B-Type Natriuretic Peptide—A Paradox in the Diagnosis of Acute Heart Failure with Preserved Ejection Fraction in Obese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Approval
2.2. Enrollment of Patients and Data Collection
2.3. Statistical Analysis
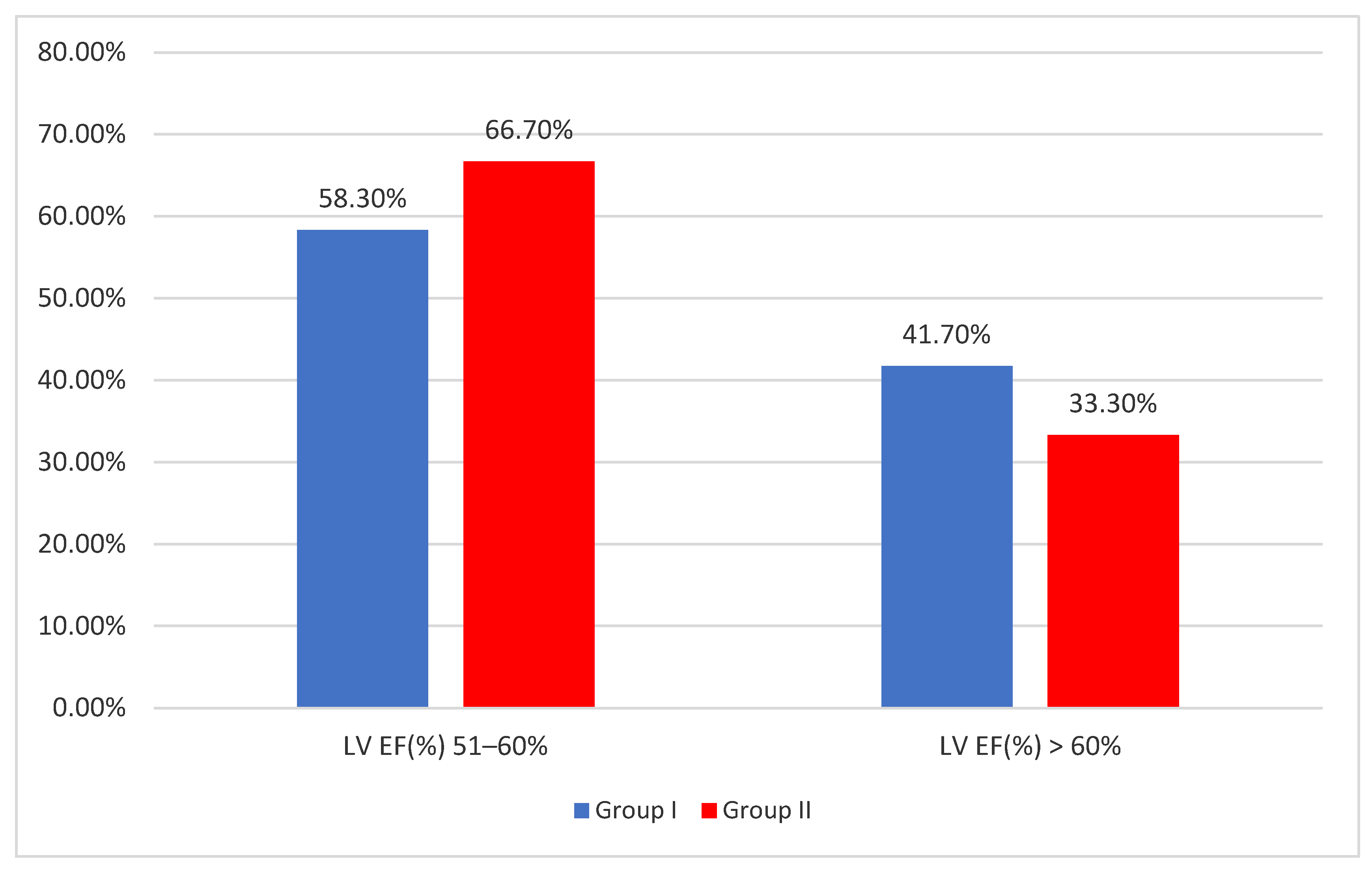
3. Results
3.1. Patients Characteristics
3.2. Clinical and Paraclinical Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L. Understanding the epidemic of heart failure: Past, present, and future. Curr. Heart Fail. Rep. 2014, 11, 404–415. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, E.E.; Hoes, A.W.; Limburg, A.; Landman, M.A.; van der Hoeven, H.; Rutten, F.H. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur. J. Heart Fail. 2014, 16, 772–777. [Google Scholar] [CrossRef]
- Halliday, B.P.; Wassall, R.; Lota, A.S.; Khalique, Z.; Gregson, J.; Newsome, S.; Jackson, R.; Rahneva, T.; Wage, R.; Smith, G.; et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. Lancet 2019, 393, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Emdin, M.; Passino, C.; Prontera, C.; Fontana, M.; Poletti, R.; Gabutti, A.; Mammini, C.; Giannoni, A.; Zyw, L.; Zucchelli, G.; et al. Comparison of brain natriuretic peptide (BNP) and amino-terminal ProBNP for early diagnosis of heart failure. Clin. Chem. 2007, 53, 1289–1297. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559573. [Google Scholar] [CrossRef]
- Packer, M.; Lam, C.S.P.; Lund, L.H.; Maurer, M.S.; Borlaug, B.A. Characterization of the inflammatory-metabolic phenotype of heart failure and a preserved ejection fraction: A hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur. J. Heart Fail. 2020, 22, 1551–1567. [Google Scholar] [CrossRef]
- Rao, V.N.; Fudim, M.; Mentz, R.J.; Michos, E.D.; Felker, G.M. Regional adiposity and heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Lavie, C.J.; Arena, R. Obesity and heart failure: Focus on the obesity paradox. Mayo Clin. Proc. 2017, 92, 266279. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the obesity paradox in heart failure. Prog. Cardiovasc. Dis. 2018, 61, 151156. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, C.; Meyer, P.; Desai, R.V.; Karatzidou, K.; Ovalle, F.; White, M.; Aban, I.; Love, T.E.; Deedwania, P.; Anker, S.D.; et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: A propensity-matched study. Eur. J. Heart Fail. 2011, 13, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Mueller, C.; Adams, K.; Jr Anker, S.D.; Aspromonte, N.; Cleland, J.G.; Cohen-Solal, A.; Dahlstrom, U.; De Maria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.P.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. Acute heart failure congestion and perfusion status—Impact of the clinical classification on in-hospital and long-term outcomes: Insights from the ESC-EORP-HFA heart failure long-term registry. Eur. J. Heart Fail. 2019, 21, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Javaloyes, P.; Miro, O.; Gil, V.; Martin-Sanchez, F.J.; Jacob, J.; Herrero, P.; Takagi, K.; Alquezar-Arbe, A.; Lopez Diez, M.P.; Martin, E.; et al. Clinical phenotypes of acute heart failure based on signs and symptoms of perfusion and congestion at emergency department presentation and their relationship with patient management and outcomes. Eur. J. Heart Fail. 2019, 21, 1353–1365. [Google Scholar] [CrossRef]
- Masip, J.; Peacock, W.F.; Price, S.; Cullen, L.; Martin-Sanchez, F.J.; Seferovic, P.; Maisel, A.S.; Miro, O.; Filippatos, G.; Vrints, C.; et al. Indications and practical approach to non-invasive ventilation in acute heart failure. Eur. Heart J. 2018, 39, 17–25. [Google Scholar] [CrossRef]
- Harjola, V.P.; Mebazaa, A.; Celutkiene, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.P.; Antohi, E.L.; Arrigo, M.; Gal, T.B.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Mebazaa, A.; Combes, A.; van Diepen, S.; Hollinger, A.; Katz, J.N.; Landoni, G.; Hajjar, L.A.; Lassus, J.; Lebreton, G.; Montalescot, G.; et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 2018, 44, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Sundgaard-Riise, K.; Reisin, E.D.; Dreslinski, G.R.; Ventura, H.O.; Oigman, W.; Frohlich, E.D.; Dunn, F.G. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann. Intern. Med. 1983, 99, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Paci, V.M.; Dessi-Fulgheri, P.; Espinosa, E.; Rappelli, A. Comparative analysis of atrial natriuretic peptide receptor expression in rat tissues. J. Hypertens. 1993, 11 (Suppl. 5), S214–S215. [Google Scholar] [CrossRef]
- Sarzani, R.; Dessi-Fulgheri, P.; Paci, V.M.; Espinosa, E.; Rappelli, A. Expression of natriuretic peptide receptors in human adipose and other tissues. J. Endocrinol. Investig. 1996, 19, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Leptin-aldosterone-neprilysin axis: Identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation 2018, 137, 1614–1631. [Google Scholar] [CrossRef]
- Gentili, A.; Frangione, M.R.; Albini, E.; Vacca, C.; Ricci, M.A.; De Vuono, S.; Boni, M.; Rondelli, F.; Rotelli, L.; Lupattelli, G.; et al. Modulation of natriuretic peptide receptors in human adipose tissue: Molecular mechanisms behind the “natriuretic handicap” in morbidly obese patients. Transl. Res. 2017, 186, 52–61. [Google Scholar] [CrossRef]
- Meléndez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Rappelli, A. Hypertension and obesity after the menopause. J. Hypertens. 2002, 20 (Suppl. 2), 26–28. [Google Scholar]
- Frohlich, E.D.; Messerli, F.H.; Reisin, E.; Dunn, F.G. The problem of obesity and hypertension. Hypertension 1983, 5 (Suppl. III), 71–78. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, T.; Kishimoto, I.; Kokubo, Y.; Makino, H.; Miyamoto, Y.; Yoshimasa, Y. Association of plasma B-type natriuretic peptide levels with obesity in a general urban Japanese population:the Suita Study. Endocr. J. 2010, 57, 727–733. [Google Scholar] [CrossRef]
- Daniels, L.B.; Clopton, P.; Bhalla, V.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am. Heart J. 2006, 151, 999–1005. [Google Scholar] [CrossRef]
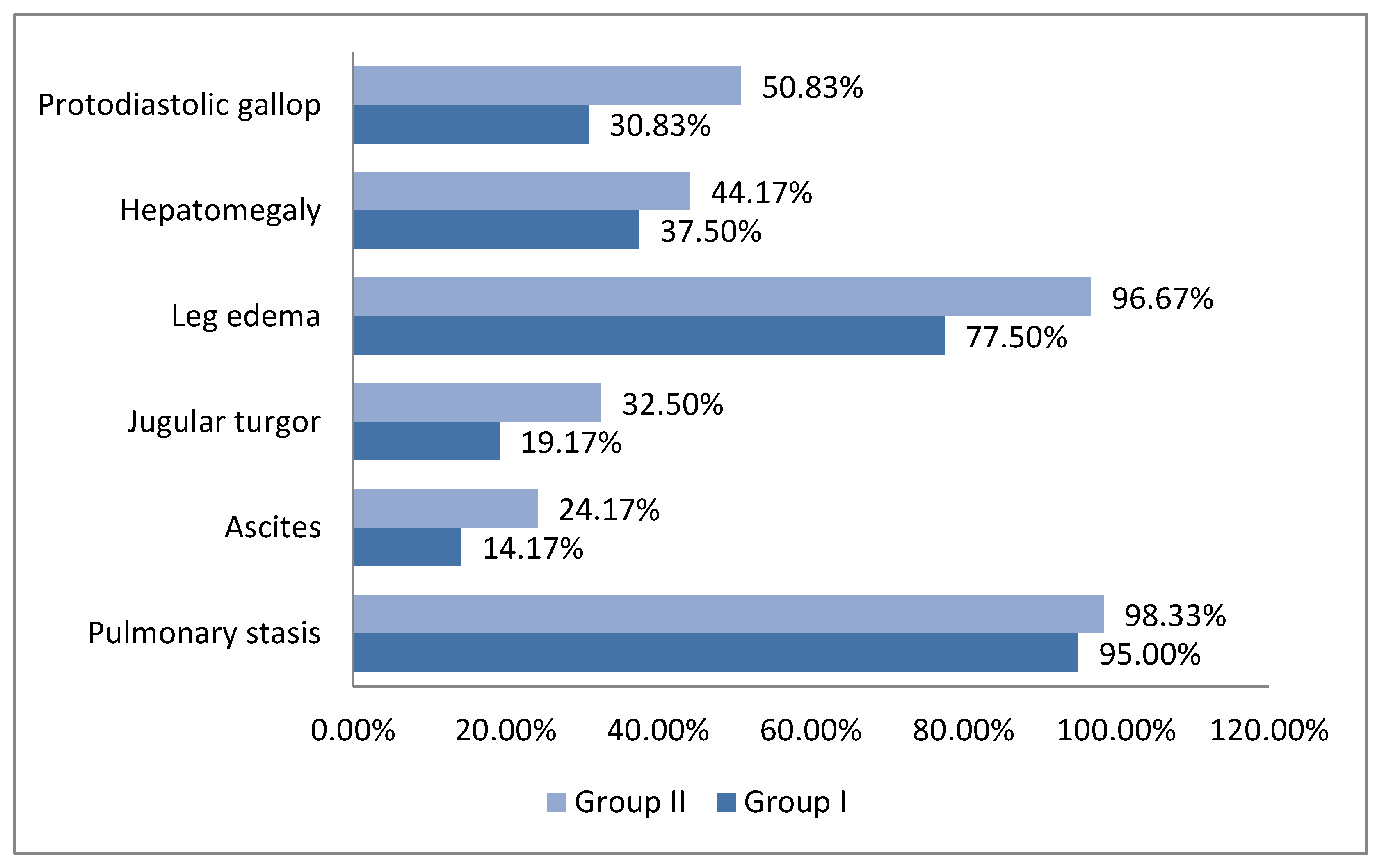

| Gender/BMI | Female | Male | p | ||||
| Nr. | Percent % | Nr. | Percent % | ||||
| <30 kg/m2 | 63 | 52.5 | 57 | 47.5 | 0.089 | ||
