A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings
Abstract
:1. Introduction
2. Search Strategy
3. Results
3.1. The Structure of the Olfactory System and Pathogenesis of Olfactory Disorders in COVID-19
3.1.1. The Structure of the Olfactory System
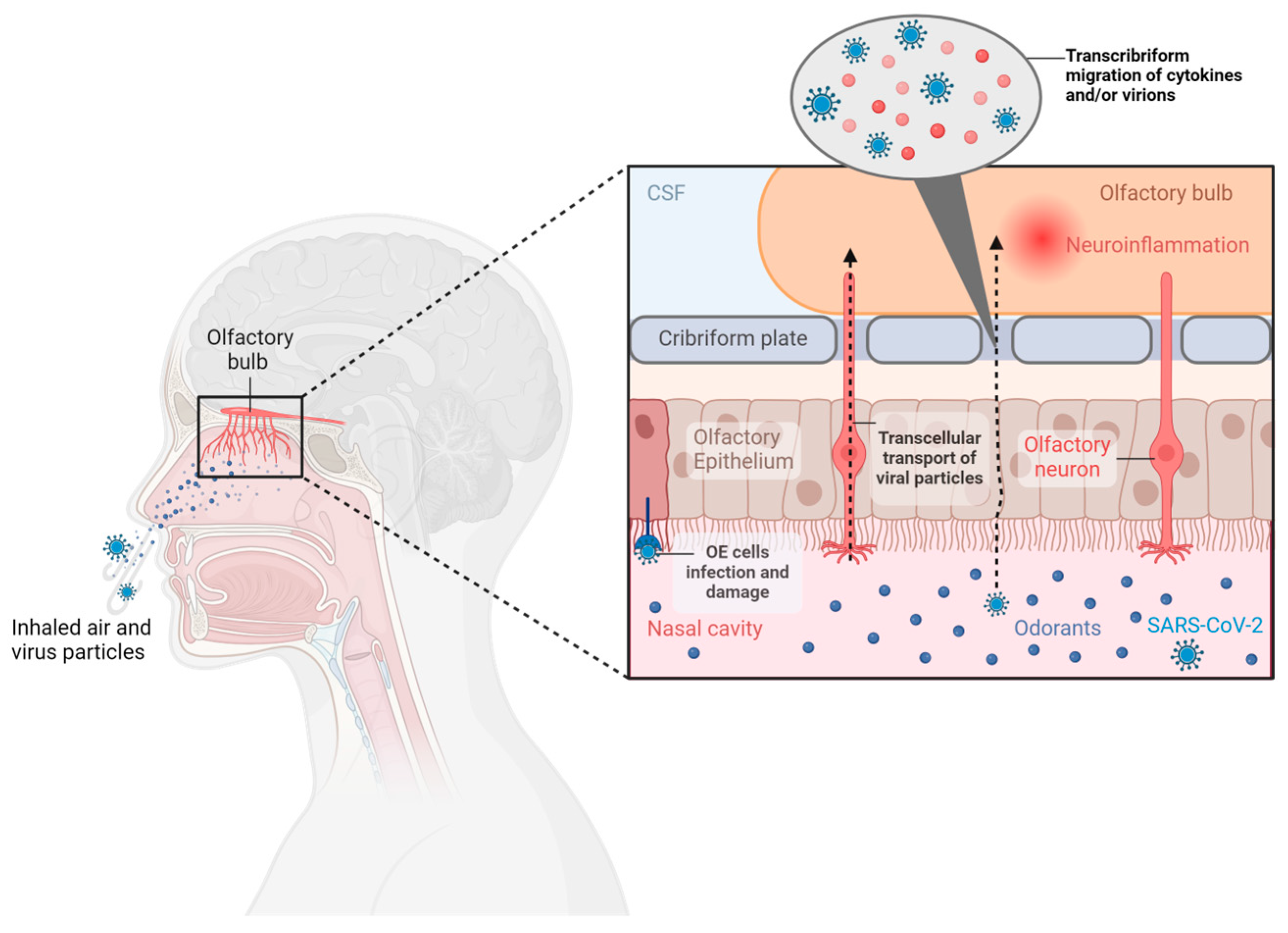
3.1.2. Pathogenesis of Olfactory Disorders in COVID-19
Direct Damage to Olfactory Neurons
Damage to the OE
Inflammation
3.2. Magnetic Resonance Imaging Olfactory System Findings
3.2.1. Correlation between Olfactory System MRI Findings and OD in COVID-19 Patients
3.2.2. OBV and OS Depth Changes
3.2.3. OBs Signal Intensity
3.2.4. OBs Morphological Abnormalities
3.2.5. Olfactory Neuron Filia Abnormalities
3.2.6. Olfactory Cortex and White Matter Olfactory Region Abnormalities
| Authors | Scanner Field | Sequence | Patients | Controls | Findings |
|---|---|---|---|---|---|
| Strauss S.B. et al. (2020) [4] | 3T (Signa Architect and Discovery 750W, GE Healthcare Waukesha, WI, USA) 3T (Skyra, Siemens Healthineers, Erlangen, Germany) |
| Patients with COVID-19 and neurological symptoms (n = 12 including 1 with OD) | Patients with non-COVID-19 OD (n = 12) | Increase signal intensity in OB |
| Kandemirli S.D. et al. (2021) [39] | 3T (Magnetom, SiemensHealthineers, Erlangen, Germany) |
| Patients with persistent COVID-19-related OD (n = 23) | NO | Decrease in OBV; Abnormality in OB signal intensity; Clumping of olfactory filia |
| Yildirim D. et al. (2021) [35] | 3T (Magnetom, Siemens Healthineers, Erlangen, Germany) |
| Patients with persistent related COVID-19 OD (n = 31) | Patients with post-infectious OD other than COVID-19 (n = 97) | Decrease in OBV; Increased OB signal intensity; Clumping of olfactory filia |
| Li et al. (2021) [44] | Not reported |
| Patient with COVID-19 and OD (n = 1) | NO | Decrease in right OBV; Hyperintensities in bilateral olfactory nerves |
| Brudasca I. (2022) [34] | Not reported |
| Patients with related COVID-19 OD (n = 67) | NO | No OBV significant differences in between subgroups with mild, moderate, or severe hyposmia |
| Gore M.R. (2022) [38] | Not reported |
