Enhancement of Ambulatory Glucose Profile for Decision Assistance and Treatment Adjustments
Abstract
:1. Introduction
- The existing metrics are insufficient to analyze a complete glucose profile, which fails to capture the temporal patterns of glucose fluctuations that may lead to increased risks of hyperglycemia and hypoglycemia, as well as cardiovascular, neuropathic, and nephropathic issues.
- With just the combination of the MG, GV, TIR, and GMI values and the graph from the AGP report, deeper insights into patients’ glucose profiles are not impossible, such as their interday GV, intraday GV, and glucose fluctuations, resulting in incomplete assessments and poor diabetes control.
- The AGP report is a 14-day comprehensive view of the effect of a treatment regimen and lifestyle changes, including patients’ meal intake and activities; however, it is crucial to analyze glucose data every day based on the severity of complications and during emergencies.
- Patients’ response to medication, the persistence of glycemic peaks or lows, and reoccurrences of hyperglycemia and hypoglycemia are crucial factors that are hard to analyze using the metrics and the complex graphical representation in the AGP report.
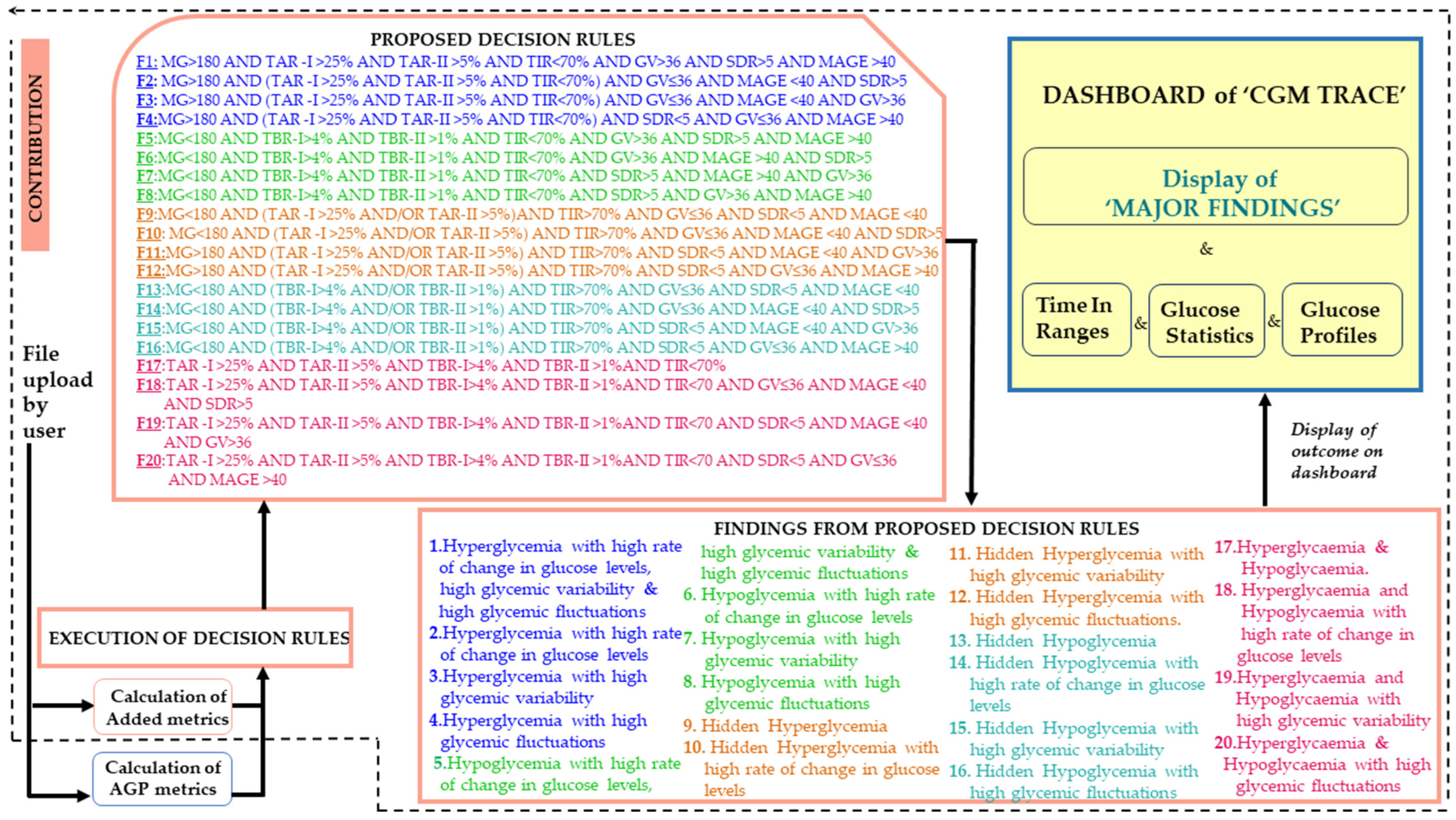
- Decision rules are formulated with a combined assessment of metrics to assist doctors in gaining insights into glucose variability, temporal patterns of glucose fluctuations, patients’ response to medication, persistence in glycemic peaks or lows, and reoccurrences of hyperglycemia and hypoglycemia to create accurate treatment regimens.
