cleanliness in capsule endoscopy based on a novel CNN architecture. Sci. Rep. 2020, 10, 17706. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Oh, H.S.; Lee, Y.J.; Jung, H.; Lee, J.H.; Kang, B.; Choi, S.; Kim, J.H.; Kim, K.O.; Chung, Y.J. Clean mucosal area detection of gastroenterologists versus artificial intelligence in small bowel capsule endoscopy. Medicine 2023, 102, e32883. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.; Margalit-Yehuda, R.; Gatt, K.; Sciberras, M.; Girelli, C.; Saurin, J.C.; Valdivia, P.C.; Cotter, J.; Eliakim, R.; Caprioli, F.; et al. Scoring systems in clinical small-bowel capsule endoscopy: All you need to know! Endosc. Int. Open 2021, 9, E802–E823. [Google Scholar] [CrossRef]
- Van Weyenberg, S.J.; De Leest, H.T.; Mulder, C.J. Description of a novel grading system to assess the quality of bowel preparation in video capsule endoscopy. Endoscopy 2011, 43, 406–411. [Google Scholar] [CrossRef]
- Ponte, A.; Pinho, R.; Rodrigues, A.; Silva, J.; Rodrigues, J.; Carvalho, J. Validation of the computed assessment of cleansing score with the Mirocam® system. Rev. Esp. Enferm. Dig. 2016, 108, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Abou Ali, E.; Histace, A.; Camus, M.; Gerometta, R.; Becq, A.; Pietri, O.; Nion-Larmurier, I.; Li, C.; Chaput, U.; Marteau, P.; et al. Development and validation of a computed assessment of cleansing score for evaluation of quality of small-bowel visualization in capsule endoscopy. Endosc. Int. Open 2018, 6, E646–E651. [Google Scholar] [CrossRef] [PubMed]
- Oumrani, S.; Histace, A.; Abou Ali, E.; Pietri, O.; Becq, A.; Houist, G.; Nion-Larmurier, I.; Camus, M.; Florent, C.; Dray, X. Multi-criterion, automated, high-performance, rapid tool for assessing mucosal visualization quality of still images in small bowel capsule endoscopy. Endosc. Int. Open 2019, 7, E944–E948. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, R.; Souchaud, M.; Houist, G.; Le Mouel, J.P.; Saurin, J.C.; Cholet, F.; Rahmi, G.; Leandri, C.; Histace, A.; Dray, X. A neural network-based algorithm for assessing the cleanliness of small bowel during capsule endoscopy. Endoscopy 2021, 53, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Hwang, Y.; Oh, D.J.; Park, J.; Kim, K.B.; Jung, M.K.; Lim, Y.J. Development of a deep learning-based software for calculating cleansing score in small bowel capsule endoscopy. Sci. Rep. 2021, 11, 4417. [Google Scholar] [CrossRef]
- Ju, J.W.; Jung, H.; Lee, Y.J.; Mun, S.W.; Lee, J.H. Semantic Segmentation Dataset for AI-Based Quantification of Clean Mucosa in Capsule Endoscopy. Medicina 2022, 58, 397. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Mascarenhas Saraiva, M.J.; Afonso, J.; Cardoso, P.; Mendes, F.; Martins, M.; Andrade, A.P.; Cardoso, H.; Mascarenhas Saraiva, M.; Ferreira, J.; et al. Design of a Convolutional Neural Network as a Deep Learning Tool for the Automatic Classification of Small-Bowel Cleansing in Capsule Endoscopy. Medicina 2023, 59, 810. [Google Scholar] [CrossRef] [PubMed]