| ≥30 kg/m2 | 77 | 64.2 | 43 | 35.8 | |||
| BMI/NYHA class | NYHA II | NYHA III | NYHA IV | p | |||
| Nr. | Percent % | Nr. | Percent % | Nr. | Percent % | ||
| <30 kg/m2 | 30 | 25 | 55 | 45.8 | 35 | 29.2 | <0.001 |
| ≥30 kg/m2 | 0 | 0 | 80 | 66.7 | 40 | 33.3 | |
| BMI Group/BNP | Average ± SD | Median (IQR) | Mean Rank | p * |
| <30 kg/m2 | 107.12 ± 11.34 | 108.5 (106–112) | 177.92 | <0.001 |
| ≥30 kg/m2 | 62.09 ± 15.1 | 56 (53–67) | 63.08 | |
| Gender/BNP | Average ± SD | Median (IQR) | Mean Rank | p * |
| Female | 78.85 ± 26.1 | 67 (55–108) | 107.49 | 0.001 |
| Male | 92.66 ± 24.25 | 105 (75–110) | 138.72 | |
| Hypertension/BNP | Average ± SD | Median (IQR) | Mean Rank | p * |
| Absent | 80.47 ± 26.12 | 69.5 (55–107) | 109.93 | 0.020 |
| Present | 88.6 ± 25.72 | 105 (58–109) | 130.73 | |
| Diabetes/BNP | Average ± SD | Median (IQR) | Mean Rank | p * |
| Absent | 85.14 ± 26.3 | 95 (56–109) | 122.52 | 0.291 |
| Present | 81.65 ± 25.76 | 84 (55–106.5) | 109.42 | |
| CKD/BNP | Average ± SD | Median (IQR) | Mean Rank | p * |
| Absent | 83.68 ± 26.11 | 87 (56–109) | 120.47 | 0.994 |
| Present | 85.61 ± 26.37 | 98 (55–108) | 120.53 | |
| Rhythm status/BNP | Average ± SD | Median (IQR) | Mean Rank | p * |
| Sinus rhythm | 81.53 ± 26.63 | 76 (54–108) | 112.37 | 0.0504 |
| Atrial fibrillation | 88.18 ± 25.34 | 102 (58–110) | 129.95 | |
| NYHA/BNP | Average ± SD | Median (IQR) | Mean Rank | p ** |
| Class II | 105.07 ± 14.74 | 109 (98–112) | 171.58 | <0.001 *** |
| Class III | 82.13 ± 25.9 | 84 (55–108) | 114.19 | |
| Class IV | 80.88 ± 26.87 | 75 (55–108) | 111.43 |
| BMI index | Average ± SD | Median (IQR) | Average Rank | p * |
| <30 kg/m2 | 107.12 ± 11.34 | 108.5 (106–112) | 177.92 | <0.001 |
| ≥30 kg/m2 | 62.09 ± 15.1 | 56 (53–67) | 63.08 | |
| NYHA class | Average ± SD | Median (IQR) | Average Rank | p * |
| NYHA III | 64.7 ± 16.81 | 56 (53.2–69.5) | 64.71 | 0.061 |
| NYHA IV | 56.88 ± 9 | 55 (51–63) | 52.09 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| β (95% C.I.) | p | β (95% C.I.) | p | |