| Patients with cardiac arrhythmia (n = 44), Patients with COVID-19 (n = 11) | Healthy control (n = 43) | Decrease in OBV and OS depths |
| Abdou E.H.E. et al. (2023) [41] | 1.5T (Ingenia, Philips Medical Systems, Eindhoven, The Netherland) |
| Patients with related COVID-19 OD (n = 110) | Healthy control (n= 50) | Increased OBV and OB dimensions (length × width × height) |
| Capelli S. (2023) [2] | 3T (Discovery 750W, GE Healthcare Waukesha, WI, USA) |
| Patients with COVID-19 (n = 196, n = 78 of them with OD) | Healthy control (n= 39) | Decrease in OBV; No significant differences in OT signal intensity |
| Iravani K. et al. (2023) [36] | 1.5T (Magnetom Avento, SiemensHealthineers, Erlangen, Germany) |
| Patients with related COVID-19 OD (n = 15) | Healthy control (n= 5) | Decreased activity in the superior frontal lobe and basal ganglia |
| Parlak A.E. et al. (2023) [37] | 3T (Ingenia, Philips Medical Systems, Eindhoven, The Netherland) |
| Patients with COVID-19, anosmia, and hyposmia (n = 31) | Healthy control (n= 35) | Decrease in OBV and OS depth |
| Perlaki G. (2023) [46] | 3T (Magnetom PrismaFit, SiemensHealthineers, Erlangen, Germany) |
| Patients who recovered from mild COVID infection (n = 38) | Healthy control (n = 37) | Lower bilateral mean cortical thickness, lower subcortical gray matter, and lower right OBV |
| Seleim A.M.A (2023) [40] | 1.5T (Achieva, Philips Medical Systems, Eindhoven, The Netherland) | Not reported | Patients with related COVID-19 anosmia (n = 20) | NO | Decreases in OBV and OS depth |
| Campabadal A. (2023) [45] | 3T (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany) |
| Patient with COVID-19 and OD (n = 23) | Patient with COVID-19 without OD (n = 25) | Decrease in GM volume (areas reported in the main text); Increase MD in olfactory system |
3.3. Correlation between OD and Neurocognitive Deficits
3.3.1. Cognitive Impairment
3.3.2. Mood Disorders
3.4. Treatment Approaches for COVID-19-Related OD
4. Discussion and Future Directions
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://www.covid19.who.int/2023 (accessed on 11 November 2023).
