Abstract
It has been shown that patients with NYHA class I and II have a high morbidity and mortality burden. We investigated the value of a new tissue Doppler index, E/(e′ × s′), to predict cardiac events in the long-term follow-up of patients at an early stage of heart failure (HF). Sequential echocardiography was conducted on a consecutive cohort of 212 hospitalized HF patients, pre-discharged and with three-month follow-up. The primary end point consisted of cardiac death or readmission due to HF worsening. During follow-up, cardiac events occurred in 99 patients (46.7%). The first cardiac event was represented by cardiac death in 8 patients (3.8%) and readmission for HF in 91 patients (42.9%). A Kaplan–Meier analysis did not show a significantly different event-free survival rate between patients with NYHA class I and II. The composite end point was significantly higher in patients with an E/(e′ × s′) >1.6. The E/(e′ × s′) at discharge was the best independent predictor of cardiac events. Those exhibiting an E/(e′ × s′) > 1.6 at discharge, with a subsequent deterioration after three months, displayed the poorest prognosis concerning cardiac events, HF-related rehospitalization, and cardiac mortality (all p < 0.05). In early-stage HF patients, an E/(e′ × s′) > 1.6 emerged as a robust predictor of clinical outcomes, especially when coupled with a deterioration in condition.
1. Introduction
Heart failure (HF) is a chronic disease with a similar, if not worse, prognosis than other serious and life-threatening conditions, such as chronic kidney disease or cancer [1,2]. Despite the development of novel drugs and devices responsible for significant improvements in patient survival, the mortality rate remains high, with one in three patients dying within 1 year of hospitalization for HF and 40–50% within 5 years of diagnosis [1,2,3]. The poor outcome associated with left ventricular (LV) dysfunction results in the need to obtain prognostic information as soon as possible.
In daily practice, patients with HF are divided into functional classes based on the New York Heart Association (NYHA) classification. This symptomatic classification has been a major entry criterion for the clinical trials that support current HF treatment guidelines. It has been shown that patients with HF have increased mortality rates, irrespective of symptomatology and LV ejection fraction (EF). Providers may falsely believe that patients with milder symptoms have low morbidity and mortality, and those patients with advanced disease may be beyond help [3]. Patients with HF with preserved LVEFs and signs of an elevated left atrial (LA) pressure on echocardiography potentially have an increased risk of death or HF rehospitalization [4,5]. Even patients at asymptomatic early stages of HF show evidence of ongoing adaptive and maladaptive pathways [1,6]. Accordingly, patients with NYHA class I or II still have a relatively high morbidity and mortality burden. In a sub-analysis of the Digitalis Investigational Group trial, when 1863 subjects with NYHA I and II were matched to the same number of subjects with NYHA III and IV, the mortality rates were 34% versus 42%, and all-cause hospitalizations were 66% versus 71%, respectively [7]. Patients at an early stage of HF are ideal targets for complication prevention.
The entire echocardiographic evaluation of a patient referred with suspected HF must include a thorough assessment of LV function. All modalities, including tissue Doppler imaging (TDI) and conventional echocardiography, offer diagnostic hints [8]. As the most accurate echocardiographic predictor in an LV filling pressure assessment in various clinical contexts, the ratio of the early diastolic velocity of mitral annular motion (e′) to the early diastolic velocity of mitral inflow (E) has been proposed [9,10]. This ratio shows a continuous progression with increasing mean pulmonary capillary wedge pressure.
The analysis of the LV long-axis function demonstrates valuable additive information for a noninvasive assessment of HF prognosis [11]. Several years ago, our group proposed a new TDI index, E/(e′ × s′), that associates a marker of diastolic function (E/e′) and a parameter that explores LV systolic performance (systolic mitral annular velocity, s′) to assess the LV filling pressure [12]. We identified that a value > 1.6 for E/(e′ × s′) can be a good predictor of clinical outcome in patients with HF [13].
Our purpose has been to investigate the value of the E/(e′ × s′) ratio in predicting cardiac events in the long-term follow-up of patients at an early stage of HF (NYHA class I and II).
2. Materials and Methods
2.1. Study Population
Patients
We prospectively analyzed 500 consecutive patients with HF, in sinus rhythm, hospitalized in our clinic. Only patients with NYHA functional class I or II were selected for this study. Exclusion criteria were represented by inadequate echocardiographic images, symptoms or clinical and instrumental signs of acute coronary syndrome or myocarditis, coronary revascularization during follow-up, significant primary valvular heart disease (except mild forms not hemodynamically relevant), cardiac pacemaker/defibrillator, congenital heart disease, renal failure (serum creatinine > 1.3 mg/dL), malignant neoplasia, anemia (hemoglobin < 12 mg/dL in women and <13 mg/dL in men), and endocrine disorders (hypo- and hyperthyroidism, hyperaldosteronism). Pericardial disease, primary pulmonary hypertension, and aortic diseases were excluded based on echocardiography. The remaining 212 patients formed our study group. The study complied with the Declaration of Helsinki and was approved by the local research ethics committee. Patient group criteria are detailed in Table 1.

Table 1.
Inclusion and exclusion criteria in the patient group.
2.2. Echocardiography
Using Milwaukee, WI-based Vivid 9 General Electric equipment, echocardiography was performed within 24 h of hospital release after appropriate medical care. During the apical four-chamber and apical two-chamber views during ventricular end-systole (maximum LA size), the biplane area-length method was used to compute the LA volume, which was then indexed for body surface area. With the modified Simpson’s rule, LVEF was computed from apical two- and four-chamber images [14]. The mitral regurgitation’s regurgitant orifice area (ROA) and regurgitant volume (RV) were estimated [15]. With an apical four-chamber window and a 5 mm pulsed-sample Doppler volume placed in between the mitral valve tips, the transmitral flow patterns were recorded. During end-expiratory apnea, the E and late transmitral flow (A) were measured throughout the course of five cardiac cycles, and the average values were noted [10]. The global myocardial index (GMI) was calculated by dividing the ejection time by the sum of the isovolumic contraction and relaxation times [16]. In using continuous Doppler to measure the maximal regurgitant velocity at the tricuspid valve, the systolic pulmonary artery pressure (SPAP) was determined.
The TDI program was run in pulsed-wave Doppler mode, putting a 5 mm sample volume sequentially at the mitral annulus’ lateral and septal corners inside the apical four-chamber view [10]. During end-expiratory apnea, the peak e′ and s′ were recorded throughout the course of five consecutive cardiac cycles, and their averages were then calculated. The average velocities at both the septal and lateral locations were used to calculate E/e′ and E/(e′ × s′). Patients were divided into two groups after being discharged from the hospital according to their E/(e′ × s′) values: Group I had E/(e′ × s′) ≤ 1.6, and Group II had E/(e′ × s′) >1.6. The TDI measurements were taken again 90 ± 10 days later, or around three months after the hospital discharge. E/(e′ × s′) worsening was described as a value higher than the discharge value that had been previously established.
An expert echocardiographer conducted each measurement. We looked at the variabilities between and among observers for E/e’, s’, and E/(e′ × s′). One observer conducted measurements in a group of thirty randomly chosen subjects twice; the other two investigators were in the dark about each other’s measurements and the research time point.
2.3. Clinical Variables Recorded
The prognostic model took into account the clinical characteristics that were noted upon hospital discharge, including age, sex, body mass index, heart rate, mean arterial pressures, etiology of heart failure, NYHA functional class, and N-terminal pro-brain natriuretic peptide (NT-proBNP) values. Additionally, the primary treatment classes’ prescriptions for HF were documented.
2.4. Clinical Outcome
Both a cardiac mortality and a hospital admission brought on by worsening HF were designated as a major event. Cardiac death included deaths that were directly related to heart conditions, such as congestive heart failure, or unexpected deaths. Electronic medical records and phone conversations with patients or their families were used to gather follow-up data. In this study, we aimed to present the 3-year follow-up outcomes of patients with acute heart failure admitted to the Institute of Cardiovascular Diseases, Timișoara.