- Houdeville, C.; Leenhardt, R.; Souchaud, M.; Velut, G.; Carbonell, N.; Nion-Larmurier, I.; Nuzzo, A.; Histace, A.; Marteau, P.; Dray, X. Evaluation by a Machine Learning System of Two Preparations for Small Bowel Capsule Endoscopy: The BUBS (Burst Unpleasant Bubbles with Simethicone) Study. J. Clin. Med. 2022, 11, 2822. [Google Scholar] [CrossRef]
- Wu, X.; Chen, H.; Gan, T.; Chen, J.; Ngo, C.W.; Peng, Q. Automatic Hookworm Detection in Wireless Capsule Endoscopy Images. IEEE Trans. Med. Imaging 2016, 35, 1741–1752. [Google Scholar] [CrossRef]
- Gan, T.; Yang, Y.; Liu, S.; Zeng, B.; Yang, J.; Deng, K.; Wu, J.; Yang, L. Automatic Detection of Small Intestinal Hookworms in Capsule Endoscopy Images Based on a Convolutional Neural Network. Gastroenterol. Res. Pr. 2021, 2021, 5682288. [Google Scholar] [CrossRef] [PubMed]
- Spyridonos, P.; Vilariño, F.; Vitrià, J.; Azpiroz, F.; Radeva, P. Anisotropic feature extraction from endoluminal images for detection of intestinal contractions. Med. Image Comput. Comput. Assist. Interv. 2006, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Malagelada, C.; De Iorio, F.; Azpiroz, F.; Accarino, A.; Segui, S.; Radeva, P.; Malagelada, J.R. New insight into intestinal motor function via noninvasive endoluminal image analysis. Gastroenterology 2008, 135, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Teshima, C.W.; Kuipers, E.J.; van Zanten, S.V.; Mensink, P.B. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: An updated meta-analysis. J. Gastroenterol. Hepatol. 2011, 26, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Rondonotti, E.; Despott, E.J.; Dray, X.; Keuchel, M.; Moreels, T.; Sanders, D.S.; Spada, C.; Carretero, C.; Cortegoso Valdivia, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2022. Endoscopy 2023, 55, 58–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Rajan, E.; Cheng, S.; Shen, R.; Zhang, C.; Zhang, S.; Wu, Y.; Zhong, J. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am. J. Gastroenterol. 2006, 101, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Ohata, K.; Nakajima, A.; Matsuhashi, N. Diagnosis and therapeutic strategies for small bowel vascular lesions. World J. Gastroenterol. 2019, 25, 2720–2733. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas Saraiva, M.; Ribeiro, T.; Afonso, J.; Andrade, P.; Cardoso, P.; Ferreira, J.; Cardoso, H.; Macedo, G. Deep Learning and Device-Assisted Enteroscopy: Automatic Detection of Gastrointestinal Angioectasia. Medicina 2021, 57, 1378. [Google Scholar] [CrossRef]
- Yen, H.H.; Chang, C.W.; Chou, J.W.; Wei, S.C. Balloon-Assisted Enteroscopy and Capsule Endoscopy in Suspected Small Bowel Crohn’s Disease. Clin. Endosc. 2017, 50, 417–423. [Google Scholar] [CrossRef]
- Jang, H.J.; Choi, M.H.; Eun, C.S.; Choi, H.; Choi, K.Y.; Park, D.I.; Park, J.H.; Chang, D.K.; Kim, J.O.; Ko, B.M.; et al. Clinical usefulness of double balloon enteroscopy in suspected Crohn’s disease: The KASID multi-center trial. Hepatogastroenterology 2014, 61, 1292–1296. [Google Scholar]