- Capelli, S.; Caroli, A.; Barletta, A.; Arrigoni, A.; Napolitano, A.; Pezzetti, G.; Longhi, L.G.; Zangari, R.; Lorini, F.L.; Sessa, M.; et al. MRI evidence of olfactory system alterations in patients with COVID-19 and neurological symptoms. J. Neurol. 2023, 270, 1195–1206. [Google Scholar] [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef]
- Strauss, S.B.; Lantos, J.E.; Heier, L.A.; Shatzkes, D.R.; Phillips, C.D. Olfactory Bulb Signal Abnormality in Patients with COVID-19 Who Present with Neurologic Symptoms. AJNR Am. J. Neuroradiol. 2020, 41, 1882–1887. [Google Scholar] [CrossRef]
- Tan, C.J.; Tan, B.K.J.; Tan, X.Y.; Liu, H.T.; Teo, C.B.; See, A.; Xu, S.; Toh, S.T.; Kheok, S.W.; Charn, T.C.; et al. Neuroradiological Basis of COVID-19 Olfactory Dysfunction: A Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 1260–1274. [Google Scholar] [CrossRef]
- de Melo, G.D.; Perraud, V.; Alvarez, F.; Vieites-Prado, A.; Kim, S.; Kergoat, L.; Coleon, A.; Trueb, B.S.; Tichit, M.; Piazza, A.; et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat. Commun. 2023, 14, 4485. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.K.; Gupta, N.; Verma, R.R.; Sharma, J.; Sah, M.; Jain, S.; Kashyap, D. Olfactory and taste dysfunction in COVID-19-incidence and recovery. Egypt J. Otolaryngol. 2023, 39, 18. [Google Scholar] [CrossRef]
- Cavazzana, A.; Larsson, M.; Munch, M.; Hahner, A.; Hummel, T. Postinfectious olfactory loss: A retrospective study on 791 patients. Laryngoscope 2018, 128, 10–15. [Google Scholar] [CrossRef]
- Nordin, S.; Bramerson, A. Complaints of olfactory disorders: Epidemiology, assessment and clinical implications. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Carpio-Orantes, L.D.; Garcia-Mendez, S.; Diaz, J.S.S.; Solis-Sanchez, I.; Aguilar-Silva, A. Anosmia and dysgeusia as markers of severity and prognosis in COVID-19. Brain Circ. 2023, 9, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, J.; Maremmani, C.; Salvadori, S.; Silani, V.; Ticozzi, N. Testing olfactory dysfunction in acute and recovered COVID-19 patients: A single center study in Italy. Neurol. Sci. 2021, 42, 2183–2189. [Google Scholar] [CrossRef]
- Frosolini, A.; Parrino, D.; Fabbris, C.; Fantin, F.; Inches, I.; Invitto, S.; Spinato, G.; Filippis, C. Magnetic Resonance Imaging Confirmed Olfactory Bulb Reduction in Long COVID-19: Literature Review and Case Series. Brain Sci. 2022, 12, 430. [Google Scholar] [CrossRef]
- Patt, Y.S.; Fisher, L.; David, P.; Bergwerk, M.; Shoenfeld, Y. Autoimmunity, COVID-19 Omicron Variant, and Olfactory Dysfunction: A Literature Review. Diagnostics 2023, 13, 641. [Google Scholar] [CrossRef]
- Han, S.A.; Kim, J.K.; Cho, D.Y.; Patel, Z.M.; Rhee, C.S. Olfactory System: Basic Anatomy and Physiology for General Otorhinolaryngologists. Clin. Exp. Otorhinolaryngol. 2023, 16, 308. [Google Scholar] [CrossRef] [PubMed]
- Manzini, I.; Schild, D.; Di Natale, C. Principles of odor coding in vertebrates and artificial chemosensory systems. Physiol. Rev. 2022, 102, 61–154. [Google Scholar] [CrossRef]
- Liang, F. Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes 2020, 11, 493. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.S.; McNamara, J.O.; Williams, S.M. The Olfactory Epithelium and Olfactory Receptor Neurons, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Wilson, D.A.; Xu, W.; Sadrian, B.; Courtiol, E.; Cohen, Y.; Barnes, D.C. Cortical odor processing in health and disease. Prog. Brain Res. 2014, 208, 275–305. [Google Scholar] [CrossRef]
- Doty, R.L. The Olfactory Dysfunction of COVID-19. Semin. Neurol. 2023, 43, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Research 2021, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Tsukahara, T.; Brann, D.H.; Datta, S.R. Mechanisms of SARS-CoV-2-associated anosmia. Physiol. Rev. 2023, 103, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Benjamin, L.; Lunn, M.P.; Bharucha, T.; Zandi, M.S.; Hoskote, C.; McNamara, P.; Manji, H. Pathophysiology, diagnosis, and management of neuroinflammation in COVID-19. BMJ 2023, 382, e073923. [Google Scholar] [CrossRef] [PubMed]