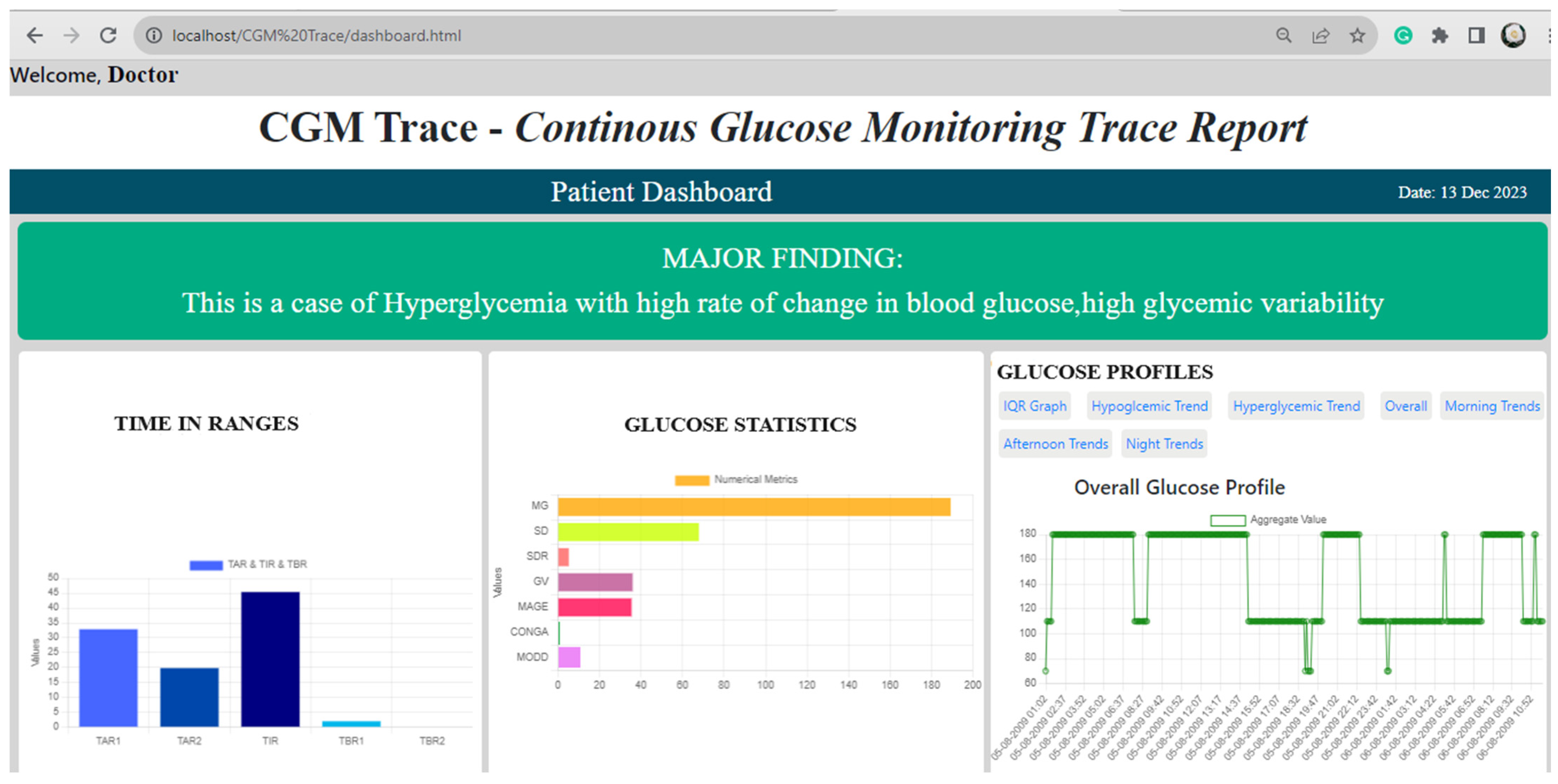
- This work presents the findings from the five standard AGP metrics, the five added statistical metrics, and the five graphical trends in a place that lacks an AGP report on the “CGM Trace” dashboard system.
- The decision rules are statistically validated using MANOVA, in which all metrics obtained a p value of <0.05, implying a significant correlation between the metrics and the findings.
- “CGM Trace” enhances the AGP report by generating five graphical presentations for identifying glucose trends and patterns, providing a distinct view of glycemic excursions.