2.5. Statistical Analysis
Numerical data were presented as the mean ± standard deviation for continuous variables, whereas proportions were represented as categorical variables. Continuous variables were compared through an independent sample t-test, while categorical variables were analyzed using the chi-square test. The prognostic factors that were shown to be strongly connected with cardiac events in a univariate analysis were explored using a multivariate approach using a Cox proportional hazard model that used a forward stepwise method. Heart event-free survival rates were computed using Kaplan–Meier analysis, and event rates were compared using log-rank testing. At the time of death, patients who died from non-cardiac reasons were censored, or deemed non-events. E/e′, s′, and E/(e′ × s′) intra-observer and inter-observer variabilities were calculated using the root-mean-square method to calculate the coefficient of variation (CV) and the intraclass correlation coefficient. All statistical tests assumed a significance level of 0.05. Statistical analyses were conducted using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA).
3. Results
The current study included 212 consecutive, hospitalized patients, with HF and NYHA class I or II, in sinus rhythm (61 ± 11.4 years; 64 women). The mean LVEF was 46 ± 14.3%. The underlying causes of HF were attributed to coronary artery disease in 148 patients, non-ischemic cardiomyopathy in 34 patients, or systemic hypertension in 30 patients. All patients had recordable mitral annular velocities at both sites. Patient group characteristics are detailed in Table 2. Notably, Group II patients exhibited significantly elevated SPAP and NT-proBNP levels, along with larger LA and LV dimensions. Additionally, they demonstrated higher values for E, the E/A ratio, and the E/e′ ratio, while displaying a lower E-deceleration time, LVEF, e′, and s′. Factors such as age, sex, heart rate, body mass index, NYHA functional class, RV, ROA, and GMI showed no notable differences between the groups.

Table 2.
Baseline characteristics of the study groups. A = late transmitral flow velocity; ACEI = angiotensin-converting enzyme inhibitor; E = early diastolic transmitral flow velocity; e′ = early mitral annular diastolic velocity; LV = left ventricle; NT-proBNP = N-terminal pro-brain natriuretic peptide; s′ = systolic velocity of mitral annulus.
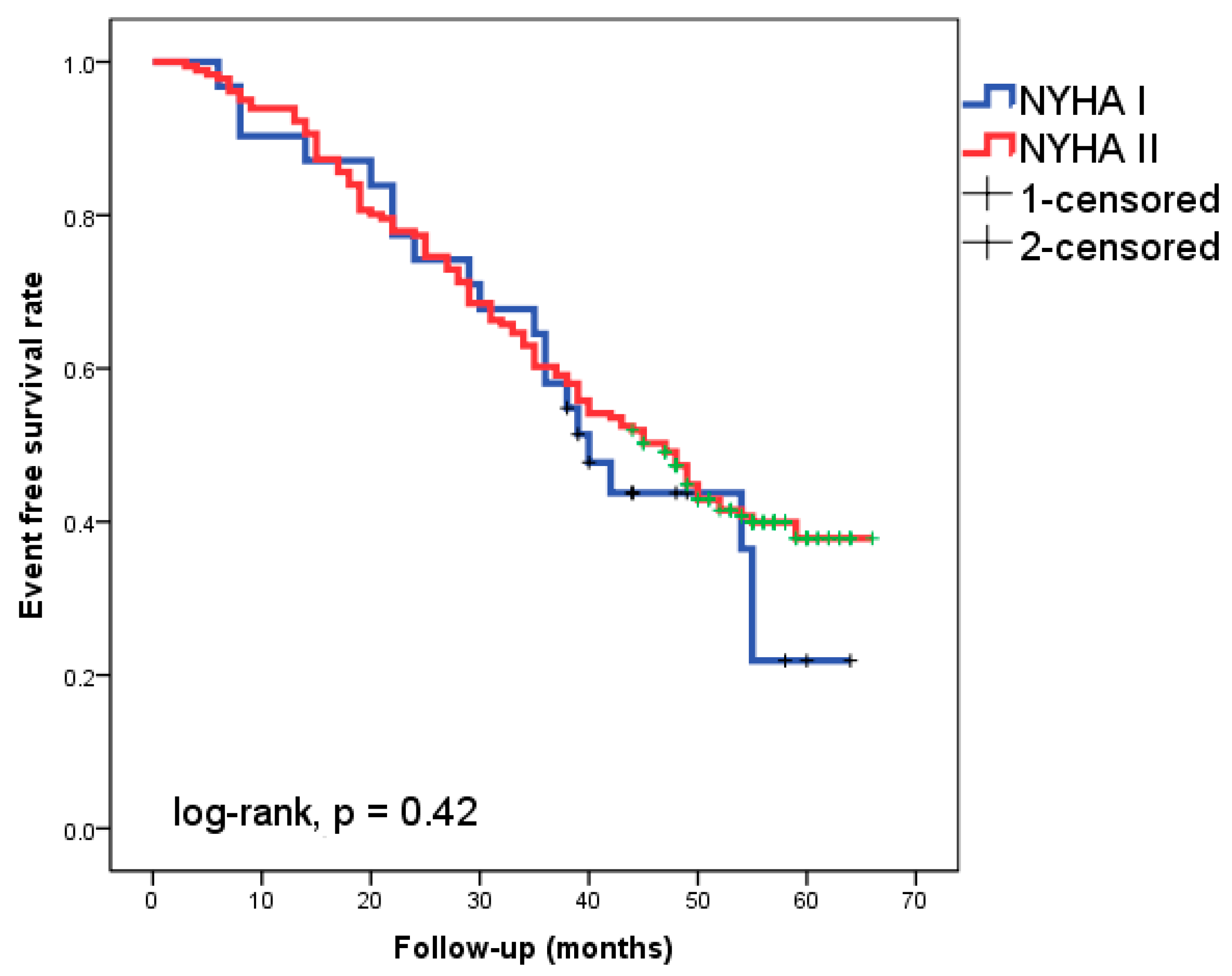
Of the patients followed-up with for 49 ± 11 months, 46.7% experienced cardiac events. Eight patients (3.8%) experienced cardiac death as the initial cardiac episode, while 91 patients (42.9%) experienced HF readmission. The Kaplan–Meier analysis did not show a significantly different event-free survival rate between patients with NYHA class I and II, respectively (Figure 1, log-rank, p = 0.42).
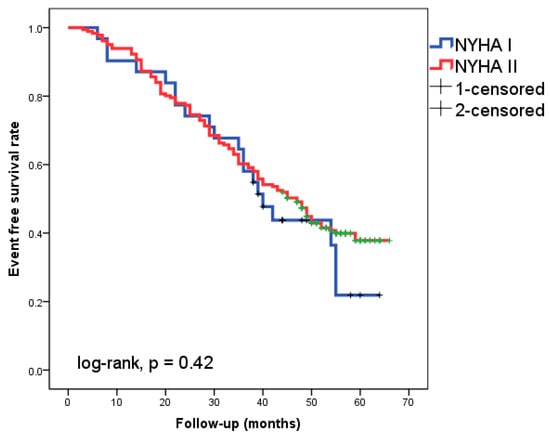
Figure 1.
Kaplan–Meier survival curves in the 212 patients with heart failure according to New York Heart Association (NYHA) functional class I or II.
