Rim Enhancement on Contrast-Enhanced CT as a Predictor of Prognosis in Patients with Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
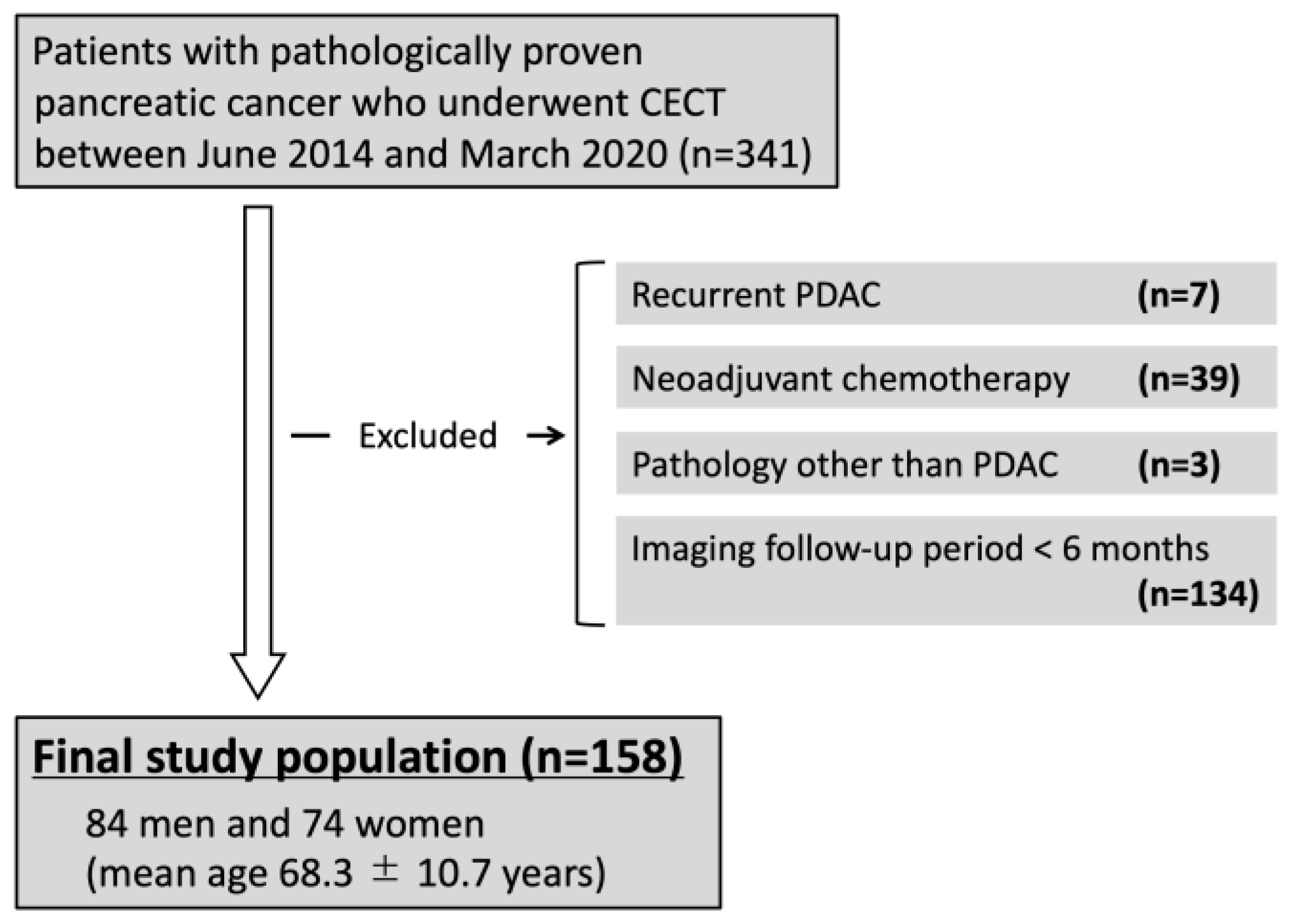
2.1. Study Design and Population
2.2. CT Examination
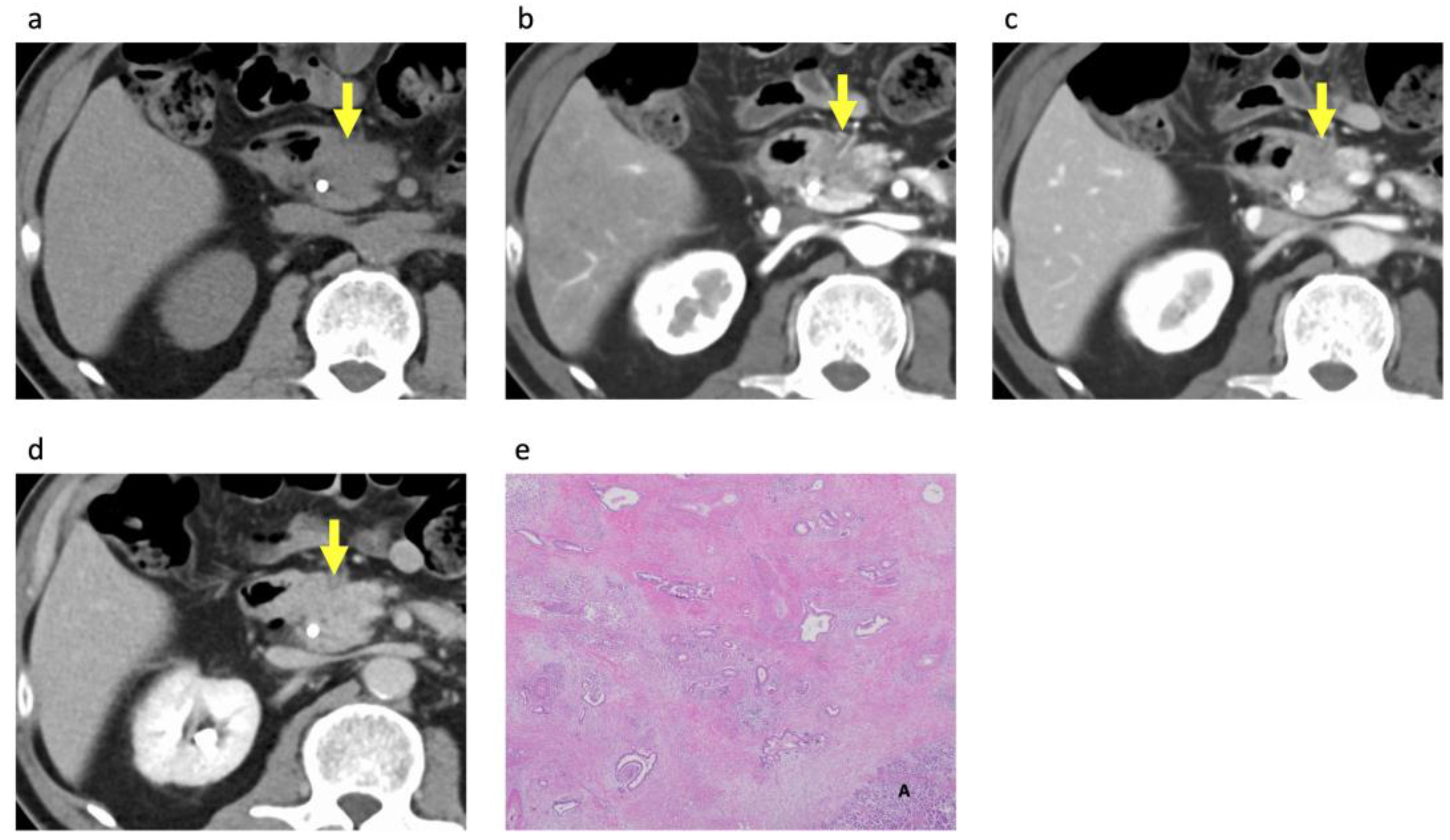
2.3. Image Analysis
2.4. Clinical Data Collection and Survival Analysis
2.5. Histopathological Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Patients and PDACs
3.2. CECT Imaging Findings
3.3. Histopathological Analysis of the PDACs According to Rim Enhancement
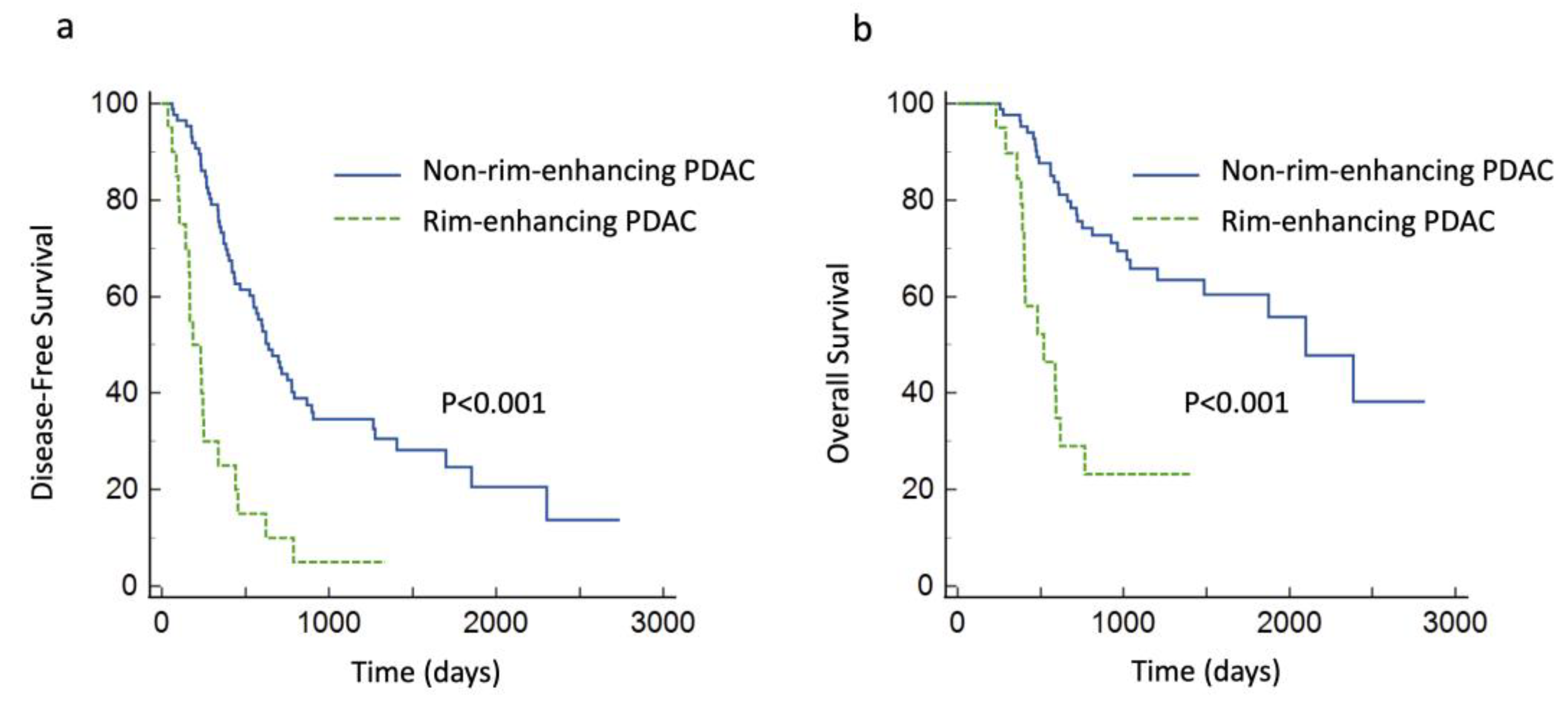
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617, Erratum in N. Engl. J. Med. 2010, 363, 298. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, D.; Saied, A.; Kocher, H.M. Analysis of mortality rates for pancreatic cancer across the world. HPB 2008, 10, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Demachi, H.; Matsui, O.; Kobayashi, S.; Akakura, Y.; Konishi, K.; Tsuji, M.; Miwa, A.; Miyata, S. Histological influence on contrast-enhanced CT of pancreatic ductal adenocarcinoma. J. Comput. Assist. Tomogr. 1997, 21, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.H.; Yu, E.S.; Kim, M.H.; Kim, J.; Byun, J.H.; Lee, S.S.; Hwang, H.J.; Hwang, J.Y.; Lee, S.S.; et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: Frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010, 257, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fukukura, Y.; Takumi, K.; Higashi, M.; Shinchi, H.; Kamimura, K.; Yoneyama, T.; Tateyama, A. Contrast-enhanced CT and diffusion-weighted MR imaging: Performance as a prognostic factor in patients with pancreatic ductal adenocarcinoma. Eur. J. Radiol. 