2. Materials and Methods
2.1. CGM Data Collection
2.2. Metrics Incorporated into “CGM Trace”
Added Metrics
- The standard deviation rate of change (SDR): The SDR is defined as the standard deviation (SD) calculated on the rate of change of glucose levels [14,15,16]. As the SD is highly asymmetric, with an unequal distribution of hypoglycemic and hyperglycemic ranges leading toward biased assessment, the SDR is advantageous in indicating fluctuations and higher variability in glucose levels over time. The CGM sensor collects data every 5 min; therefore, the SDR is computed with SD over 5 min. An SDR of 5 is a threshold to indicate glucose levels with higher fluctuations [17]. The metric SDR is helpful for the doctor to identify hypoglycemia [14] and it is a crucial tool for predicting post-prandial GV. It is an important tool to make informed decisions regarding insulin dosage [15]. Correlating SDR with TIR and TAR helps in identifying chronic kidney disease, myocardial infarction, heart failure, and stroke [18]. Incorporating SDR into “CGM Trace” will provide information about the glucose levels’ improvement, deterioration, or stability, for effective decision-making and treatment adjustments of increasing, decreasing, or continuing the exact drug dosage.
- The interquartile range (IQR): The non-uniform distribution of a hypoglycemic and hyperglycemic range of the glucose margin creates an asymmetrical distribution, where the IQR is more important than the SD for non-symmetrical distributions [19]. Incorporating the IQR into “CGM Trace” will provide insight for the doctor to understand the spread of glucose levels visually. A wider IQR indicates a spread of glucose, indicating instability and inconsistency, thus implying a need to improve/increase the drug dosage for glycemic control.
- The hypoglycemic events graph: Hypoglycemia is a condition of low blood glucose levels when blood glucose is <70 mg/dL [20]. Incorporating a hypoglycemic graph into “CGM Trace” is beneficial for the doctor to identify the events of hypoglycemia and its specific hidden patterns, which cannot be inferred from the AGP report. It will provide the doctor with a vision of the nocturnal lows, information on the effect of insulin, medications, meal plans, physical activity, and underlying diseases, such as high arterial stiffness risk of albuminuria, retinopathy, cardiovascular disease mortality, all-cause mortality, and abnormal carotid intima-media thickness [18,20,21,22].
- The hyperglycemic events graph: Hyperglycemia is a condition of high blood glucose levels when blood glucose is >180 mg/dL [19,23]. Incorporating hyperglycemic events into “CGM Trace” will help the doctor to assess the post-meal spikes, glucose concentration, effectiveness of drug dosage, uncontrolled diabetes, decisions for new treatment approaches, and identification of microvascular and macrovascular complications [24,25].
- The intraday glucose trend: Intraday is the glucose trend divided into bolus infusion timings, that is, during the morning, afternoon, and at midnight. The intraday trend represents the glucose concentration through the Poincaré plot [19,23]. Incorporating the intraday glucose trend into “CGM Trace” will allow the doctor to make an informed decision on the drug dosage and correct the dose of insulin depending on factors such as recurring patterns, meal intake, response to drugs, and physical activity. It can assist the doctor in recommending meal plans based on post-meal spikes or midnight lows.
- The overall glucose trend: The overall glucose trend represents interstitial glucose in a selected time series [19]. Incorporating the intraday glucose trend into “CGM Trace” will allow doctors to decide on treatment adjustments by analyzing the glycemic control over some time. Assistance regarding meal plans and treatment adjustments can be provided based on the number of times the patient has achieved target glucose levels (<180 mg/dL), post-meal spikes, and midnight lows.
- The standard deviation (SD): A measure of the spread of glucose obtained around the average, where an SD < 33 is desirable [20]. Incorporating SD into “CGM Trace” will allow the doctor to identify the higher or lower glucose variability and decide on changes in the treatment regimen.
- The mean of daily differences (MODD): A measure of the interday GV estimation. It is calculated as the mean of the absolute differences between glucose concentrations measured at the same time of the day for two consecutive days [19]. Incorporating MODD into “CGM Trace” will allow clinicians to decide on drug dosage based on the food consumed and the bolus insulin/medications received. There is no threshold for MODD; however, a higher MODD is indicative of irregular food and lifestyle habits [24]. The MODD of the patients can be compared from the last visit to the current visit to identify the improvement in the patient’s lifestyle and diabetic management. It helps the doctor to assist patients with proper eating and lifestyle habits to achieve a lower MODD and to identify microvascular and macrovascular complications [25,26].
- The continuous overall net glycemic action (CONGA): The CONGA is the SD measured as the difference between a current glycemic observation and another observation n hours apart [19]. Incorporating CONGA into “CGM Trace” will allow doctors to make data-driven decisions based on the food consumed and the bolus insulin/medications received. There is no threshold for CONGA; however, it was observed that CONGA increased gradually from 1 to 8 h in the case of higher variability in glucose. It was also observed to be stable at 4 h if the glucose level is not fluctuating [27]. The CONGA of the patients can be compared from the last visit to the current visit to identify the improvement in the patient’s lifestyle and diabetic management. It will also enable doctors to predict long-term hyperglycemic or hypoglycemic events, make treatment adjustments, and reduce suboptimal glucose control, which is related to identifying microvascular and macrovascular complications [24,26].
- The mean amplitude of glycemic excursions (MAGE): Calculated as the arithmetic mean of the differences between the consecutive peaks and troughs of differences greater than one time the SD of the mean glycemia. It estimates major glucose swings and excludes minor swings [2,25]. There is no threshold for MAGE; however, 40 mg/dL was found in a clinical study to be indicative of suboptimal glucose control and was considered as a reference in this work [28]. Incorporating MAGE into “CGM Trace” will allow clinicians to analyze the glycemic excursions to identify specific periods of peaks and lows for creating a tailored treatment plan to achieve target glucose levels.