- Bryche, B.; St Albin, A.; Murri, S.; Lacote, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Rai, P.; Gautam, A.; Kaur, H.; Kapoor, S.; Suttee, A.; Jaiswal, P.K.; Sharma, A.; Singh, G.; Barnwal, R.P. Neurological manifestations of SARS-CoV-2: Complexity, mechanism and associated disorders. Eur. J. Med. Res. 2023, 28, 307. [Google Scholar] [CrossRef]
- Vargas, G.; Medeiros Geraldo, L.H.; Gedeao Salomao, N.; Viana Paes, M.; Regina Souza Lima, F.; Carvalho Alcantara Gomes, F. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives. Brain Behav. Immun. Health 2020, 7, 100127. [Google Scholar] [CrossRef]
- Burks, S.M.; Rosas-Hernandez, H.; Alejandro Ramirez-Lee, M.; Cuevas, E.; Talpos, J.C. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav. Immun. 2021, 95, 7–14. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wang, Y. COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Viruses 2021, 13, 2225. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021, 20, 753–761. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Schwabenland, M.; Salie, H.; Tanevski, J.; Killmer, S.; Lago, M.S.; Schlaak, A.E.; Mayer, L.; Matschke, J.; Puschel, K.; Fitzek, A.; et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 2021, 54, 1594–1610.e11. [Google Scholar] [CrossRef]
- Brudasca, I.; Lisan, Q.; Tournegros, R.; Bensafi, M.; Ferdenzi, C.; Fournel, A.; Denoix, L.; Tringali, S.; Fieux, M. Systematic MRI in persistent post-COVID-19 olfactory dysfunction should be reassessed. Int. Forum Allergy Rhinol. 2023, 13, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, D.; Kandemirli, S.G.; Tekcan Sanli, D.E.; Akinci, O.; Altundag, A. A Comparative Olfactory MRI, DTI and fMRI Study of COVID-19 Related Anosmia and Post Viral Olfactory Dysfunction. Acad. Radiol. 2022, 29, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Iravani, K.; Malekpour, B.; Rasekhi, A.; Faramarzi, A.; Soltaniesmaeili, A.; Golkhar, B.; Jahanandish, F.; Babaei, A. Functional magnetic resonance imaging in coronavirus disease 2019 induced olfactory dysfunction. J. Laryngol. Otol. 2024, 138, 178–183. [Google Scholar] [CrossRef]
- Parlak, A.E.; Selcuk, O.T.; Yilmaz, G.O.; Aydenizoz, D.; Selcuk, N.T.; Ocal, R.; Seyman, D.; Yilmaz, M.; Eyigor, H. Olfactory Bulb Volume and Morphology Changes in COVID-19 Patients With Olfactory Disorders Using Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Gore, M.R. Olfactory Radioanatomical Findings in Patients With Cardiac Arrhythmias, COVID-19, and Healthy Controls. Cureus 2022, 14, e26564. [Google Scholar] [CrossRef]
- Kandemirli, S.G.; Altundag, A.; Yildirim, D.; Tekcan Sanli, D.E.; Saatci, O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2021, 28, 28–35. [Google Scholar] [CrossRef]
- Elfeshawy, M.S. Radiological Evaluation of COVID-19 Anosmic Patients By MRI of The Olfactory Bulb and Computed Tomography of the Paranasal Sinuses. Egypt. J. Ear Nose Throat Allied Sci. 2023, 24, 1–5. [Google Scholar] [CrossRef]
- Abdou, E.H.E.; Ebada, H.A.; Salem, M.A.; Ghoneim, M.M.R.; Sherif, F.; Kamal, E. Clinical and Imaging Evaluation of COVID-19-Related Olfactory Dysfunction. Am. J. Rhinol. Allergy 2023, 37, 456–463. [Google Scholar] [CrossRef]
- Ammar, A.; Distinguin, L.; Chetrit, A.; Safa, D.; Hans, S.; Carlier, R.; Lechien, J.R.; Edjlali, M. Transient modifications of the olfactory bulb on MR follow-up of COVID-19 patients with related olfactory dysfunction. J. Neuroradiol. 2022, 49, 329–332. [Google Scholar] [CrossRef]