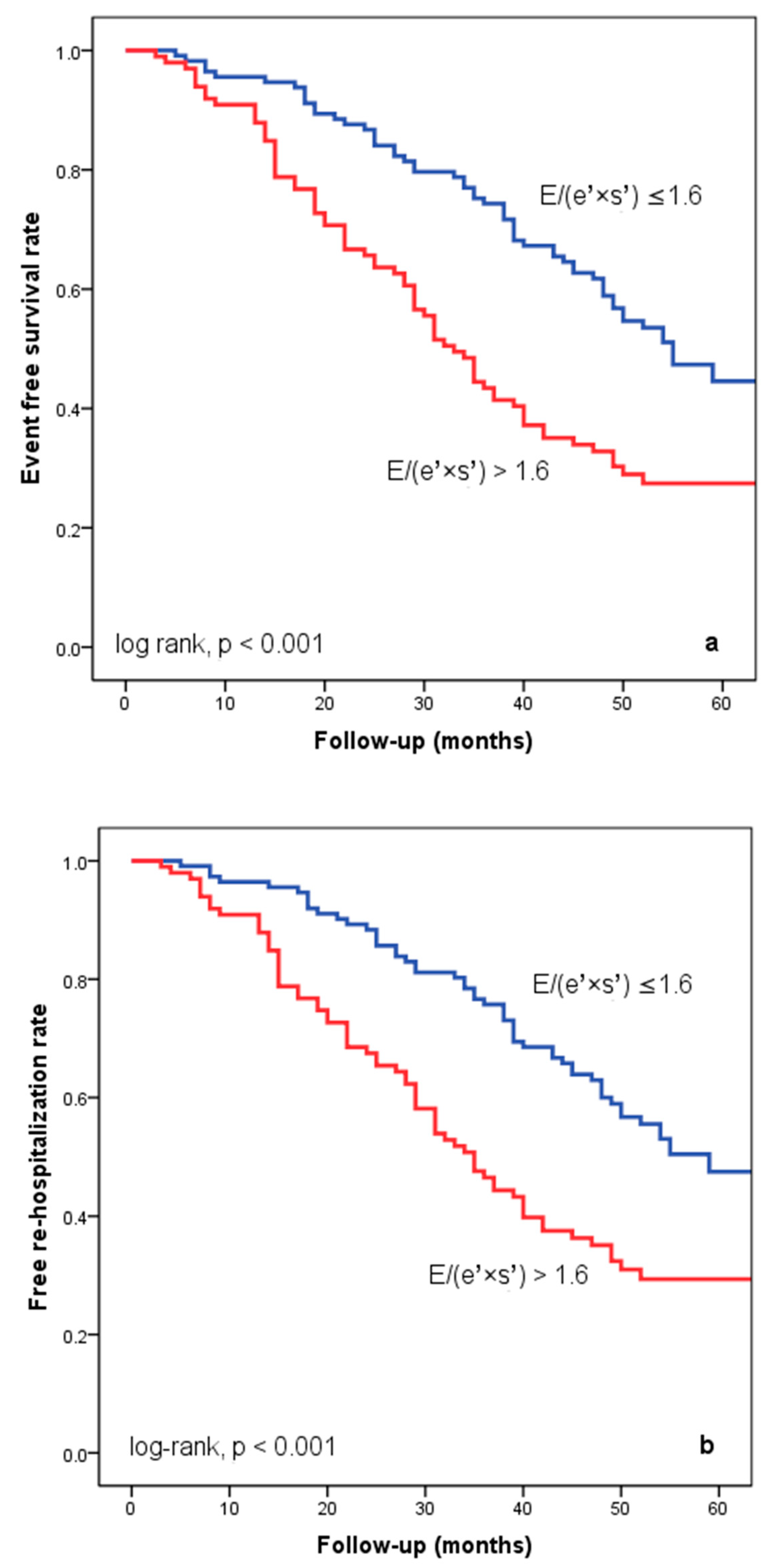
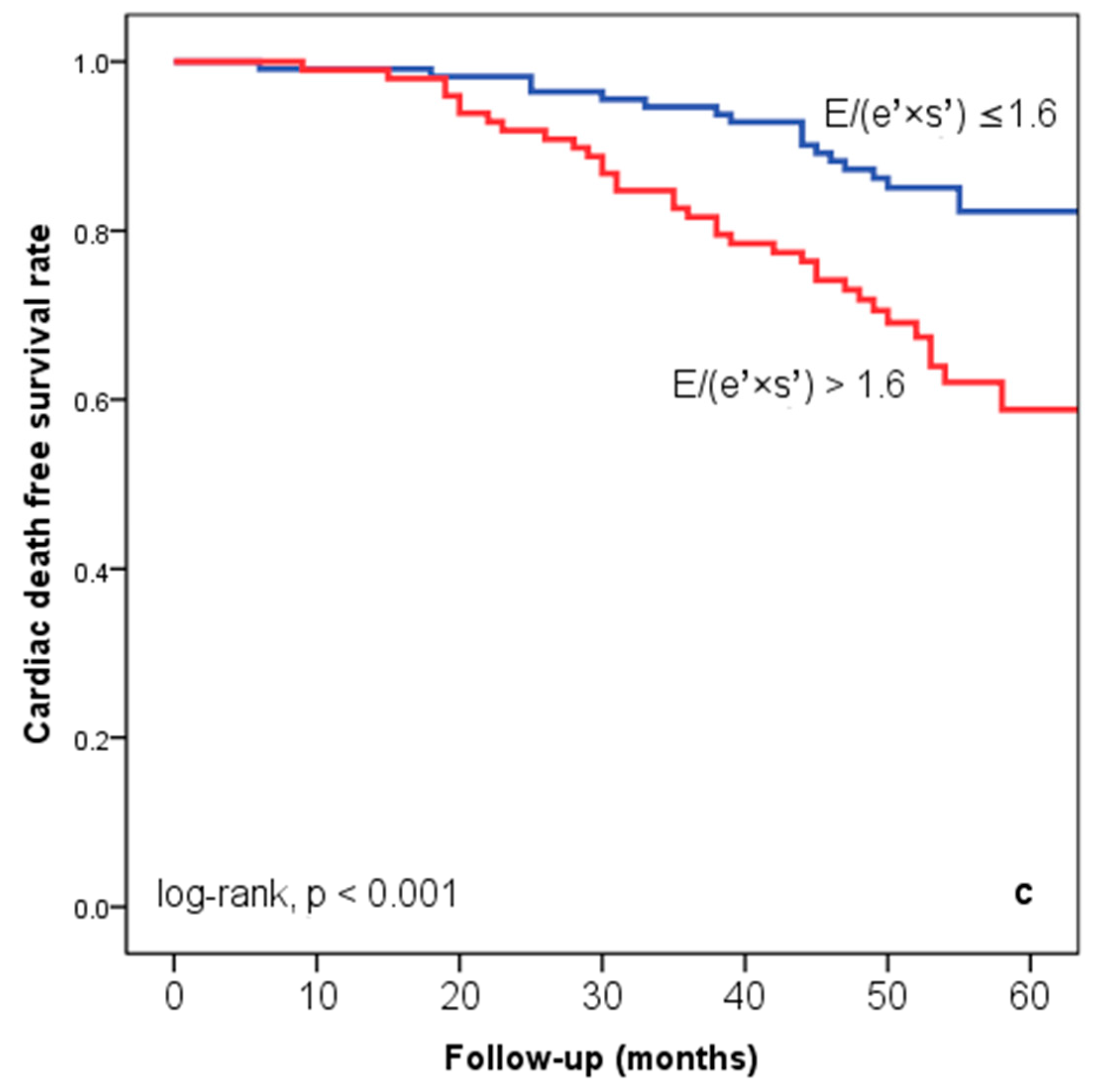
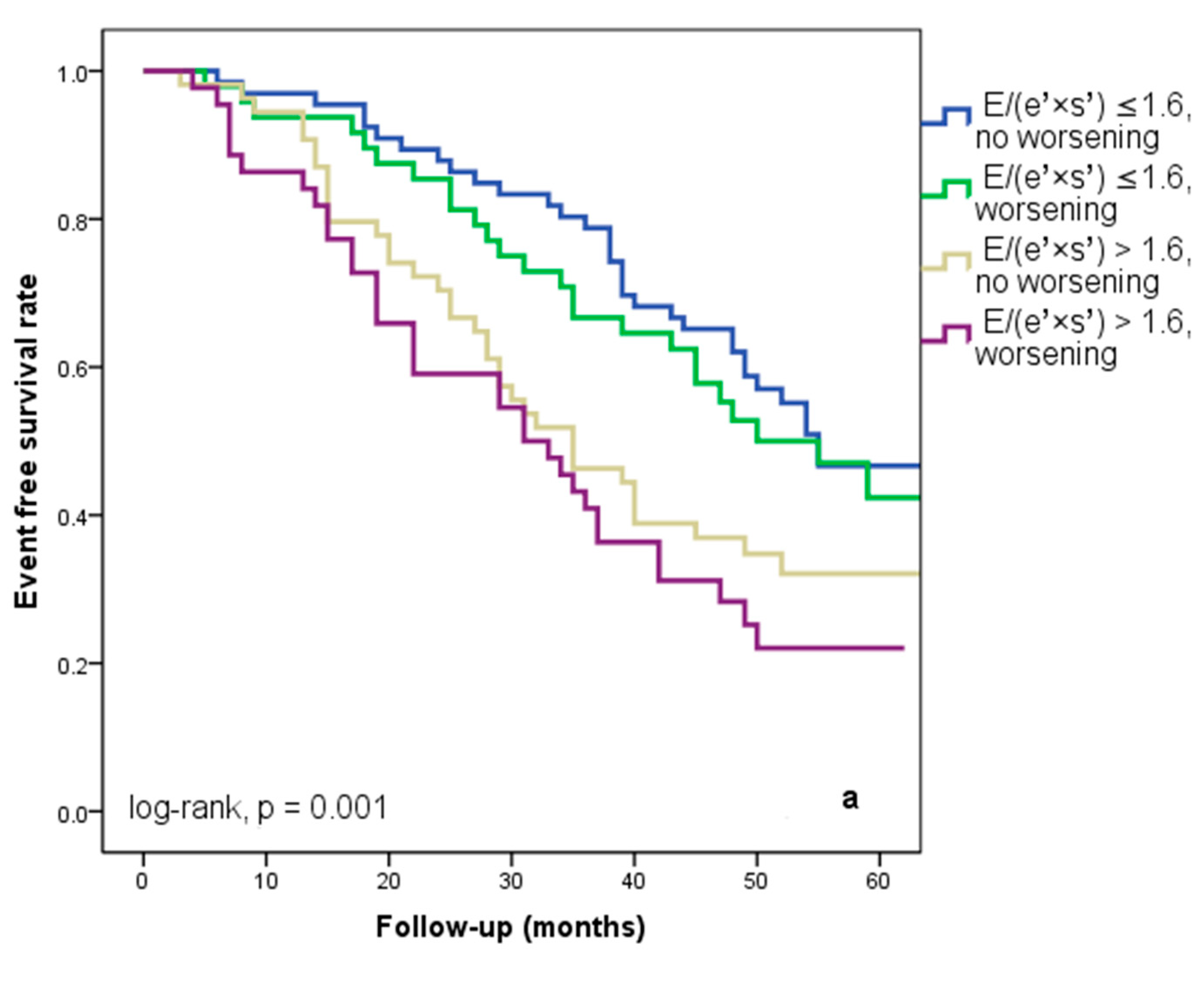
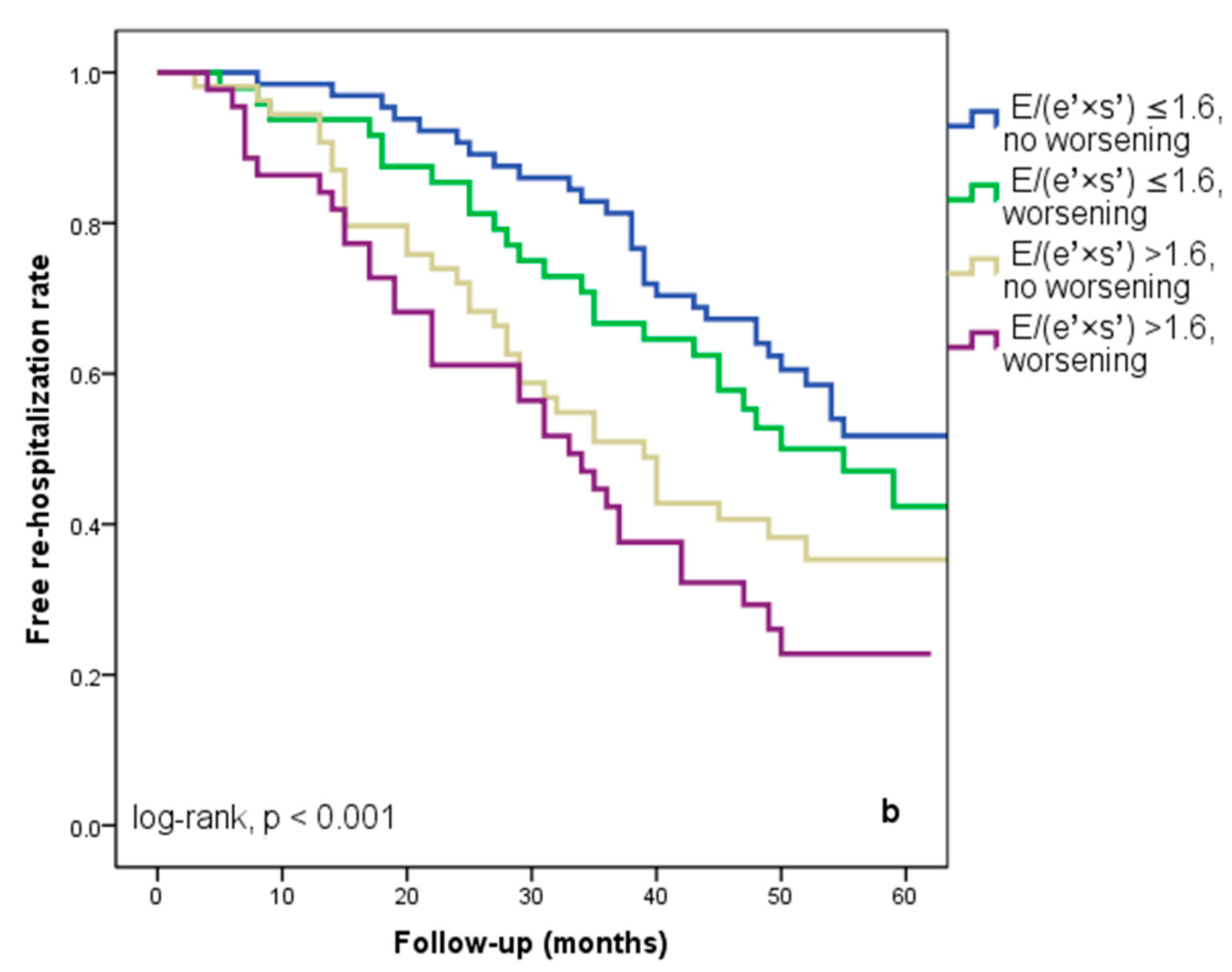
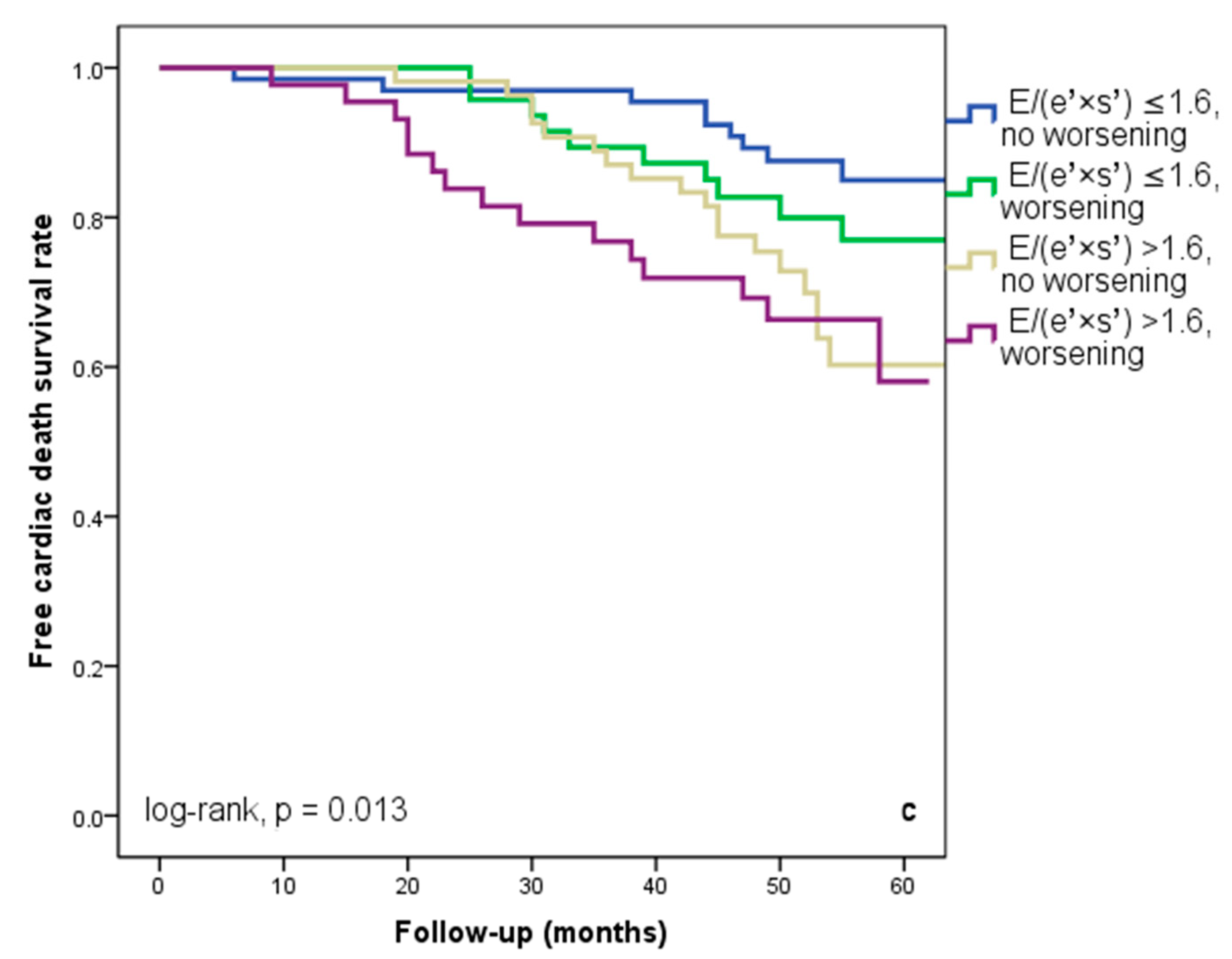
At hospital release, Group II’s mean E/(e′ × s′) was 2.8 ± 0.95, whereas Group I’s mean E/(e′ × s′) was 0.91 ± 0.35 (p < 0.001). Group II had significantly more occurrences of the composite endpoint (79 events, 70.7% versus 57 events, 50.4%; p < 0.001) than Group I. The group exhibiting E/(e′ × s′) > 1.6 (log-rank, p < 0.001) had a significantly lower cardiac event-free survival rate over the follow-up period, according to the Kaplan–Meier analysis. (Figure 2a). The same group had higher re-hospitalization and cardiac death rates (Figure 2b,c). The cohort patient group had 76 patients with HF with a reduced EF (LVEF ≤ 40%) (24 patients with functional class NYHA I, and 