2.3. Proposed Decision Rules
3. Results
3.1. Validation of Decision Rules
3.2. Web Interface and Data Analyzation from “CGM Trace”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasudevan, D.M.; Sreekumari Vaidyanathan, K. Carbohydrates–III: Regulation of blood glucose: Diabetes mellitus. In Textbook of Biochemistry for Dental Students; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2017; p. 53. [Google Scholar]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- AGP Reports. Available online: http://www.agpreport.org/agp/agpreports (accessed on 12 September 2023).
- Kröger, J.; Siegmund, T.; Schubert-Olesen, O.; Keuthage, W.; Lettmann, M.; Richert, K.; Pfeiffer, A.F. AGP and Nutrition—Analysing postprandial glucose courses with CGM. Diabetes Res. Clin. Pract. 2021, 174, 108738. [Google Scholar] [CrossRef]
- Johnson, M.L.; Martens, T.W.; Criego, A.B.; Carlson, A.L.; Simonson, G.D.; Bergenstal, R.M. Utilizing the Ambulatory Glucose Profile to Standardize and Implement Continuous Glucose Monitoring in Clinical Practice. Diabetes Technol. Ther. 2019, 21 (Suppl. S2), S2-17–S2-25. [Google Scholar] [CrossRef] [PubMed]
- Bergenstal, R.M.; Ahmann, A.J.; Bailey, T.; Beck, R.W.; Bissen, J.; Buckingham, B.; Deeb, L.; Dolin, R.H.; Garg, S.K.; Goland, R.; et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: The ambulatory glucose profile. J. Diabetes Sci. Technol. 2013, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.B.; Verderese, C.A. Professional flash continuous glucose monitoring with ambulatory glucose profile reporting to supplement A1c: Rationale and practical implementation. Endocr. Pract. 2017, 23, 1333–1344. [Google Scholar] [CrossRef]
- Tokutsu, A.; Okada, Y.; Torimoto, K.; Tanaka, Y. Relationship between interstitial glucose variability in ambulatory glucose profile and standardized continuous glucose monitoring metrics; a pilot study. Diabetol. Metab. Syndr. 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Brown, F.; Ekinci, E.I. The ambulatory glucose profile and its interpretation. Med. J. Aust. 2022, 217, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, D. The ambulatory glucose profile: Opportunities for enhancement. Diabetes Technol. Ther. 2021, 23, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, D.; Rooney, M.R.; Echouffo-Tcheugui, J.B.; Coresh, J.; Aurora, R.N.; Punjabi, N.M.; Selvin, E. Performance of the glucose management indicator (GMI) in type 2 diabetes. Clin. Chem. 2023, 69, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D.R. Glycemic Variability: The Third Component of the Dysglycemia in Diabetes. Is it Important? How to Measure it? J. Diabetes Sci. Technol. 2008, 2, 1094–1100. [Google Scholar] [CrossRef]
- Czupryniak, L.; Dzida, G.; Fichna, P.; Jarosz-Chobot, P.; Gumprecht, J.; Klupa, T.; Mysliwiec, M.; Szadkowska, A.; Bomba-Opon, D.; Czajkowski, K.; et al. Ambulatory glucose profile (AGP) report in daily care of patients with diabetes: Practical tips and recommendations. Diabetes Ther. 2022, 13, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.C.; Eastman, R.C.; Tamada, J.A. Rates of Glucose Change Measured by Blood Glucose Meter and the GlucoWatch Biographer During Day, Night, and Around Mealtimes. Diabetes Care 2004, 27, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Majithia, A.R.; Wiltschko, A.B.; Zheng, H.; Walford, G.A.; Nathan, D.M. Rate of Change of Premeal Glucose Measured by Continuous Glucose Monitoring Predicts Postmeal Glycemic Excursions in Patients With Type 1 Diabetes: Implications for Therapy. J. Diabetes Sci. Technol. 2018, 12, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Pleus, S.; Schoemaker, M.; Morgenstern, K.; Schmelzeisen-Redeker, G.; Haug, C.; Link, M.; Zschornack, E.; Freckmann, G. Rate-of-Change Dependence of the Performance of Two CGM Systems During Induced Glucose Swings. J. Diabetes Sci. Technol. 2015, 9, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.B. Glycemic Variability and Diabetes Complications: Does It Matter? Of Course It Does! Diabetes Care 2015, 38, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Breyton, A.E.; Lambert-Porcheron, S.; Laville, M.; Vinoy, S.; Nazare, J.A. CGMS and glycemic variability, relevance in clinical research to evaluate interventions in T2D, a literature review. Front. Endocrinol. 2021, 12, 666008. [Google Scholar] [CrossRef] [PubMed]
- Czerwoniuk, D.; Fendler, W.; Walenciak, L.; Mlynarski, W. GlyCulator: A glycemic variability calculation tool for continuous glucose monitoring data. J. Diabetes Sci. Technol. 2011, 5, 447–451. [Google Scholar] [CrossRef]
- Wakasugi, S.; Mita, T.; Katakami, N.; Okada, Y.; Yoshii, H.; Osonoi, T.; Kuribayashi, N.; Taneda, Y.; Kojima, Y.; Gosho, M.; et al. Associations between continuous glucose monitoring-derived metrics and arterial stiffness in Japanese patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 15. [Google Scholar] [CrossRef]