- Rahman, A.; Ross, A.; Leighton, J.A.; Schembre, D.; Gerson, L.; Lo, S.K.; Waxman, I.; Dye, C.; Semrad, C. Double-balloon enteroscopy in Crohn’s disease: Findings and impact on management in a multicenter retrospective study. Gastrointest. Endosc. 2015, 82, 102–107. [Google Scholar] [CrossRef]
- Bourreille, A.; Ignjatovic, A.; Aabakken, L.; Loftus, E.V., Jr.; Eliakim, R.; Pennazio, M.; Bouhnik, Y.; Seidman, E.; Keuchel, M.; Albert, J.G.; et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: An international OMED-ECCO consensus. Endoscopy 2009, 41, 618–637. [Google Scholar] [CrossRef]
- Martins, M.; Mascarenhas, M.; Afonso, J.; Ribeiro, T.; Cardoso, P.; Mendes, F.; Cardoso, H.; Andrade, P.; Ferreira, J.; Macedo, G. Deep-Learning and Device-Assisted Enteroscopy: Automatic Panendoscopic Detection of Ulcers and Erosions. Medicina 2023, 59, 172. [Google Scholar] [CrossRef]
- Cardoso, P.; Saraiva, M.M.; Afonso, J.; Ribeiro, T.; Andrade, P.; Ferreira, J.; Cardoso, H.; Macedo, G. Artificial Intelligence and Device-Assisted Enteroscopy: Automatic Detection of Enteric Protruding Lesions Using a Convolutional Neural Network. Clin. Transl. Gastroenterol. 2022, 13, e00514. [Google Scholar] [CrossRef]
- Mendes, F.; Mascarenhas, M.; Ribeiro, T.; Afonso, J.; Cardoso, P.; Martins, M.; Cardoso, H.; Andrade, P.; Ferreira, J.P.S.; Mascarenhas Saraiva, M.; et al. Artificial Intelligence and Panendoscopy—Automatic Detection of Clinically Relevant Lesions in Multibrand Device-Assisted Enteroscopy. Cancers 2024, 16, 208. [Google Scholar] [CrossRef]
- Leenhardt, R.; Koulaouzidis, A.; Histace, A.; Baatrup, G.; Beg, S.; Bourreille, A.; de Lange, T.; Eliakim, R.; Iakovidis, D.; Dam Jensen, M.; et al. Key research questions for implementation of artificial intelligence in capsule endoscopy. Ther. Adv. Gastroenterol. 2022, 15, 17562848221132683. [Google Scholar] [CrossRef]
- Lavin, A.; Gilligan-Lee, C.M.; Visnjic, A.; Ganju, S.; Newman, D.; Ganguly, S.; Lange, D.; Baydin, A.G.; Sharma, A.; Gibson, A.; et al. Technology readiness levels for machine learning systems. Nat. Commun. 2022, 13, 6039. [Google Scholar] [CrossRef]
- Meher, D.; Gogoi, M.; Bharali, P.; Anirvan, P.; Singh, S.P. Artificial intelligence in small bowel endoscopy: Current perspectives and future directions. J. Dig. Endosc. 2020, 11, 245–252. [Google Scholar] [CrossRef]
- Leenhardt, R.; Fernandez-Urien Sainz, I.; Rondonotti, E.; Toth, E.; Van de Bruaene, C.; Baltes, P.; Rosa, B.J.; Triantafyllou, K.; Histace, A.; Koulaouzidis, A.; et al. PEACE: Perception and Expectations toward Artificial Intelligence in Capsule Endoscopy. J. Clin. Med. 2021, 10, 5708. [Google Scholar] [CrossRef]
- Messmann, H.; Bisschops, R.; Antonelli, G.; Libânio, D.; Sinonquel, P.; Abdelrahim, M.; Ahmad, O.F.; Areia, M.; Bergman, J.; Bhandari, P.; et al. Expected value of artificial intelligence in gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022, 54, 1211–1231. [Google Scholar] [CrossRef]