- Laurendon, T.; Radulesco, T.; Mugnier, J.; Gerault, M.; Chagnaud, C.; El Ahmadi, A.A.; Varoquaux, A. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 2020, 95, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Syue, L.S.; Tsai, Y.S.; Li, M.C.; Lo, C.L.; Tsai, C.S.; Chen, P.L.; Ko, W.C.; Lee, N.Y. Anosmia and olfactory tract neuropathy in a case of COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Campabadal, A.; Oltra, J.; Junque, C.; Guillen, N.; Boti, M.A.; Sala-Llonch, R.; Monte-Rubio, G.C.; Lledo, G.; Bargallo, N.; Rami, L.; et al. Structural brain changes in post-acute COVID-19 patients with persistent olfactory dysfunction. Ann. Clin. Transl. Neurol. 2023, 10, 195–203. [Google Scholar] [CrossRef]
- Perlaki, G.; Darnai, G.; Arato, A.; Alhour, H.A.; Szente, A.; Afra, E.; Nagy, S.A.; Horvath, R.; Kovacs, N.; Doczi, T.; et al. Gray Matter Changes Following Mild COVID-19: An MR Morphometric Study in Healthy Young People. J. Magn. Reson. Imaging 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Perrottelli, A.; Sansone, N.; Giordano, G.M.; Caporusso, E.; Giuliani, L.; Melillo, A.; Pezzella, P.; Bucci, P.; Mucci, A.; Galderisi, S. Cognitive Impairment after Post-Acute COVID-19 Infection: A Systematic Review of the Literature. J. Pers. Med. 2022, 12, 2070. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Pedersen, J.K.; Gunnarsson, D.V.; Roikjer, T.K.; Podlekareva, D.; Hansen, H.; Dall, C.H.; Johnsen, S. Cognitive impairments among patients in a long-COVID clinic: Prevalence, pattern and relation to illness severity, work function and quality of life. J. Affect. Disord. 2023, 324, 162–169. [Google Scholar] [CrossRef]
- Schild, A.K.; Goereci, Y.; Scharfenberg, D.; Klein, K.; Lulling, J.; Meiberth, D.; Schweitzer, F.; Sturmer, S.; Zeyen, P.; Sahin, D.; et al. Multidomain cognitive impairment in non-hospitalized patients with the post-COVID-19 syndrome: Results from a prospective monocentric cohort. J. Neurol. 2023, 270, 1215–1223. [Google Scholar] [CrossRef]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef]
- Shin, T.; Kim, J.; Ahn, M.; Moon, C. Olfactory Dysfunction in CNS Neuroimmunological Disorders: A Review. Mol. Neurobiol. 2019, 56, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Churnin, I.; Qazi, J.; Fermin, C.R.; Wilson, J.H.; Payne, S.C.; Mattos, J.L. Association Between Olfactory and Gustatory Dysfunction and Cognition in Older Adults. Am. J. Rhinol. Allergy 2019, 33, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Domellof, M.E.; Lundin, K.F.; Edstrom, M.; Forsgren, L. Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease. Park. Relat. Disord. 2017, 38, 41–47. [Google Scholar] [CrossRef]
- Faulet, T.; Gasnier, M.; Lecoq, A.L.; Morin, L.; Choucha, W.; Montani, D.; Monnet, X.; Becquemont, L.; Corruble, E.; Colle, R.; et al. Anosmia during acute COVID-19 and psychiatric outcomes 4 months later: Results from the prospective COMEBAC cohort. Gen. Hosp. Psychiatry 2023, 84, 260–261. [Google Scholar] [CrossRef]
- Ruggeri, M.; Ricci, M.; Pagliaro, M.; Gerace, C. Anosmia predicts memory impairment in post-COVID-19 syndrome: Results of a neuropsychological cohort study. Eur. Arch. Psychiatry Clin. Neurosci. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- de Araujo, I.E.; Rolls, E.T.; Velazco, M.I.; Margot, C.; Cayeux, I. Cognitive modulation of olfactory processing. Neuron 2005, 46, 671–679. [Google Scholar] [CrossRef]
- Soudry, Y.; Lemogne, C.; Malinvaud, D.; Consoli, S.M.; Bonfils, P. Olfactory system and emotion: Common substrates. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 18–23. [Google Scholar] [CrossRef]
- Ariza, M.; Cano, N.; Segura, B.; Adan, A.; Bargallo, N.; Caldu, X.; Campabadal, A.; Jurado, M.A.; Mataro, M.; Pueyo, R.; et al. Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaints. Front. Aging Neurosci. 2022, 14, 1029842. [Google Scholar] [CrossRef]
- Quan, M.; Wang, X.; Gong, M.; Wang, Q.; Li, Y.; Jia, J. Post-COVID cognitive dysfunction: Current status and research recommendations for high risk population. Lancet Reg. Health West. Pac. 2023, 38, 100836. [Google Scholar] [CrossRef]