- Yapanis, M.; James, S.; Craig, M.E.; O’Neal, D.; Ekinci, E.I. Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. J. Clin. Endocrinol. Metab. 2022, 107, e2221–e2236. [Google Scholar] [CrossRef]
- Sun, B.; Luo, Z.; Zhou, J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc. Diabetol. 2021, 20, 9. [Google Scholar] [CrossRef]
- Clarke, W.; Kovatchev, B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol. Ther. 2009, 11 (Suppl. S1), S-45–S-54. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, R.A.; Shi, H.; Yuan, L.H.; Brehm, W.; Pop-Busui, R.; Nelson, P.W. Translating glucose variability metrics into the clinic via Continuous Glucose Monitoring: A Graphical User Interface for Diabetes Evaluation (CGM-GUIDE©). Diabetes Technol. Ther. 2011, 13, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef]
- Krentz, A.J.; Clough, G.; Byrne, C.D. Interactions between microvascular and macrovascular disease in diabetes: Pathophysiology and therapeutic implications. Diabetes Obes. Metab. 2007, 9, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.J.; Donath, S.M.; Baghurst, P.A. Measuring glycaemic variation. Curr. Diabetes Rev. 2010, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.R. Mean Amplitude of Glycemic Excursions, a Measure of Diabetic Instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef]
- Martinez, M.; Santamarina, J.; Pavesi, A.; Musso, C.; Umpierrez, G.E. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002032. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.E.; Morgan, K., Jr.; Fu, D.K.; Wilkins, N.; Guffey, W.J. Time in Range: How to Measure It, How to Report It, and Its Practical Application in Clinical Decision-Making. Clin. Diabetes Publ. Am. Diabetes Assoc. 2020, 38, 439–448. [Google Scholar] [CrossRef]
- Saisho, Y.; Tanaka, C.; Tanaka, K.; Roberts, R.; Abe, T.; Tanaka, M.; Meguro, S.; Irie, J.; Kawai, T.; Itoh, H. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim. Care Diabetes 2015, 9, 290–296. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Owens, D.R. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018, 44, 313–319. [Google Scholar] [CrossRef]
- Madhu, S.V.; Muduli, S.K.; Avasthi, R. Abnormal glycemic profiles by CGMS in obese first-degree relatives of type 2 diabetes mellitus patients. Diabetes Technol. Ther. 2013, 15, 461–465. [Google Scholar] [CrossRef]
- Kohnert, K.D.; Heinke, P.; Fritzsche, G.; Vogt, L.; Augstein, P.; Salzsieder, E. Evaluation of the mean absolute glucose change as a measure of glycemic variability using continuous glucose monitoring data. Diabetes Technol. Ther. 2013, 15, 448–454. [Google Scholar] [CrossRef]
- Suh, S.; Kim, J.H. Glycemic Variability: How Do We Measure It and Why Is It Important? Diabetes Metab. J. 2015, 39, 273. [Google Scholar] [CrossRef]
- Ceriello, A.; Monnier, L.; Owens, D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019, 7, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.W.; Bibby, B.M. Rebound Hypoglycemia and Hyperglycemia in Type 1 Diabetes. J. Diabetes Sci. Technol. 2023, 19322968231168379. [Google Scholar] [CrossRef] [PubMed]
- Maposa, D.; Mudimu, E.; Ngwenya, O. A multivariate analysis of variance (MANOVA) of the performance of sorghum lines in different agroecological regions of Zimbabwe. Afr. J. Agric. Res. 2010, 5, 196–203. [Google Scholar]
- Huang, F.L. MANOVA: A Procedure Whose Time Has Passed? Gift. Child Q. 2019, 64, 56–60. [Google Scholar] [CrossRef]
- Mo, Y.; Zhou, J.; Li, M.; Wang, Y.; Bao, Y.; Ma, X.; Li, D.; Lu, W.; Hu, C.; Li, M.; et al. Glycemic variability is associated with subclinical atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc. Diabetol. 2013, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okada, Y.; Mori, H.; Tanaka, Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12, 1. [Google Scholar] [CrossRef]
- Su, G.; Mi, S.; Tao, H.; Li, Z.; Yang, H.; Zheng, H.; Zhou, Y.; Ma, C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 19. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; PKovatchev, B. Glycemic Variability: How to Measure and Its Clinical Implication for Type 2 Diabetes. Am. J. Med. Sci. 2018, 356, 518–527. [Google Scholar] [CrossRef]
- Gabbay, M.A.L.; Rodacki, M.; Calliari, L.E.; Vianna, A.G.D.; Krakauer, M.; Pinto, M.S.; Reis, J.S.; Puñales, M.; Miranda, L.G.; Ramalho, A.C.; et al. Time in range: A new parameter to evaluate blood glucose control in patients with diabetes. Diabetol. Metab. Syndr. 2020, 12, 22. [Google Scholar] [CrossRef]
- Klein, H.E. Glycemia Risk Index Measures Hypoglycemia, Hyperglycemia Risk in Patients with T1D. AJMC. Published 17 February 2023. Available online: https://www.ajmc.com/view/glycemia-risk-index-measures-hypoglycemia-hyperglycemia-risk-in-patients-with-t1d (accessed on 22 September 2023).