2014, 83, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, X.; Xue, H.; Wu, H.; Chen, G.; Sun, H.; He, Y.; Jin, Z.; Liang, Z.; Zhang, Z. CT Imaging Biomarkers Predict Clinical Outcomes after Pancreatic Cancer Surgery. Medicine 2016, 95, e2664, Erratum in Medicine 2016, 95, e29ce. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.H.; Park, H.K.; Jang, K.T.; Hwang, J.A.; Kim, S. Pancreatic Ductal Adenocarcinoma: Rim Enhancement at MR Imaging Predicts Prognosis after Curative Resection. Radiology 2018, 288, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. Residual tumor (R) classification and prognosis. Semin. Surg. Oncol. 1994, 10, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Geer, R.J.; Brennan, M.F. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am. J. Surg. 1993, 165, 68–72. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Chien, M.W.; Earle, C.C. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann. Surg. 2003, 237, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, K.F.; de Castro, S.M.; Wesseling, J.G.; ten Kate, F.J.; Offerhaus, G.J.; Busch, O.R.; van Gulik, T.M.; Obertop, H.; Gouma, D.J. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur. J. Cancer 2004, 40, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, S.; Hasebe, T.; Iwasaki, M.; Kinoshita, T.; Ochiai, A.; Shimizu, N. Important prognostic histological parameters for patients with invasive ductal carcinoma of the pancreas. Cancer Sci. 2005, 96, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wu, L.; Dai, J.; Wang, K.; Shi, H.; Lu, Z.; Ji, G.; Yu, J.; Xu, Q. Rim Enhancement and Peripancreatic Fat Stranding in Preoperative MDCT as Predictors for Occult Metastasis in PDAC Patients. Acad. Radiol. 