- Yus, M.; Matias-Guiu, J.A.; Gil-Martinez, L.; Gomez-Ruiz, N.; Polidura, C.; Jorquera, M.; Delgado-Alonso, C.; Diez-Cirarda, M.; Matias-Guiu, J.; Arrazola, J. Persistent olfactory dysfunction after COVID-19 is associated with reduced perfusion in the frontal lobe. Acta Neurol. Scand. 2022, 146, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Alobid, I.; Fuentes, M.; Lopez-Chacon, M.; Mullol, J. Olfactory Dysfunction in Mental Illness. Curr. Allergy Asthma Rep. 2023, 23, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, L.; Sighinolfi, G.; Mitolo, M.; Ferri, L.; Jane Rochat, M.; Pensato, U.; Taruffi, L.; Testa, C.; Masullo, M.; Cortelli, P.; et al. Cognitive and functional connectivity impairment in post-COVID-19 olfactory dysfunction. Neuroimage Clin. 2023, 38, 103410. [Google Scholar] [CrossRef] [PubMed]
- Llana, T.; Mendez, M.; Garces-Arilla, S.; Hidalgo, V.; Mendez-Lopez, M.; Juan, M.C. Association between olfactory dysfunction and mood disturbances with objective and subjective cognitive deficits in long-COVID. Front. Psychol. 2023, 14, 1076743. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Canu, E.; Basaia, S.; Barbieri, A.; Cardamone, R.; Bernasconi, M.P.; Castelnovo, V.; Cividini, C.; Cursi, M.; et al. Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J. Neurol. 2022, 269, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; La Rocca, M.; Quaranta, N.; Iannuzzi, L.; Vecchio, E.; Brunetti, A.; Gentile, E.; Dibattista, M.; Lobasso, S.; Bevilacqua, V.; et al. Prefrontal dysfunction in post-COVID-19 hyposmia: An EEG/fNIRS study. Front. Hum. Neurosci. 2023, 17, 1240831. [Google Scholar] [CrossRef]
- Di Stadio, A.; Brenner, M.J.; De Luca, P.; Albanese, M.; D’Ascanio, L.; Ralli, M.; Roccamatisi, D.; Cingolani, C.; Vitelli, F.; Camaioni, A.; et al. Olfactory Dysfunction, Headache, and Mental Clouding in Adults with Long-COVID-19: What Is the Link between Cognition and Olfaction? A Cross-Sectional Study. Brain Sci. 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Dudine, L.; Canaletti, C.; Giudici, F.; Lunardelli, A.; Abram, G.; Santini, I.; Baroni, V.; Paris, M.; Pesavento, V.; Manganotti, P.; et al. Investigation on the Loss of Taste and Smell and Consequent Psychological Effects: A Cross-Sectional Study on Healthcare Workers Who Contracted the COVID-19 Infection. Front. Public Health 2021, 9, 666442. [Google Scholar] [CrossRef] [PubMed]
- Karamali, K.; Elliott, M.; Hopkins, C. COVID-19 related olfactory dysfunction. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 19–25. [Google Scholar] [CrossRef]
- Whitcroft, K.L.; Hummel, T. Olfactory Dysfunction in COVID-19: Diagnosis and Management. JAMA 2020, 323, 2512–2514. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, A.A.; Aldrweesh, A.K.; Aldoughan, A.F.; Alarfaj, S.M.; Alabdulqader, F.K.; Alyahya, K.A. Olfactory Dysfunction following COVID-19 and the Potential Benefits of Olfactory Training. J. Clin. Med. 2023, 12, 4761. [Google Scholar] [CrossRef] [PubMed]
- Donelli, D.; Antonelli, M.; Valussi, M. Olfactory training with essential oils for patients with post-COVID-19 smell dysfunction: A case series. Eur. J. Integr. Med. 2023, 60, 102253. [Google Scholar] [CrossRef]
- Hummel, T.; Rissom, K.; Reden, J.; Hahner, A.; Weidenbecher, M.; Huttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef]
- Le Bon, S.D.; Konopnicki, D.; Pisarski, N.; Prunier, L.; Lechien, J.R.; Horoi, M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur. Arch. Otorhinolaryngol. 2021, 278, 3113–3117. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.; Dixit, A. Olfactory training for olfactory dysfunction in COVID-19: A promising mitigation amidst looming neurocognitive sequelae of the pandemic. Clin. Exp. Pharmacol. Physiol. 2022, 49, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Kondo, K.; Ueha, R.; Kashiwadani, H.; Heinbockel, T. Possible Use of Phytochemicals for Recovery from COVID-19-Induced Anosmia and Ageusia. Int. J. Mol. Sci. 2021, 22, 8912. [Google Scholar] [CrossRef]