112, NYHA II) and 136 patients with an LVEF > 40% (7 patients with NYHA I, and 69, NYHA II) (p = 0.108). Irrespective of the LVEF value, an index > 1.6 was associated with an unfavorable prognosis (Figure 3).
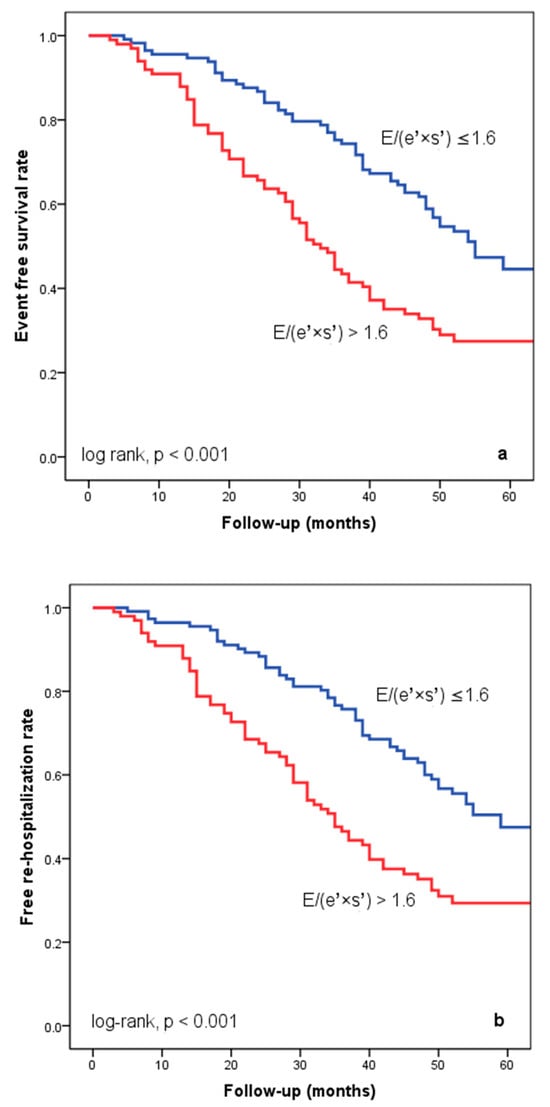
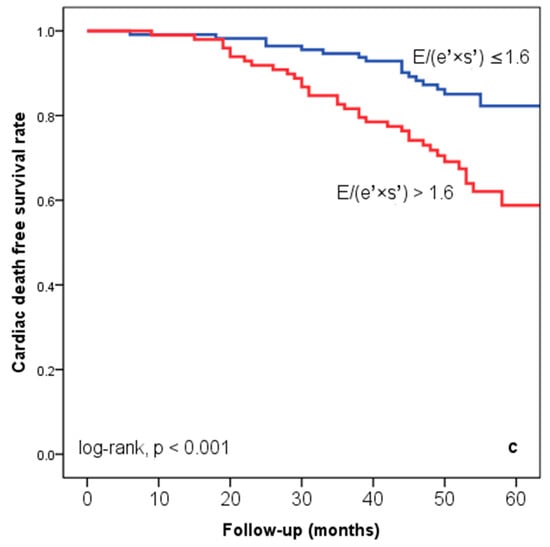
Figure 2.
Kaplan–Meier event-free survival curves for cardiac events (a), re-hospitalization (b), and cardiac death (c) in patients at an early stage of heart failure based on the E/(e′ × s′) ratio at hospital discharge, with values either below or above 1.6. E represents early diastolic transmitral velocity, e′ signifies early mitral annular diastolic velocity, and s′ denotes systolic mitral annular velocity.
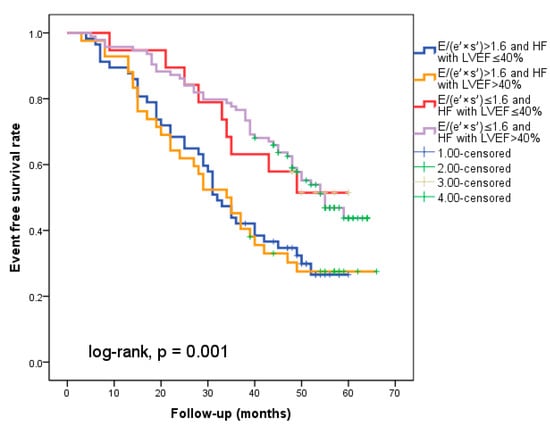
Figure 3.
The figure shows that regardless of the type of heart failure (with reduced EF (</=40%) or over 40%), an index over 1.6 indicates a poor prognosis.
The variables that showed predictive value for cardiac events (p < 0.05) in univariate Cox regression analysis are listed in Table 3: LA volume, SPAP, E, E/A ratio, E/e′, s′, and E/(e′ × s′) are among the NT-proBNP levels. On the other hand, the univariate analysis revealed that cardiac events were not significantly correlated with age, sex, heart rate, blood pressure, NYHA class, aetiology of HF (coronary artery disease, etc.), treatment, indexed LA volume, LVEF, LV end-diastolic volume index, LV end-systolic volume index, E-deceleration time, A, e′, RV, or ROA. Subsequently, a multivariate analysis was conducted using univariate important predictors to track the occurrence of cardiac events. The best independent predictor at 36 months of follow-up was found to be the E/(e′ × s′) prior to discharge (HR = 1.55, 95%, CI = 1.100–1.202, p = 0.012).