- Lee, J.; Wallace, M.B. State of the Art: The Impact of Artificial Intelligence in Endoscopy 2020. Curr. Gastroenterol. Rep. 2021, 23, 7. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Ribeiro, T.; Afonso, J.; Mendes, F.; Cardoso, P.; Martins, M.; Ferreira, J.; Macedo, G. Smart Endoscopy Is Greener Endoscopy: Leveraging Artificial Intelligence and Blockchain Technologies to Drive Sustainability in Digestive Health Care. Diagnostics 2023, 13, 3625. [Google Scholar] [CrossRef]
- Namikawa, K.; Hirasawa, T.; Yoshio, T.; Fujisaki, J.; Ozawa, T.; Ishihara, S.; Aoki, T.; Yamada, A.; Koike, K.; Suzuki, H.; et al. Utilizing artificial intelligence in endoscopy: A clinician’s guide. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 689–706. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Afonso, J.; Ribeiro, T.; Andrade, P.; Cardoso, H.; Macedo, G. The Promise of Artificial Intelligence in Digestive Healthcare and the Bioethics Challenges It Presents. Medicina 2023, 59, 790. [Google Scholar] [CrossRef]


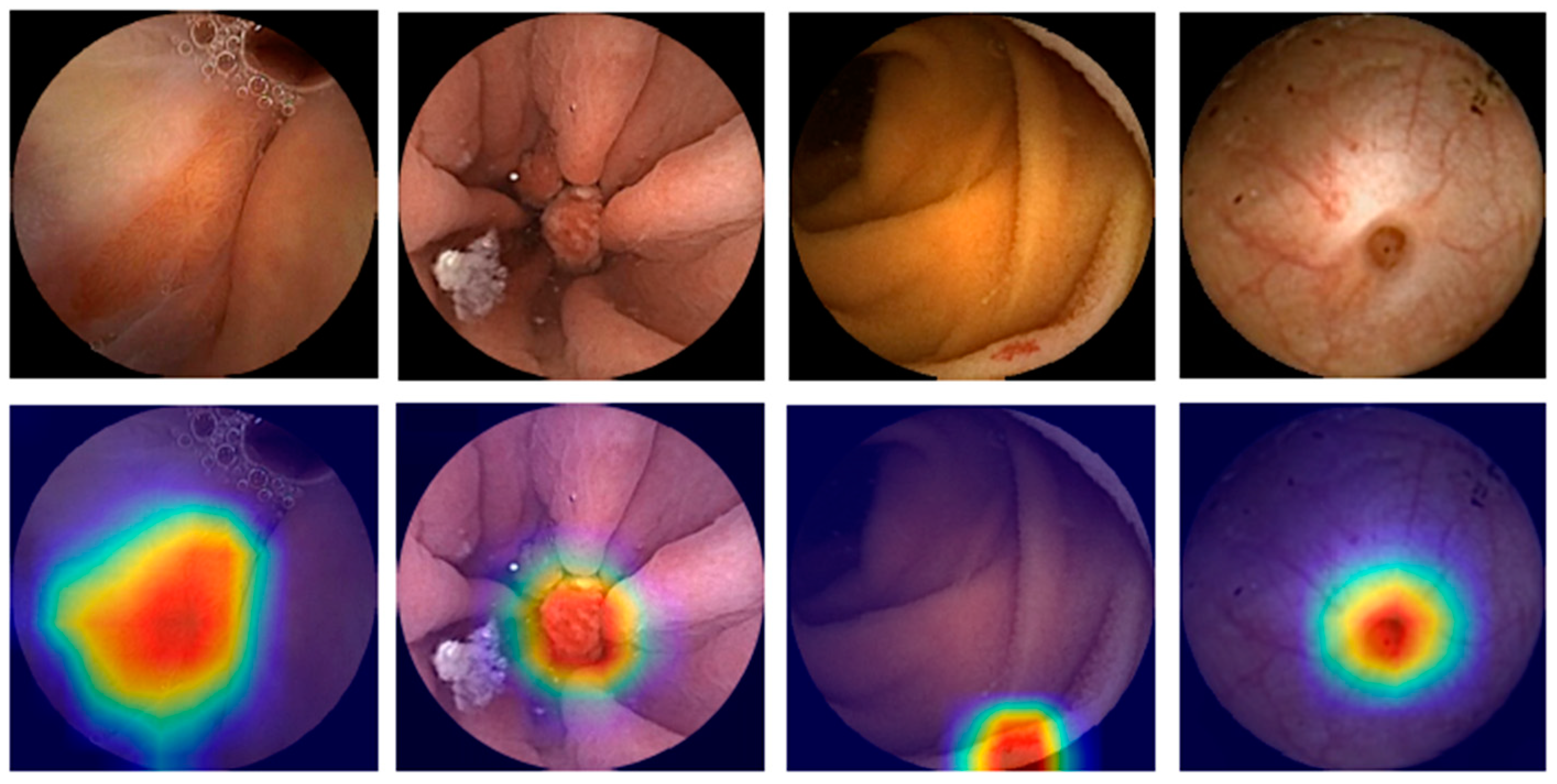

| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Pan et al. [15] | GI bleeding | 2010 | Retrospective | Detect a bleeding image | 150 full videos | - | 3172 bleeding frames 11,458 normal frames | CNN | S: 93.1%, Sp: 85.6% |
| Fu et al. [16] | GI bleeding | 2014 | Retrospective | Detect a bleeding image | 20 different WCE videos | 10,000 bleeding and 20,000 non-bleeding frames | 10,000 bleeding and 40,000 non-bleeding frames | SVM | S: 99%, Sp: 94%, Acc: 95% |
| Jia et al. [17] | GI bleeding | 2016 | Retrospective | Detection of GI bleeding | 10,000 images | 050 GI bleeding frames and 6150 normal frames | 800 GI bleeding frames and 1000 normal frames | CNN | S:99%, Sp:100% |
| Fan et al. [18] | GI bleeding | 2018 | Retrospective | Detection of ulcers and erosions in SB mucosa | 144 full WCE videos | Ulcers: 2000 images of ulcers and 2400 images of normal mucosa | Ulcers: 500 images of ulcers and 600 images of normal mucosa | CAD DL framework | Ulcers: Acc: 95.2% S: 96.8% Sp 94.8% |
| Erosions: 2720 images of erosions and 3200 images of normal mucosa | Erosions: 1500 images of erosions and 4000 images of normal mucosa | Erosions: Acc: 95.3% S: 93.7% Sp 96.0% | |||||||
| Aoki et al. [19] | Obscure GI bleeding | 2019 | Retrospective | Detection of ulcers and erosions the SB | 15,800 images from 180 patients | 5360 images of ulcers and erosions (115 patients) | 440 images of ulcers and erosions, 10,000 normal images (65 patients) | CNN | AUC of 0.958. Sensitivity of 88.2%, specificity of 90.9%, and accuracy of 90.8% |
| Wang et al. [20] | Obscure GI bleeding | 2019 | Retrospective | Detection of ulcers and localization | 1504 patients (1076 with ulcers) | 15,781 ulcer frames and 17,138 normal frames | 4917 ulcer frames and 5007 normal frames | CNN | S: 89.7%, Sp: 90.5%, Acc: 90.1% |
| Aoki et al. [21] | GI bleeding | 2019 | Retrospective | Validation of a CNN method as a first reader for ulcer detection | 20 full videos | - | - | CNN | Significantly shorter reading time with screening by the CNN, without reducing ng the detection rate of mucosal breaks |