2023, 30, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Lüttges, J.; Schemm, S.; Vogel, I.; Hedderich, J.; Kremer, B.; Klöppel, G. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J. Pathol. 2000, 191, 154–161. [Google Scholar] [CrossRef]
- Hiraoka, N.; Ino, Y.; Sekine, S.; Tsuda, H.; Shimada, K.; Kosuge, T.; Zavada, J.; Yoshida, M.; Yamada, K.; Koyama, T.; et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br. J. Cancer 2010, 103, 1057–1065. [Google Scholar] [CrossRef] [PubMed]

| Aquilion 64 | Aquilion One | Aquilion Precision | SOMATOM Force | |
|---|---|---|---|---|
| Acquisition parameters | ||||
| Number of channels | 64 | 320 | 160 | 192 |
| Tube voltage (kVp) | 120 | 120 | 120 | 120 |
| Detector configuration (mm) | 64 × 0.5 | 80 × 0.5 | 80 × 0.5 | 192 × 0.6 |
| Acquisition matrix | 512 × 512 | 512 × 512 | 512 × 512 | 512 × 512 |
| Pitch factor | 0.641 | 0.813 | 0.813 | 0.6 |
| Rotation time (s) | 0.5 | 0.5 | 0.5 | 0.5 |
| Tube current–time product | AEC | AEC | AEC | AEC |
| Reconstruction parameters | ||||
| Reconstruction plane | Axial | Axial | Axial | Axial |
| Section thickness (mm) | 5 | 5 | 5 | 5 |
| Reconstruction interval (mm) | 5 | 5 | 5 | 5 |
| Thin-slice reconstruction parameters | ||||
| Reconstruction plane | Axial | Axial | Axial | Axial |
| Section thickness (mm) | 0.5 | 0.5 | 0.5 | 0.6 |
| Reconstruction interval (mm) | 0.3 | 0.3 | 0.3 | 0.4 |