- Vergès, B.; Pignol, E.; Rouland, A.; Bouillet, B.; Baillot-Rudoni, S.; Quilot, E.; Djeffal, A.; Petit, J.M. Glycemic variability assessment with a 14-day continuous glucose monitoring system: When and how long to measure MAGE (mean amplitude of glucose excursion) for optimal reliability? J. Diabetes Sci. Technol. 2022, 16, 982–987. [Google Scholar] [CrossRef]
- Otowa-Suematsu, N.; Sakaguchi, K.; Komada, H.; Nakamura, T.; Sou, A.; Hirota, Y.; Kuroda, M.; Shinke, T.; Hirata, K.; Ogawa, W. Comparison of the relationship between multiple parameters of glycemic variability and coronary plaque vulnerability assessed by virtual histology–intravascular ultrasound. J. Diabetes Investig. 2018, 9, 610–615. [Google Scholar] [CrossRef]
- Rodbard, D. Glucose Variability: A Review of Clinical Applications and Research Developments. Diabetes Technol. Ther. 2018, 20 (Suppl. S2), S2-5–S2-15. [Google Scholar] [CrossRef] [PubMed]



| Age (in groups) (1–30): (30–60): (60–90): | 12 to 65 years M: 29, F: 29 M: 4, F: 3 M: 1, F: 1 |
| Gender | M = 34, F = 33 |
| Diabetes duration | At least 1 year of clinical diagnosis with diabetes |
| HbA1C | Measured between 5.0 and 10.5 |
| Inclusion criteria | Patients on CGM sensor with proper mental health and cognition |
| Exclusion criteria | Pregnant and lactating women, patients with diabetic ketoacidosis, seizure disorder, active infection, muscular condition, cancer patients, and cystic fibrosis |
| R.No | Decision Rule for Different Findings |
|---|---|
| R1 | If MG > 180 AND TAR-I > 25% AND TAR-II > 5% AND TIR < 70% AND GV > 36 AND SDR > 5 AND MAGE > 40 Then This is a case of hyperglycemia with a high rate of change in glucose levels, high glycemic variability, and high glucose fluctuations |
| R2 | If MG > 180 AND (TAR-I > 25% AND TAR-II > 5% AND TIR < 70%) AND GV ≤ 36 AND MAGE < 40 AND SDR > 5 Then This is a case of hyperglycemia with a high rate of change in glucose |
| R3 | If MG > 180 AND (TAR-I > 25% AND TAR-II > 5% AND TIR < 70%) AND SDR < 5 AND MAGE < 40 AND GV > 36 Then This is a case of hyperglycemia with a high glycemic variability |
| R4 | If MG > 180 AND (TAR-I > 25% AND TAR-II >5% AND TIR < 70%) AND SDR < 5 AND GV ≤ 36 AND MAGE > 40 Then This is a case of hyperglycemia with high glucose fluctuations |
| R5 | If MG < 180 AND TBR-I > 4% AND TBR-II > 1% AND TIR < 70% AND GV > 36 AND SDR > 5 AND MAGE > 40 Then This is a case of hypoglycemia with a high rate of change in glucose levels, high glycemic variability, and high glucose fluctuations |
| R6 | If MG < 180 AND TBR-I > 4% AND TBR-II > 1% AND TIR < 70% AND GV ≤ 36 AND MAGE < 40 AND SDR > 5 Then This is a case of hypoglycemia with a high rate of change in glucose |
| R7 | If MG < 180 AND TBR-I > 4% AND TBR-II > 1% AND TIR < 70% AND SDR < 5 AND MAGE <40 AND GV > 36 Then This is a case of hypoglycemia with high glycemic variability |
| R8 | If MG < 180 AND TBR-I > 4% AND TBR-II > 1% AND TIR < 70% AND SDR < 5 AND GV ≤ 36 AND MAGE > 40 Then This is a case of hypoglycemia with high glucose fluctuations |
| R9 | If MG < 180 AND (TAR-I > 25% OR TAR-II >5%) AND TIR > 70% AND GV ≤ 36 AND SDR < 5 AND MAGE <40 Then This is a case of hidden hyperglycemia |