| Aoki et al. [22] | GI bleeding | 2020 | Retrospective | Detect GI bleeding | 27,847 images from 41 patients | 27,847 images (6503 images with blood content from 29 patients and 21,344 normal images from 12 patients) | 10,208 images (208 images from 5 patients with blood content and 10,000 images from 20 patients with normal mucosa) | CNN | S: 96.6% Sp: 99.9% Acc: 99.9% |
| Ghosh et al. [23] | Obscure GI bleeding | 2021 | - | Detect bleeding zones | - | - | - | CNN | Acc: 94.4% |
| Afonso et al. [14] | Obscure GI bleeding | 2021 | Retrospective | Detect blood and hematic residues in the SB lumen | - | Three stages of development. In each stage, the neural architecture was adapted, and the number of CE images increased. In the final stage, 23,190 frames were used. | CNN | S: 98.3% Sp: 98.4%, Acc: 98.2%. Reading time of 186 frames/second) | |
| Saraiva et al. [24] | Obscure GI bleeding | 2021 | Retrospective | Detection and differentiation of multiple SB lesions with different bleeding potential (Saurin classification) | 4319 patients | 42,844 images | 10,711 images | CNN | S: 88% Sp: 99% Acc: 99% |
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Ding et al. [43] | Multiple lesion detection | 2019 | Retrospective | Detect and distingue multiple lesions | 6970 patients | 158,235 images from 1970 exams | 113,268,334 images from 5000 patients | CNN | S near 100%. Mean reading time of 6 min per exam |
| Otani et al. [44] | Multiple lesion detection | 2020 | Retrospective | Detect and distingue multiple lesions | 167 patients | 5526 images (erosions and ulcers, vascular lesions, and tumors) and 34,437 normal images | 1247 images. | CNN | AUC: 0.996 for erosions and ulcers, 0.950 for vascular lesions, and 0.950 for tumors |
| Aoki et al. [45] | Multiple lesion detection | 2020 | Retrospective | Detect and classify multiple lesions | - | 66,028 CE images (44,684 images of lesions and 21,344 normal images) | Full videos from 379 SB CE | CNN | Acc for mucosal breaks, angioectasia, protruding lesions, and blood content were 100%, 97%, 98%, and 100%, respectively |
| Hwang et al. [42] | Multiple lesion detection | 2021 | Retrospective | Detect bleeding and ulcerative lesions separately Two models: combined and binary | - | 7556 images (half pathological and half normal) from 526 SB CE videos | 5760 images (960 abnormal and 4800 normal) from 162 videos | CNN | Both models with high accuracy for lesion detection and localization of the culprit area |
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Ciaccio et al. [8] | Celiac disease | 2010 | Retrospective | Predict celiac disease based on images of CE SBs | 11 patients and 10 controls | six celiac and five control patients’ data | five celiac and five control patients’ data | - | S: 80% Sp: 96% The incremental classifier had 88% S% and 80% Sp% |
| Zhou et al. [49] | Celiac disease | 2017 | Retrospective | Evaluate the presence and degree of intestinal villous atrophy | 21 patients | six celiac disease patients and five controls | five celiac disease patients and five control patients | CNN | S: 100% Sp: 100% Capable of correlating the Marsh score with CE images |