| BMI group (<30 kg/m2) | 45.02 (41.63–48.42) | <0.001 | 43.94 (39.54–48.35) | <0.001 |
| Gender (Male) | 13.81 (7.27–20.35) | <0.001 | 8.27 (4.81–11.72) | <0.001 |
| Hypertension | 8.12 (1.52–14.72) | 0.016 | −0.05 (−3.51–3.41) | 0.977 |
| NYHA Class | ||||
| Class II (Reference) | - | - | - | - |
| Class III | −22.94 (−32.93–−12.94) | <0.001 | 3.58 (−1.87–9) | 0.197 |
| Class IV | −24.18 (−34.88–−13.49) | <0.001 | −0.18 (−5.9–5.54) | 0.951 |
| Age | 2.83 (2.27–3.39) | <0.001 | 0.14 (−0.26–0.54) | 0.484 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, M.; Banszki, L.I.; Andronie-Cioara, F.L.; Pobirci, O.L.; Huplea, V.; Osiceanu, A.S.; Osiceanu, G.A.; Crisan, S.; Pobirci, D.D.; Guler, M.I.; et al. B-Type Natriuretic Peptide—A Paradox in the Diagnosis of Acute Heart Failure with Preserved Ejection Fraction in Obese Patients. Diagnostics 2024, 14, 808. https://doi.org/10.3390/diagnostics14080808
Rus M, Banszki LI, Andronie-Cioara FL, Pobirci OL, Huplea V, Osiceanu AS, Osiceanu GA, Crisan S, Pobirci DD, Guler MI, et al. B-Type Natriuretic Peptide—A Paradox in the Diagnosis of Acute Heart Failure with Preserved Ejection Fraction in Obese Patients. Diagnostics. 2024; 14(8):808. https://doi.org/10.3390/diagnostics14080808
Chicago/Turabian StyleRus, Marius, Loredana Ioana Banszki, Felicia Liana Andronie-Cioara, Oana Liliana Pobirci, Veronica Huplea, Alina Stanca Osiceanu, Gheorghe Adrian Osiceanu, Simina Crisan, Decebal Dumitru Pobirci, Madalina Ioana Guler, and et al. 2024. "B-Type Natriuretic Peptide—A Paradox in the Diagnosis of Acute Heart Failure with Preserved Ejection Fraction in Obese Patients" Diagnostics 14, no. 8: 808. https://doi.org/10.3390/diagnostics14080808
APA StyleRus, M., Banszki, L. I., Andronie-Cioara, F. L., Pobirci, O. L., Huplea, V., Osiceanu, A. S., Osiceanu, G. A., Crisan, S., Pobirci, D. D., Guler, M. I., & Marian, P. (2024). B-Type Natriuretic Peptide—A Paradox in the Diagnosis of Acute Heart Failure with Preserved Ejection Fraction in Obese Patients. Diagnostics, 14(8), 808. https://doi.org/10.3390/diagnostics14080808