- Berube, S.; Demers, C.; Bussiere, N.; Cloutier, F.; Pek, V.; Chen, A.; Bolduc-Begin, J.; Frasnelli, J. Olfactory Training Impacts Olfactory Dysfunction Induced by COVID-19: A Pilot Study. ORL J. Otorhinolaryngol. Relat. Spec. 2023, 85, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Yu, A.C.; Lee, J.T. Management of post-COVID-19 olfactory dysfunction. Curr. Treat. Options Allergy 2022, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Canete, A.; Cano, E.; Munoz-Chapuli, R.; Carmona, R. Role of Vitamin A/Retinoic Acid in Regulation of Embryonic and Adult Hematopoiesis. Nutrients 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.M. Re: Alexander TH, Davidson TM. Intranasal zinc and anosmia: The zinc-induced anosmia syndrome. Laryngoscope 2006;116:217-220. Laryngoscope 2006, 116, 1721–1722; discussion 1722–1723. [Google Scholar] [CrossRef]
- Equils, O.; Lekaj, K.; Wu, A.; Fattani, S.; Liu, G.; Rink, L. Intra-nasal zinc level relationship to COVID-19 anosmia and type 1 interferon response: A proposal. Laryngoscope Investig. Otolaryngol. 2021, 6, 21–24. [Google Scholar] [CrossRef]
- Figueiredo, L.P.; Paim, P.; Cerqueira-Silva, T.; Barreto, C.C.; Lessa, M.M. Alpha-lipoic acid does not improve olfactory training results in olfactory loss due to COVID-19: A double-blind randomized trial. Braz. J. Otorhinolaryngol. 2023, 90, 101356. [Google Scholar] [CrossRef]
- Hu, B.; Gong, M.; Xiang, Y.; Qu, S.; Zhu, H.; Ye, D. Mechanism and treatment of olfactory dysfunction caused by coronavirus disease 2019. J. Transl. Med. 2023, 21, 829. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Heilmann, S.; Huttenbriuk, K.B. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope 2002, 112, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Jafek, B.W.; Linschoten, M.R.; Murrow, B.W. Anosmia after intranasal zinc gluconate use. Am. J. Rhinol. 2004, 18, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Khani, E.; Khiali, S.; Beheshtirouy, S.; Entezari-Maleki, T. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. Eur. J. Pharmacol. 2021, 912, 174582. [Google Scholar] [CrossRef] [PubMed]
- Lerner, D.K.; Garvey, K.L.; Arrighi-Allisan, A.; Kominsky, E.; Filimonov, A.; Al-Awady, A.; Filip, P.; Liu, K.; Ninan, S.; Spock, T.; et al. Omega-3 Fatty Acid Supplementation for the Treatment of Persistent COVID-Related Olfactory Dysfunction. Am. J. Rhinol. Allergy 2023, 37, 531–540. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Badawi, A.M.; Mansour, H.F. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: Design, in-vitro characterization and clinical evaluation. Int. J. Pharm. 2021, 601, 120600. [Google Scholar] [CrossRef]
- Neta, F.I.; Fernandes, A.C.L.; Vale, A.J.M.; Pinheiro, F.I.; Cobucci, R.N.; Azevedo, E.P.; Guzen, F.P. Pathophysiology and possible treatments for olfactory-gustatory disorders in patients affected by COVID-19. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Nursyifa Fadiyah, N.; Megawati, G.; Erlangga Luftimas, D. Potential of Omega 3 Supplementation for Coronavirus Disease 2019 (COVID-19): A Scoping Review. Int. J. Gen. Med. 2022, 15, 3915–3922. [Google Scholar] [CrossRef]
- Rawson, N.E.; LaMantia, A.S. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Exp. Gerontol. 2007, 42, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, A. Effect of Intranasal Insulin on Olfactory Recovery in Patients with Hyposmia: A Randomized Clinical Trial. Otolaryngol. Head Neck Surg. 2018, 158, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Whitcroft, K.L.; Hummel, T. Clinical Diagnosis and Current Management Strategies for Olfactory Dysfunction: A Review. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 846–853. [Google Scholar] [CrossRef]
- Yan, C.H.; Rathor, A.; Krook, K.; Ma, Y.; Rotella, M.R.; Dodd, R.L.; Hwang, P.H.; Nayak, J.V.; Oyesiku, N.M.; DelGaudio, J.M.; et al. Effect of Omega-3 Supplementation in Patients With Smell Dysfunction Following Endoscopic Sellar and Parasellar Tumor Resection: A Multicenter Prospective Randomized Controlled Trial. Neurosurgery 2020, 87, E91–E98. [Google Scholar] [CrossRef]