| Characteristic | Finding | ||||
|---|---|---|---|---|---|
| Surgery Group | Non-Surgery Group | p Value | |||
| Patient | |||||
| Number | 106 | (67%) | 52 | (33%) | |
| Age (years) | 69.1 | ±10.5 | 66.7 | ±9.9 | 0.15 |
| Sex | 0.73 | ||||
| Men | 55 | (52%) | 29 | (56%) | |
| Women | 51 | (48%) | 23 | (44%) | |
| CA 19-9 | >0.99 | ||||
| >37 (U/mL) | 84 | (79%) | 41 | (85%) | |
| ≤37 (U/mL) | 22 | (21%) | 11 | (15%) | |
| PDAC | |||||
| Tumor size on CECT (mm) | 23.8 | ±7.1 | 37.6 | ±14.1 | <0.001 |
| Local resectability | <0.001 | ||||
| Resectable | 92 | (87%) | 17 | (33%) | |
| Borderline resectable | 14 | (13%) | 16 | (31%) | |
| Unresectable | 0 | (0%) | 19 | (36%) | |
| Attenuation in pancreatic phase | 0.09 | ||||
| Hypoattenuation | 99 | (93%) | 52 | (100%) | |
| Iso- or hyperattenuation | 7 | (7%) | 0 | (0%) | |
| Attenuation in equilibrium phase | 0.001 | ||||
| Hypoattenuation | 35 | (33%) | 32 | (62%) | |
| Iso- or hyperattenuation | 71 | (67%) | 20 | (38%) | |
| Rim enhancement | 0.001 | ||||
| Presence | 20 | (19%) | 23 | (44%) | |
| Absence | 86 | (81%) | 29 | (56%) | |
| Rim-Enhancing PDAC (n = 20) | Non-Rim-Enhancing PDAC (n = 86) | p Value | |||
|---|---|---|---|---|---|
| Tumor size on histopathologic analysis (mm) | 34.0 | ±12.4 | 28.1 | ±9.5 | 0.06 |
| Histologic grade | <0.001 | ||||
| Well-differentiated | 0 | (0%) | 22 | (26%) | |
| Moderately differentiated | 9 | (45%) | 56 | (65%) | |
| Poorly differentiated or adenosquamous carcinoma | 11 | (55%) | 8 | (9%) | |
| T stage | 0.22 | ||||
| T1 | 1 | (5%) | 17 | (20%) | |
| T2 | 15 | (75%) | 59 | (69%) | |