| R10 | If MG < 180 AND (TAR-I > 25% OR TAR-II > 5%) AND TIR > 70% AND GV ≤ 36 AND MAGE < 40 AND SDR > 5 Then This is a case of hidden hyperglycemia with a high rate of change in glucose levels |
| R11 | If MG > 180 AND (TAR-I > 25% OR TAR-II > 5%) AND TIR > 70% AND SDR < 5 AND MAGE < 40 AND GV > 36 Then This is a case of hidden hyperglycemia with high glycemic variability |
| R12 | If MG > 180 AND (TAR-I > 25% OR TAR-II > 5%) AND TIR > 70% AND SDR < 5 AND GV ≤ 36 AND MAGE > 40 Then This is a case of hidden hyperglycemia with high glucose fluctuations |
| R13 | If MG < 180 AND (TBR-I > 4% OR TBR-II > 1%) AND TIR > 70% AND GV ≤ 36 AND SDR < 5 AND MAGE < 40 Then This is a case of hidden hypoglycemia |
| R14 | If MG < 180 AND (TBR-I > 4% OR TBR-II > 1%) AND TIR > 70% AND GV ≤ 36 AND MAGE <40 AND SDR > 5 Then This is a case of hidden hypoglycemia with a high rate of change in glucose levels |
| R15 | If MG < 180 AND (TBR-I > 4% OR TBR-II > 1%) AND TIR > 70% AND SDR < 5 AND MAGE <40 AND GV > 36 Then This is a case of hidden hypoglycemia with high glycemic variability |
| R16 | If MG < 180 AND (TBR-I > 4% OR TBR-II > 1%) AND TIR > 70% AND SDR < 5 AND GV ≤ 36 AND MAGE > 40 Then This is a case of hidden hypoglycemia with high glucose fluctuations |
| R17 | If (TAR-I > 25% OR TAR-II >5%) AND (TBR-I > 4% OR TBR-II > 1%) AND TIR < 70% Then This is a case of hyperglycemia and hypoglycemia at different intervals |
| R18 | If (TAR-I > 25% AND TAR-II > 5%) AND (TBR-I > 4% OR TBR-II > 1%) AND TIR < 70 AND GV ≤ 36 AND MAGE <40 AND SDR > 5 Then This is a case of hyperglycemia and hypoglycemia at different intervals with a high rate of change in glucose levels |
| R19 | If (TAR -I > 25% OR TAR-II > 5%) AND (TBR-I > 4% AND TBR-II > 1%) AND TIR < 70 AND SDR < 5 AND MAGE <40 AND GV > 36 Then This is a case of hyperglycemia and hypoglycemia at different intervals with high glycemic variability |
| R20 | If (TAR-I > 25% OR TAR-II > 5%) AND (TBR-I > 4% AND TBR-II > 1%) AND TIR < 70 AND SDR < 5 AND GV ≤ 36 AND MAGE > 40 Then This is a case of hyperglycemia and hypoglycemia at different intervals with high glucose fluctuations |
| No. | Assumptions | Test Performed | Transformation Performed | p-Value |
|---|---|---|---|---|
| 1 | Normality distribution | Normality test | Inverse DF | - |
| 2 | Homogeneity of covariance matrices | Box’s M test | - | 0.531 |
| 3 | Effect of independent variables on dependent variables | Tests of Between-Subjects Effects | - | 0.791 |
| 4 | Overall significance | Wilk’s Lambda | - | 0.001 |
| P | Metrics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDR | MG | SD | GV (%) | TIR (%) | TAR-I (%) | TAR-II (%) | TBR-I (%) | TBR-II (%) | MODD | CONGA | MAGE | |
| 1 | 5.4 | 96.6 | 42.2 | 34.9 | 65.3 | 3.07 | 0 | 11.5 | 18.4 | 7.4 | 0 | 73.5 |
| 2 | 5.5 | 117.7 | 48.9 | 31.1 | 64.4 | 22.2 | 5 | 5.9 | 1.7 | 7.7 | 2.6 | 96.2 |
| 3 | 4.0 | 126.1 | 36.1 | 29.4 | 86.1 | 8.8 | 0 | 5.5 | 0 | 6.7 | 8.8 × 10−16 | 44.9 |
| 4 | 3.94 | 154.4 | 33.0 | 36 | 76.5 | 23.4 | 0 | 0 | 0 | 4.2 | 1.0 | 19.7 |
| 5 | 6.0 | 96.0 | 27.3 | 37.4 | 68.6 | 0 | 0 | 18.7 | 12.1 | 4.7 | 1.7 × 10−15 | 51.5 |