| Koh et al. [50] | Celiac disease | 2018 | Retrospective | Identify patients with celiac disease | 13 control subjects and 13 celiac patients | - | - | SVM | S: 88.4% Sp: 84.6% Acc: 86.5% |
| Wang et al. [51] | Celiac disease | 2020 | Identify patients with celiac disease | 107 exams | 1100 images with healthy mucosa and 1040 lesion images | CNN | S: 97.2% Sp: 95.6% Acc: 95.9% | ||
| Stoleru et al. [52] | Celiac disease | 2022 | Retrospective | Diagnose celiac disease with CE images, without complex algorithm | 105 exams | 51 videos (of 100 frames) | 51 videos (of 100 frames) | ML | Acc: 94.1% |
| Zammit et al. [53] | Celiac disease | 2023 | Retrospective | Evaluate and grade celiac disease severity, compare with expert classification | - | 334,080 frames from 35 patients with celiac disease. 110,579 frames from 13 patients without celiac disease | 63 VCE videos from 63 patients with celiac disease | ML | Strong correlation between celiac severity scores provided by the algorithm and the average expert reader scores |
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Klang et al. [56] | Inflammatory bowel disease | 2019 | Retrospective | Detection of SB ulcers in Crohn’s disease patients | 17,640 CE images from 49 patients | - | - | CNN | Acc over 95% AUC over 0.94 |
| Barash et al. [58] | Inflammatory bowel disease | 2020 | Retrospective | Detect and grade the severity of ulcers in Crohn’s disease and access the inter-reader variability and the agreement of two experts and the AI method | 17,640 CE images from 49 patients | Pre-train with 17,640 CE images (7391 images with mucosal ulcers and 10,249 images of normal mucosa). Train with 1242 images. | 248 images | CNN | Overall agreement between the consensus reading and the automatic algorithm of 67% but an inter-reader agreement of only 31% |
| Klang et al. [59] | Inflammatory bowel disease | 2020 | Retrospective | Detect strictures in CE images of Crohn’s disease patients | 27,892 CE images | - | - | DL | Acc: 93.5% Excellent discrimination between strictures, normal mucosa, and different grades of ulcers |
| Author Ref. | Field | Pub. Year | Study Design | Aim | Number of Subjects | Training Dataset | Validation and Testing Dataset | AI Type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Small-Bowel Capsule Endoscopy | |||||||||
| Van Weyen-berg et al. [63] | SB cleanliness | 2011 | Retrospective | Design an objective score of quality of SB visualization—computer assessment of cleansing (CAC) score | 40 VCE segments from 10 VCE studies | - | - | Computer evaluation | Show feasibility of using the CAC score in the assessment of the quality of intestinal preparation in PillCam® CE system |
| Ponte et al. [64] | SB cleanliness | 2016 | Retrospective | Adapt the CAC score to the Mirocam® CE system | 30 VCE | - | - | Computer evaluation | Results slightly inferior to those of Van Weyenberg but significant |
| Abou Ali et al. [65] | SB cleanliness | 2018 | Retrospective | Develop and validate a CAC score at the image level by defining the threshold for an adequate SB visualization | 33 VCE | - | - | Computer evaluation | S: 91.3% Sp: 94.7% |