| T3 | 4 | (20%) | 10 | (12%) | |
| N stage | 0.97 | ||||
| N0 | 7 | (35%) | 28 | (33%) | |
| N1 | 9 | (45%) | 39 | (45%) | |
| N2 | 4 | (20%) | 19 | (22%) | |
| AJCC stage | 0.84 | ||||
| I | 7 | (35%) | 26 | (30%) | |
| II | 9 | (45%) | 39 | (45%) | |
| III | 4 | (20%) | 18 | (21%) | |
| IV | 0 | (0%) | 3 | (3%) | |
| Stromal volume | 0.13 | ||||
| Medullary type | 2 | (10%) | 1 | (1%) | |
| Intermediate type | 14 | (70%) | 62 | (72%) | |
| Scirrhous type | 4 | (20%) | 14 | (16%) | |
| N/A | 0 | (0%) | 9 | (10%) | |
| Residual tumor classification | 0.48 | ||||
| R0 | 18 | (90%) | 72 | (84%) | |
| R1 | 2 | (10%) | 14 | (16%) | |
| R2 | 0 | (0%) | 0 | (0%) | |
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | p Value | Hazard Ratio | p Value | |||
| Age (years) | 0.99 | (0.97–1.01) | 0.42 | |||
| Sex (men) | 0.97 | (0.62–1.51) | 0.90 | |||
| CA 19-9 (>37 U/mL) | 1.05 | (0.60–1.82) | 0.86 | |||
| Tumor size on CECT (mm) | 1.04 | (1.00–1.07) | 0.04 | 1.02 | (0.98–1.06) | 0.30 |
| Hypoattenuation in pancreatic phase | 1.08 | (0.47–2.50) | 0.86 | |||
| Hypoattenuation in equilibrium phase | 1.64 | (1.04–2.60) | 0.03 | 1.19 | (0.70–2.01) | 0.49 |
| Rim enhancement | 3.56 | (2.09–6.06) | <0.001 | 3.03 | (1.66–5.54) | <0.001 |
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | p Value | Hazard Ratio | p Value | |||
| Age (years) | 1.00 | (0.97–1.02) | 0.75 | |||
| Sex (men) | 1.01 | (0.56–1.82) | 0.98 | |||
| CA 19-9 (>37 U/mL) | 1.00 | (0.48–2.09) | 0.99 | |||
| Tumor size on CECT (mm) | 1.10 | (1.06–1.15) | <0.001 | 1.08 | (1.03–1.13) | <0.001 |