- Winn, P.Z.; Hlaing, T.; Tun, K.M.; Lei, S.L. Effect of any form of steroids in comparison with that of other medications on the duration of olfactory dysfunction in patients with COVID-19: A systematic review of randomized trials and quasi-experimental studies. PLoS ONE 2023, 18, e0288285. [Google Scholar] [CrossRef]
- Chung, T.W.; Zhang, H.; Wong, F.K.; Sridhar, S.; Lee, T.M.; Leung, G.K.; Chan, K.H.; Lau, K.K.; Tam, A.R.; Ho, D.T.; et al. A Pilot Study of Short-Course Oral Vitamin A and Aerosolised Diffuser Olfactory Training for the Treatment of Smell Loss in Long COVID. Brain Sci. 2023, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Patel, Z.M. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int. Forum Allergy Rhinol. 2018, 8, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Pendolino, A.L.; Ottaviano, G.; Nijim, J.; Scarpa, B.; De Lucia, G.; Berro, C.; Nicolai, P.; Andrews, P.J. A multicenter real-life study to determine the efficacy of corticosteroids and olfactory training in improving persistent COVID-19-related olfactory dysfunction. Laryngoscope Investig. Otolaryngol. 2022, 8, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Azar, C.; Goektas, O. Treatment of Olfactory Disorders After SARS—CoViD 2 Virus Infection. Ear. Nose Throat J. 2023, 1–6. [Google Scholar] [CrossRef]
- Gupta, S.; Lee, J.J.; Perrin, A.; Khan, A.; Smith, H.J.; Farrell, N.; Kallogjeri, D.; Piccirillo, J.F. Efficacy and Safety of Saline Nasal Irrigation Plus Theophylline for Treatment of COVID-19-Related Olfactory Dysfunction: The SCENT2 Phase 2 Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A.; Ahmed, M.A.A. The effectiveness of cerebrolysin, a multi-modal neurotrophic factor, for treatment of post-COVID-19 persistent olfactory, gustatory and trigeminal chemosensory dysfunctions: A randomized clinical trial. Expert Rev. Clin. Pharmacol. 2023, 16, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Henkin, R.I. Comparative monitoring of oral theophylline treatment in blood serum, saliva, and nasal mucus. Ther. Drug Monit. 2012, 34, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Ghosh, S.; Guan, C.; Fries, B.L.; Burke, F.W. Acute on Chronic Theophylline Toxicity in an Elderly Patient. Cureus 2021, 13, e13484. [Google Scholar] [PubMed]
- Riccardi, G.; Niccolini, G.F.; Bellizzi, M.G.; Fiore, M.; Minni, A.; Barbato, C. Post-COVID-19 Anosmia and Therapies: Stay Tuned for New Drugs to Sniff Out. Diseases 2023, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Liebell, D. Part Two: Perspectives on Olfactory and Gustatory Dysfunction Pathophysiology, Management, and Relevance to COVID-19: Current Approaches. Altern. Ther. Health Med. 2023, 29, 30–35. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonini, L.; Frijia, F.; Ait Ali, L.; Foffa, I.; Vecoli, C.; De Gori, C.; De Cori, S.; Baroni, M.; Aquaro, G.D.; Maremmani, C.; et al. A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings. Diagnostics 2024, 14, 359. https://doi.org/10.3390/diagnostics14040359
Simonini L, Frijia F, Ait Ali L, Foffa I, Vecoli C, De Gori C, De Cori S, Baroni M, Aquaro GD, Maremmani C, et al. A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings. Diagnostics. 2024; 14(4):359. https://doi.org/10.3390/diagnostics14040359
Chicago/Turabian StyleSimonini, Ludovica, Francesca Frijia, Lamia Ait Ali, Ilenia Foffa, Cecilia Vecoli, Carmelo De Gori, Sara De Cori, Monica Baroni, Giovanni Donato Aquaro, Carlo Maremmani, and et al. 2024. "A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings" Diagnostics 14, no. 4: 359. https://doi.org/10.3390/diagnostics14040359
APA StyleSimonini, L., Frijia, F., Ait Ali, L., Foffa, I., Vecoli, C., De Gori, C., De Cori, S., Baroni, M., Aquaro, G. D., Maremmani, C., & Lombardo, F. (2024). A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings. Diagnostics, 14(4), 359. https://doi.org/10.3390/diagnostics14040359