Table 3.
Clinical, laboratory, and echocardiographic variables at hospital discharge associated with cardiac events in the Cox univariate and multivariate analysis. A = late diastolic transmitral velocity; CI = confidence interval; E = early diastolic transmitral velocity; e′ = mitral annular diastolic velocity; HR = hazard ratio; LVEF = left ventricular ejection fraction; s′ = systolic velocity of mitral annulus; NA = not applicable; NT-proBNP = N-terminal pro-brain natriuretic peptide; SPAP = systolic pulmonary artery pressure.
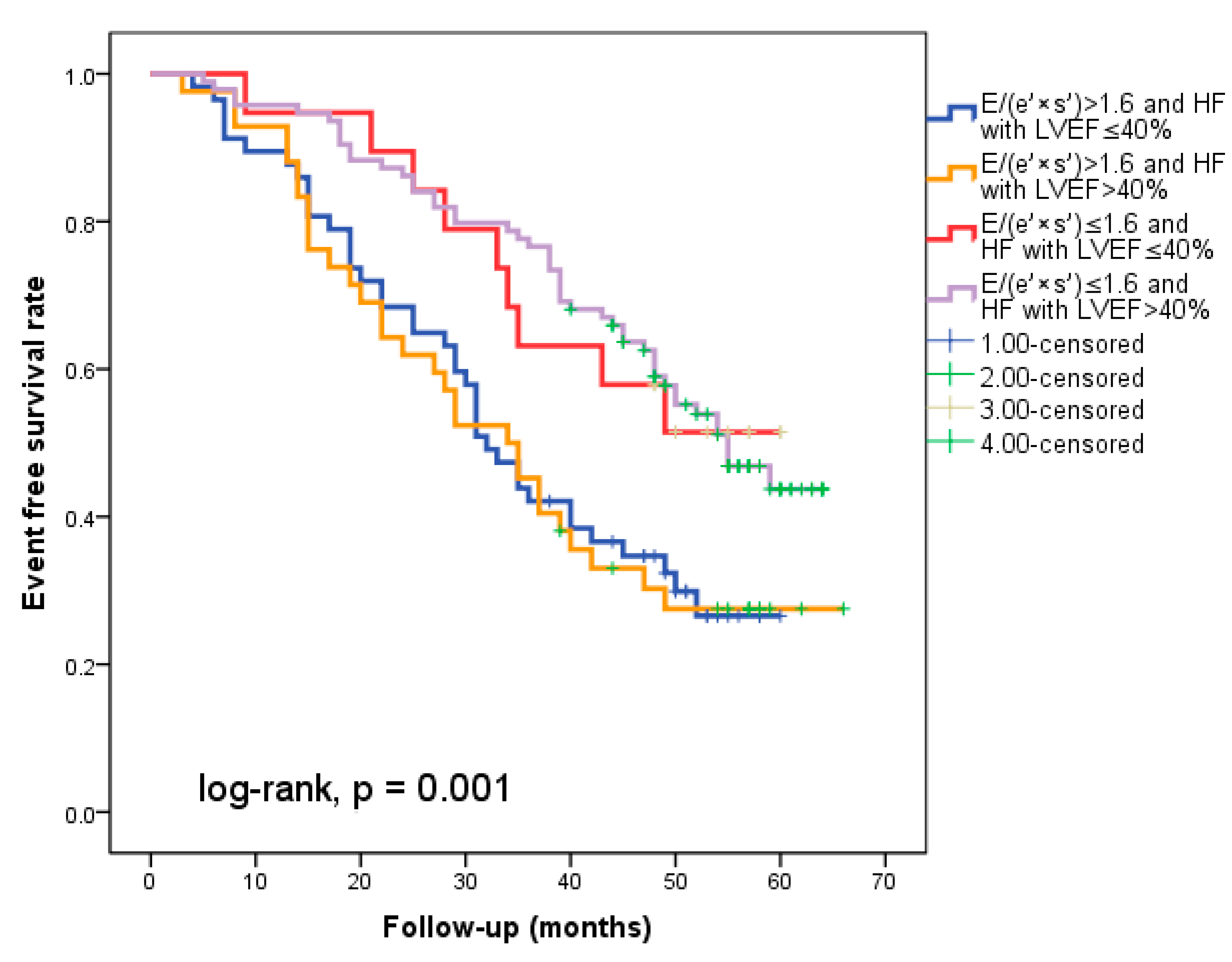
We found that 92 patients (43.3%) worsened in their E/(e′ × s′) ratio three months after being discharged from the hospital. Out of these individuals, 44 (20.7%) had an initial E/(e′ × s′) value that was higher than 1.6. Regardless of the E/(e′ × s′) value at study inclusion, as Figure 3a illustrates, E/(e′ × s′) worsening was linked to a lower cardiac event-free survival rate (25% versus 33.3% in patients with the initial E/(e′ × s′) > 1.6 and 47% versus 50% in those with E/(e′ × s′) ≤ 1.6 at hospital discharge, log-rank, p = 0.001). The patients who initially had an E/(e′ × s′) ratio greater than 1.6 and whose ratio continued to worsen after three months were the subgroup with the worst prognosis for cardiac events, HF rehospitalizations, and cardiac fatalities (Figure 4a–c). In total, 52 patients, or 24.5% of the sample, experienced cardiac-related deaths throughout the course of the follow-up period. Group I and Group II had comparable rates of non-cardiac death [4 (3.5%) vs. 3 (3.03%), p = 0.72].
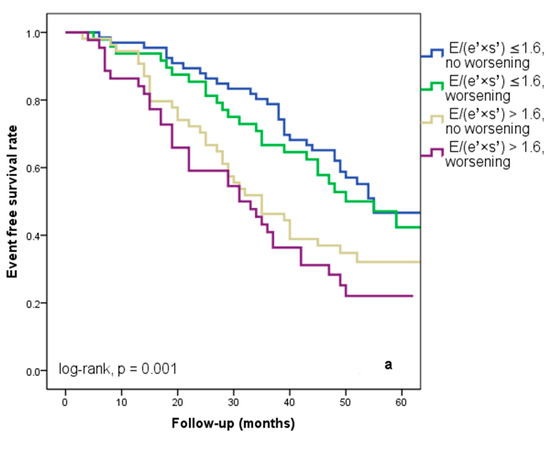
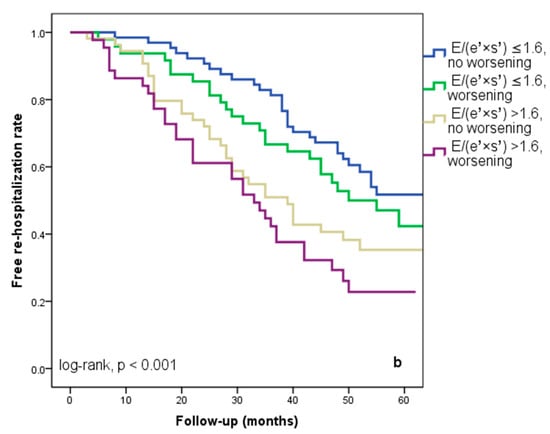
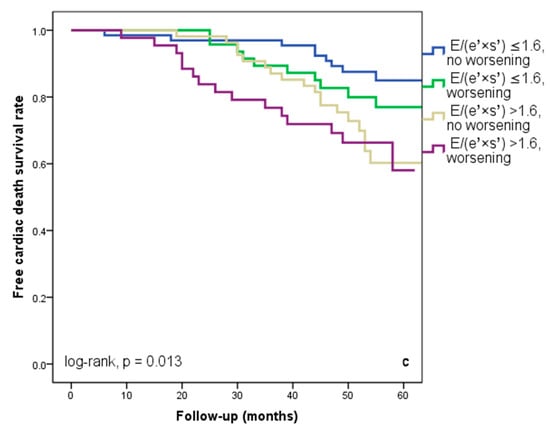
Figure 4.