| Hypoattenuation in pancreatic phase | 2.26 | (0.54–9.40) | 0.26 | |||
| Hypoattenuation in equilibrium phase | 1.28 | (0.69–2.40) | 0.43 | |||
| Rim enhancement | 4.28 | (2.21–8.28) | <0.001 | 2.99 | (1.47–6.09) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, T.; Sofue, K.; Ueshima, E.; Sugiyama, N.; Yabe, S.; Ueno, Y.; Masuda, A.; Toyama, H.; Kodama, T.; Komatsu, M.; et al. Rim Enhancement on Contrast-Enhanced CT as a Predictor of Prognosis in Patients with Pancreatic Ductal Adenocarcinoma. Diagnostics 2024, 14, 782. https://doi.org/10.3390/diagnostics14080782
Yamaguchi T, Sofue K, Ueshima E, Sugiyama N, Yabe S, Ueno Y, Masuda A, Toyama H, Kodama T, Komatsu M, et al. Rim Enhancement on Contrast-Enhanced CT as a Predictor of Prognosis in Patients with Pancreatic Ductal Adenocarcinoma. Diagnostics. 2024; 14(8):782. https://doi.org/10.3390/diagnostics14080782
Chicago/Turabian StyleYamaguchi, Takeru, Keitaro Sofue, Eisuke Ueshima, Naoki Sugiyama, Shinji Yabe, Yoshiko Ueno, Atsuhiro Masuda, Hirochika Toyama, Takayuki Kodama, Masato Komatsu, and et al. 2024. "Rim Enhancement on Contrast-Enhanced CT as a Predictor of Prognosis in Patients with Pancreatic Ductal Adenocarcinoma" Diagnostics 14, no. 8: 782. https://doi.org/10.3390/diagnostics14080782
APA StyleYamaguchi, T., Sofue, K., Ueshima, E., Sugiyama, N., Yabe, S., Ueno, Y., Masuda, A., Toyama, H., Kodama, T., Komatsu, M., Hori, M., & Murakami, T. (2024). Rim Enhancement on Contrast-Enhanced CT as a Predictor of Prognosis in Patients with Pancreatic Ductal Adenocarcinoma. Diagnostics, 14(8), 782. https://doi.org/10.3390/diagnostics14080782