| Oumrani et al. [66] | SB cleanliness | 2019 | Retrospective | Access the adequacy of SB mucosa visualization | 600 frames | 500 frames | 100 frames | ML | S: 90.0% Sp: 87.7% |
| Noorda et al. [60] | SB cleanliness | 2020 | Retrospective | Access the adequacy of SB mucosa visualization with an intuitive scale | Images from 35 VCE | 26,746 clean patches and 28,547 dirty patches | 854 frames extracted from 30 different CE videos | CNN | Acc: 95.2% |
| Leenhardt et al. [67] | SB cleanliness | 2020 | Retrospective | Access SB mucosa visualization | 186 VCE | 600 still frames | two independent 78-video subsets | CNN | S: 90.3% Sp: 83.3% Acc: 89.7% |
| Nam et al. [68] | SB cleanliness | 2021 | Retrospective | Provide an objective score for quantitative evaluation of CE cleanliness | 168 CE exams | 2500 frames | 1000 frames | DL | Score had high correlation with assessment by CE experts |
| Ju et al. [61] | SB cleanliness | 2023 | Retrospective | Compare the detection of clean mucosal areas in CE using human judgment versus AI | 13,233 images from 512 CE exams | 2319 images from 12 patients | 10,914 images from 500 patients | CNN | Intra-variability within human judgment. AI judgment was consistent with the five gastroenterologists’ judgements |
| Ju et al. [69] | SB cleanliness | 2022 | Retrospective | Create a large-scale semantic segmentation dataset and combine with a CNN to evaluate SB cleanliness | 10,033 images from 179 CE studies | 7988 images from 169 patients | 2045 images from 10 patients | CNN | Acc above 94% |
| Ribeiro et al. [70] | SB cleanliness | 2023 | Retrospective | Assess the quality of intestinal preparation in CE | 4319 patients | 12,159 images | 791 images | CNN | S: 88.4% Sp: 93.6% Acc: 92.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Almeida, M.J.; Mendes, F.; Martins, M.; Ribeiro, T.; Afonso, J.; Cardoso, P.; Cardoso, H.; Andrade, P.; Ferreira, J.; et al. From Data to Insights: How Is AI Revolutionizing Small-Bowel Endoscopy? Diagnostics 2024, 14, 291. https://doi.org/10.3390/diagnostics14030291
Mota J, Almeida MJ, Mendes F, Martins M, Ribeiro T, Afonso J, Cardoso P, Cardoso H, Andrade P, Ferreira J, et al. From Data to Insights: How Is AI Revolutionizing Small-Bowel Endoscopy? Diagnostics. 2024; 14(3):291. https://doi.org/10.3390/diagnostics14030291
Chicago/Turabian StyleMota, Joana, Maria João Almeida, Francisco Mendes, Miguel Martins, Tiago Ribeiro, João Afonso, Pedro Cardoso, Helder Cardoso, Patrícia Andrade, João Ferreira, and et al. 2024. "From Data to Insights: How Is AI Revolutionizing Small-Bowel Endoscopy?" Diagnostics 14, no. 3: 291. https://doi.org/10.3390/diagnostics14030291
APA StyleMota, J., Almeida, M. J., Mendes, F., Martins, M., Ribeiro, T., Afonso, J., Cardoso, P., Cardoso, H., Andrade, P., Ferreira, J., Mascarenhas, M., & Macedo, G. (2024). From Data to Insights: How Is AI Revolutionizing Small-Bowel Endoscopy? Diagnostics, 14(3), 291. https://doi.org/10.3390/diagnostics14030291