Kaplan–Meier event-free survival curves for cardiac events (a), re-hospitalization (b), and cardiac death (c) in patients at an early stage of heart failure according to the initial E/(e′ × s′) ratio at hospital discharge (above or below 1.6) and worsening or no worsening after three months. E represents early diastolic transmitral velocity, e’ signifies early mitral annular diastolic velocity, and s’ denotes systolic mitral annular velocity.
The E/e′, s′, and E/(e′ × s′) had intra-observer intraclass coefficients of 0.94 (CV 2.7%), 0.95 (CV 3%), and 0.93 (CV 3.2%), in that order. Similarly, for the E/e′, s′, and E/(e′ × s′), the inter-observer intraclass coefficients were 0.91 (CV 2.9%), 0.92 (CV 3.2%), and 0.90 (CV 3.1%), correspondingly.
4. Discussion
In the present study, we analyzed the impact of the E/(e′ × s′) index to predict cardiac events, the worsening of HF, and cardiac death in HF patients with NYHA class I or II, in sinus rhythm. An E/(e′ × s′) ratio >1.6 emerged as a robust predictor of forthcoming cardiac events, surpassing various other echocardiographic parameters: NYHA functional class, coronary artery disease, and NT-proBNP levels. Its predictive power notably intensifies when linked with deterioration three months later.
Cardio-vascular imaging, especially echocardiography, is essential for the diagnosis and treatment of heart failure [2]. Because early treatment can positively influence this progression, the early diagnosis of LV dysfunction is essential. Previous studies have demonstrated the significant prognostic significance of the LVEF, LA size, and LV volume indices for outcomes in patients with heart failure [17,18,19]. However, after conducting multivariate analyses, variables that had been initially found in univariate analysis as outcome predictors—like the SPAP, LA volume, E, and E/A ratio—were eliminated from the study. The better performances of the TDI parameters may be partially explained by the dependence of mitral flow on variables such as age, LA pressure, volemic state, and myocardial relaxation [4].
The use of TDI, which is compatible with echocardiography equipment from many manufacturers, reduces the demand to trace endocardial contours, which is necessary for LV volumes and LVEF assessment [17]. More precise and sophisticated measurements are required in clinical trials and to guide treatment for individual patients. Several studies have demonstrated that the e′ velocity and E/e′ ratio are highly predictive of adverse events following acute myocardial infarction, and in patients with LV dysfunction, it was recently demonstrated that abnormal LV diastolic function is missed by using the reductive index E/e′ alone [4,8,20,21,22,23]. Furthermore, Sharifov et al. recently showed that there is insufficient data to substantiate the claim that E/e′ can accurately assess the LV filling pressure in patients with maintained LVEFs, which is commonly found in patients classified as NYHA class I or II [24]. This may be explained in part by the similar preload sensitivity of E and e’ so that their ratio does not change [8].
One of the main causes of the development of HF is diastolic impairment combined with systolic dysfunction. The LV long-axis function analysis provided insightful supplementary data for the noninvasive evaluation of the HF prognosis. However, the TDI enables a modern assessment of the s′ wave, or longitudinal systolic function, which is impacted differently and at distinct phases in HF [8,25]. As these interact with the prognosis, Biering-Sørensen et al. recommended evaluating TDI velocities (e′ and s′) jointly [26]. Independent of traditional echocardiographic criteria, a pattern of low systolic and diastolic performances as determined by the TDI is a critical sign of an unfavorable prognosis. Our research showed that the E/(e′ × s′) ratio—which combines an LV systolic performance marker (s′) with an HF diastolic indicator (E/e′)—proved to be a more useful metric for prognostic evaluation in an unselected HF sample [10]. Hirata et al. observed that patients with higher-risk heart failure who were at risk of readmission and cardiac death could be identified using a composite index that included an LVEF ≤ 40% and E/e′ratio > 15 [27]. Given the reduced ability of s′ to detect LV dysfunction in people with a maintained LVEF, the E/(e′ × s′) index emerges as a more robust metric [13].
Participants with NYHA I and II were matched to the same number of participants with NYHA III and IV in a sub-analysis of the Digitalis Investigational Group study. The results show that the death rates were 34% against 42%, and the all-cause hospitalization rates were 66% compared to 71%, respectively [7]. Additionally, this highlights the notably poor results for those who are purportedly less sick, suggesting that all HF patients require an equally rigorous course of treatment. There is still a comparatively high burden of morbidity and mortality among patients with NYHA class I and II. In practical practice, prognosis prediction helps identify the subset of high-risk patients who could benefit from more intensive heart failure treatment [28]. Early treatment can have a positive impact on the progression of HF. After comparing the E/(e′ × s′) index to numerous other clinical and paraclinical characteristics, especially when linked to its worsening three months later, we found that it was the strongest predictor of cardiac events, the worsening of HF, and cardiac death in NYHA class I or II.
The study group exhibited a high prevalence of coronary artery disease, raising the possibility of ischemic events contributing to the observed cardiac events [2]. Severe myocardial ischemia cause complex changes defined as LV remodeling that affect the ventricular functions and prognosis [22,29]. Biering-Sørensen et al. investigated the color Doppler parameters in patients treated with primary coronary angioplasty and found that the s’ and e’ values predict adverse cardiac events. Another small study on people with ischemic HF showed that 19% and 60% of patients with NYHA I or II, respectively, had dysfunctional but viable myocardium [26,30]. Within our study, the presence of coronary artery disease did not emerge as a predictor for cardiac events.
The severity of stable chronic heart failure as indicated by the NYHA functional classification is reflected in the NT-proBNP levels. A favorable prognosis in the medium term is predicted by high levels of NT-proBNP, while low levels are linked to an excellent prognosis [2,9]. After receiving the necessary medical care, we carried out NT-proBNP determination and echocardiography. Our data’s statistical analysis confirms that while NT-proBNP has predictive value, it is not as good as E/(e′ × s′). In a similar vein, Lim et al. showed that an aberrant echocardiography was more sensitive than plasmatic NT-proBNP levels at predicting outcomes [31].
A few limitations should be taken into account when interpreting our results. Despite the relatively small sample size in this study, we were nonetheless able to make several important findings. One drawback of the NYHA categorization, which is currently based on a physician’s experience, is its lack of consistency and accuracy. Previous studies [1] have revealed low concordance (only 54–56%) between two cardiologists when asked to characterize the NYHA class of patients with mild to moderate symptoms. One may argue that the study’s use of standard echocardiographic assessments rather than more advanced techniques (such as strain imaging) is both a drawback and a strength. The drawback is that sub-clinical impairments of both systolic and diastolic function have been found to be more sensitively detected via strain imaging. Our parameter’s strength lies in its simplicity of use, which is a result of its inclusion in the majority of contemporary echo machines. This allows it to be easily applied for patient bedside assessments. We measured only the TDI at the medial and lateral mitral annulus, and we neglected to look at the anterior and posterior velocities, which could have revealed more details. The study center functioned as a tertiary invasive center, and therefore, the study population may not reflect a general population of patients with HF.
5. Conclusions
According to our findings, the TDI-derived index E/(e′ × s′) among HF patients in NYHA class I or II in sinus rhythm is a substantial independent long-term predictor of cardiac death or HF-related hospitalization. When paired with its worsening three months later, an E/(e′ × s′) ratio > 1.6 can specifically identify high-risk patients who are susceptible to cardiac events. It is crucial to remember that our study was limited to one center; therefore, it would be more credible if it were validated at other centers or through multicenter trials.
Author Contributions
Conceptualization, C.M.; methodology, C.M. and M.A.L.; validation, T.H., C.N.D., C.T.L. and S.A.P.; formal analysis, S.C. and C.M.; data curation, I.I. and S.C.; writing—original draft preparation, I.I.; writing—review and editing, C.M., T.H. and M.A.L.; visualization, C.T.L.; supervision, C.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Romanian Academy of Medical Sciences and European Regional Development grant number [2/Axa 1/31.07.2017/SMIS 107124] and The APC was funded by [“Victor Babes” University of Medicine and Pharmacy, No. 2 Eftimie Murgu Square, 300041 Timisoara].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Timisoara Institute of Cardiovascular Diseases (1729/02.April.2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the result.