| 6 | 5.86 | 90.2 | 38.7 | 31.4 | 59.4 | 2.7 | 0 | 17.2 | 20.5 | 5.6 | 0 | 37.5 |
| 7 | 3.5 | 74.1 | 31.2 | 45.3 | 47.3 | 0 | 0 | 14.0 | 38.5 | 4.2 | 8.8 × 10−16 | 38 |
| 8 | 4.0 | 152.3 | 32.5 | 30 | 73.6 | 21.0 | 5.0 | 0 | 0 | 4.7 | 4.2 × 10−15 | 39.5 |
| 9 | 4.5 | 151.6 | 32.5 | 25.2 | 75 | 23.4 | 0 | 1.5 | 0 | 3.4 | 0 | 17.4 |
| 10 | 2.7 | 126.8 | 29.2 | 23 | 96.6 | 1.6 | 0 | 1.6 | 0 | 2.1 | 8.8 × 10−16 | 30.5 |
| 11 | 5.6 | 180.4 | 38.8 | 36.4 | 65 | 27.7 | 5.2 | 0 | 0 | 5.4 | 0 | 46.7 |
| 12 | 3.6 | 133.4 | 34.9 | 26.8 | 84.4 | 7.7 | 0 | 7.7 | 0 | 4.8 | 0 | 44.1 |
| 13 | 6 | 186.7 | 41 | 44.2 | 61.5 | 31.4 | 7 | 0 | 0 | 5.3 | 0 | 42.9 |
| 14 | 5.0 | 190.9 | 47.2 | 39.3 | 60.4 | 26.0 | 6 | 5 | 3 | 4.2 | 1.7 × 10−15 | 33.2 |
| 15 | 2.97 | 125.2 | 22.3 | 16.2 | 100 | 0 | 0 | 0 | 0 | 2.6 | 4.4 × 10−16 | 36 |
| P | Metrics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDR | MG | SD | GV (%) | TIR (%) | TAR-I (%) | TAR-II (%) | TBR-I (%) | TBR-II (%) | MODD | CONGA | MAGE | |
| 1 | 5.5 | 176.9 | 52.6 | 29.6 | 50 | 39.5 | 10.4 | 0 | 0 | 2.7 | 4.4 × 10−16 | 26.6 |
| 2 | 3.1 | 172.9 | 103 | 53.6 | 48 | 19.5 | 28.7 | 4.6 | 0 | 4.1 | 5.9 × 10−16 | 28.5 |
| 3. | 2.5 | 164.7 | 48.1 | 29.2 | 65.5 | 26.8 | 4.6 | 0 | 3 | 3.7 | 8.4 × 10−16 | 43.1 |
| 4 | 4.3 | 140.6 | 30 | 32.3 | 84.2 | 9.4 | 4.6 | 14.1 | 1.6 | 4.1 | 8.8 × 10−16 | 42.6 |
| 5 | 5.3 | 151 | 22 | 33 | 69.1 | 26.0 | 4.3 | 0.2 | 0 | 4.2 | 8.8 × 10−16 | 36.7 |
| 6 | 3 | 128.5 | 40.7 | 31.7 | 87.5 | 12.4 | 0 | 0 | 0 | 4.2 | 0 | 27.2 |
| 7 | 2.6 | 154.4 | 30.9 | 20 | 84.0 | 15.9 | 0 | 0 | 0 | 3.5 | 4.4 × 10−16 | 40 |
| 8 | 31.1 | 193.9 | 60.4 | 31.1 | 44.9 | 37.4 | 17.3 | 0.2 | 0 | 4.9 | 1.70 × 10−15 | 61.5 |
| 9 | 2.1 | 141 | 38.6 | 27.3 | 83.7 | 15.8 | 0 | 0 | 0 | 4.1 | 0 | 37 |
| 10 | 1.7 | 166.7 | 49.7 | 29.7 | 73.8 | 14.9 | 10.0 | 0 | 0 | 2.4 | 0 | 66.6 |
| 11 | 3.9 | 163.6 | 79 | 48.2 | 47.4 | 24.6 | 15 | 6.4 | 5.9 | 5.5 | 7.8 × 10−16 | 34.8 |
| 12 | 4.3 | 160 | 79.9 | 49.8 | 44.8 | 29.3 | 7.7 | 3.4 | 13.8 | 5.1 | 5.8 × 10−16 | 130.2 |
| 13 | 4.6 | 155.3 | 30.5 | 26 | 74 | 23.7 | 2.8 | 2.6 | 0 | 5.5 | 0 | 37.8 |
| 14 | 4.4 | 122 | 86.7 | 38.9 | 40.9 | 25.7 | 33.3 | 0 | 0.1 | 5 | 8.8 × 10−16 | 42 |
| 15 | 7.0 | 218.0 | 53.6 | 24.6 | 26.8 | 73.1 | 0 | 0 | 0 | 9.2 | 1.4 × 10−16 | 40.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satuluri, V.K.R.R.; Ponnusamy, V. Enhancement of Ambulatory Glucose Profile for Decision Assistance and Treatment Adjustments. Diagnostics 2024, 14, 436. https://doi.org/10.3390/diagnostics14040436
Satuluri VKRR, Ponnusamy V. Enhancement of Ambulatory Glucose Profile for Decision Assistance and Treatment Adjustments. Diagnostics. 2024; 14(4):436. https://doi.org/10.3390/diagnostics14040436
Chicago/Turabian StyleSatuluri, V. K. R. Rajeswari, and Vijayakumar Ponnusamy. 2024. "Enhancement of Ambulatory Glucose Profile for Decision Assistance and Treatment Adjustments" Diagnostics 14, no. 4: 436. https://doi.org/10.3390/diagnostics14040436
APA StyleSatuluri, V. K. R. R., & Ponnusamy, V. (2024). Enhancement of Ambulatory Glucose Profile for Decision Assistance and Treatment Adjustments. Diagnostics, 14(4), 436. https://doi.org/10.3390/diagnostics14040436