References
- Papadimitriou, L.; Moore, C.K.; Butler, J.; Long, R.C. The Limitations of Symptom-based Heart Failure Management. Card. Fail. Rev. 2019, 5, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Gheorghiade, M.; Metra, M. Moving away from symptoms-based heart failure treatment: Misperceptionsand real risks for patients with heart failure. Eur. J. Heart Fail. 2016, 18, 350–352. [Google Scholar] [CrossRef]
- Hubert, A.; Taconne, M.; Popescu, B.A.; Donal, E. Diastolic function and its non-invasive assessment. The quest for the holy grail continues. Int. J. Cardiol. 2023, 382, 96–97. [Google Scholar] [CrossRef]
- Kapłon-Cieślicka, A.; Laroche, C.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Maggioni, A.P.; Hage, C.; Lara-Padrón, A.; Fucili, A.; et al. Is heart failure misdiagnosed in hospitalized patients with preserved ejection fraction? From the European Society of Cardiology-Heart Failure Association EURObservational Research Programme Heart Failure Long-Term Registry. ESC Heart Fail. 2020, 7, 2098–2112. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Ahmed, A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am. J. Cardiol. 2007, 99, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Sunderji, I.; Singh, V.; Fraser, A.G. When does the E/e′ index not work? The pitfalls of oversimplifying diastolic function. Echocardiography 2020, 37, 1897–1907. [Google Scholar] [CrossRef]
- Hinderliter, A.L.; Blumenthal, J.A.; O’Conner, C.; Adams, K.F.; Dupree, C.S.; Waugh, R.A.; Bensimhon, D.; Christenson, R.H.; Sherwood, A. Independent prognostic value of echocardiography and N-terminal pro-B-type natriuretic peptide in patients with heart failure. Am. Heart J. 2008, 156, 1191–1195. [Google Scholar] [CrossRef][Green Version]
- Popescu, B.A.; Beladan, C.C.; Nagueh, S.F.; Smiseth, O.A. How to assess left ventricular filling pressures by echocardiography in clinical practice. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1127–1129. [Google Scholar] [CrossRef]
- Tschöpe, C.; Senni, M. Usefulness and clinical relevance of left ventricular global longitudinal systolic strain in patients with heart failure with preserved ejection fraction. Heart Fail. Rev. 2020, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mornos, C.; Cozma, D.; Rusinaru, D.; Ionac, A.; Maximov, D.; Petrescu, L.; Dragulescu, S.I. A novel index combining diastolic and systolic Tissue Doppler parameters for the non-invasive assessment of left ventricular end-diastolic pressure. Int. J. Cardiol. 2009, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mornos, C.; Petrescu, L.; Ionac, A.; Cozma, D. The prognostic value of a new tissue Doppler parameter in patients with heart failure. Int. J. Cardiovasc. Imaging 2014, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Chuwa, T.; Rodeheffer, R.J. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function: A study in normal and dilated cardiomyopathy. J. Cardiol. 1995, 26, 357–366. [Google Scholar]
- Nikitin, N.P.; Loh, P.H.; Silva, R.; Ghosh, J.; Khaleva, O.Y.; Goode, K.; Rigby, A.S.; Alamgir, F.; Clark, A.L.; Cleland, J.G.F. Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction. Heart 2006, 92, 775–779. [Google Scholar] [CrossRef]
- Leung, D.Y.; Boyd, A.; Ng, A.A.; Chi, C.; Thomas, L. Echocardiographic evaluation of left atrial size and function: Current understanding, pathophysiologic correlates, and prognostic implications. Am. Heart J. 2008, 156, 1056–1064. [Google Scholar] [CrossRef]
- Lim, T.K.; Dwivedi, G.; Hayat, S.; Majumar, S.; Senior, R. Independent value of left atrial volume index for the prediction of mortality in patients with suspected heart failure referred from the community. Heart 2009, 95, 1172–1178. [Google Scholar] [CrossRef]
- Bruch, C.; Klem, I.; Breithardt, G.; Wichter, T.; Gradaus, R. Diagnostic usefulness and prognostic implications of the mitral E/E′ ratio in patients with heart failure and severe secondary mitral regurgitation. Am. J. Cardiol. 2007, 100, 860–865. [Google Scholar] [CrossRef]
- Dokainish, H.; Zoghbi, W.A.; Lakkis, N.M.; Ambriz, E.; Patel, R.; Quinones, M.A.; Nagueh, S.F. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J. Am. Coll. Cardiol. 2005, 45, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, N.; Kimura, K.; Kosuge, M.; Tsukahara, K.; Hibi, K.; Ebina, T.; Saito, M.; Umemura, S. E/e′ two weeks after onset is a powerful predictor of cardiac death and heart failure in patients with a first-time ST elevation acute myocardial infarction. J. Am. Soc. Echocardiogr. 2012, 25, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Kim, H.; Son, J.; Yoon, H.J.; Park, H.S.; Cho, Y.K.; Han, C.D.; Nam, C.W.; Hur, S.H.; Kim, Y.N.; et al. Tissue Doppler imaging as a prognostic marker for cardiovascular events in heart failure with preserved ejection fraction and atrial fibrillation. J. Am. Soc. Echocardiogr. 2010, 23, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Sharifov, O.F.; Schiros, C.G.; Aban, I.; Denney, T.S.; Gupta, H. Diagnostic Accuracy of Tissue Doppler Index E/e′ for Evaluating Left Ventricular Filling Pressure and Diastolic Dysfunction/Heart Failure With Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002530, Erratum in: J. Am. Heart Assoc. 2016, 5, e002530. [Google Scholar] [CrossRef]
- Buzas, R.; Rogobete, A.F.; Popovici, S.E.; Mateescu, T.; Hoinoiu, T.; Sorop, V.B.; Bratu, T.; Ticlea, M.; Popoiu, C.M.; Sandesc, D. Nuclear Transcription Factor Kappa B (NF-κB) and Molecular Damage Mechanisms in Acute Cardiovascular Diseases. A Review. J. Cardiovasc. Emergencies 2018, 4, 65–72. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Jensen, J.S.; Pedersen, S.; Galatius, S.; Hoffmann, S.; Jensen, M.T.; Mogelvang, R. Doppler tissue imaging is an independent predictor of outcome in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Soc. Echocardiogr. 2014, 27, 258–267. [Google Scholar] [CrossRef]
- Hirata, K.; Hyodo, E.; Hozumi, T.; Kita, R.; Hirose, M.; Sakanoue, Y.; Nishida, Y.; Kawarabayashi, T.; Yoshiyama, M.; Yoshikawa, J.; et al. Usefulness of a combination of systolic function by left ventricular ejection fraction and diastolic function by E/E′ to predict prognosis in patients with heart failure. Am. J. Cardiol. 2009, 103, 1275–1279. [Google Scholar] [CrossRef]
- Şoşdean, R.; Mornoş, C.; Enache, B.; Macarie, R.I.; Ianoş, R.; Ştefea, A.M.; Pescariu, S. Safety and feasibility of biventricular devices reuse in general and elderly population—A single-center retrospective cohort study. Clin. Interv. Aging. 2015, 10, 1311–1318. [Google Scholar] [CrossRef]
- Kenar Tiryakioglu, S.; Ozkan, H.; Ari, H.; Yalin, K.; Coskun, S.; Tiryakioglu, O. Assessment of the Utility of the Septal E/(E′ × S′) Ratio and Tissue Doppler Index in Predicting Left Ventricular Remodeling after Acute Myocardial Infarction. Biomed. Res. Int. 2016, 2016, 4954731. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Nikitin, N.P.; Loh, H.P.; Lukaschuk, E.I.; Sherwi, N.; de Silva, R.; Tweddel, A.C.; Alamgir, M.F.; Wong, K.; Gupta, S.; et al. Prevalence of scarred and dysfunctional myocardium in patients with heart failure of ischaemic origin: A cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2012, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.; Hayat, S.A.; Gaze, D.; Celik, E.; Collinson, P.; Senior, R. Independent value of echocardiography and N-terminal pro-natriuretic peptide for the prediction of major outcomes in patients with suspected heart failure. Am. J. Cardiol. 2007, 100, 870–875. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).