Borderline Ventricles: From Evaluation to Treatment
Abstract
:1. Introduction
2. Methods
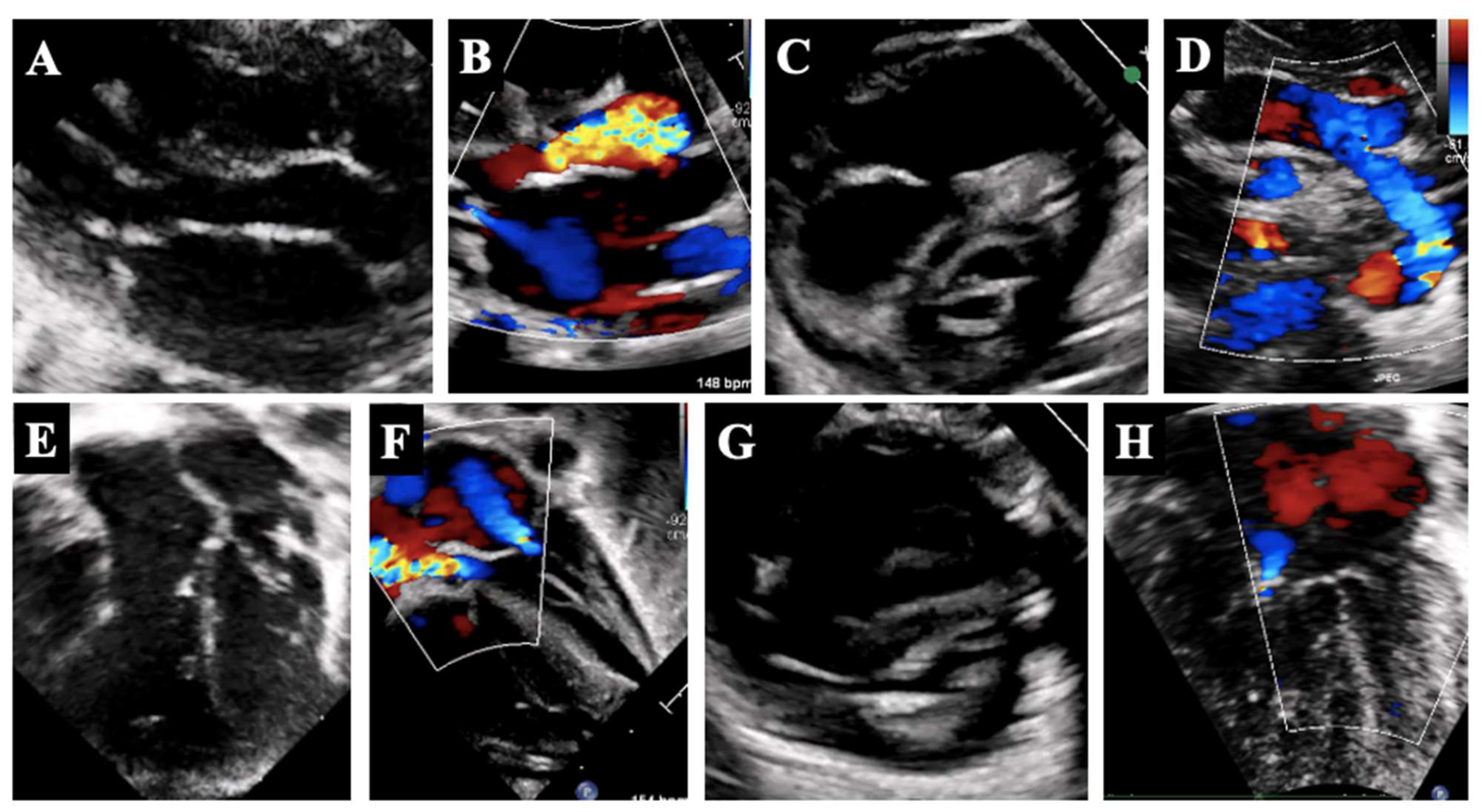
3. Borderline Left Ventricle: Predictors of Biventricular Repair
- Rhodes score (1991) [5]:
- CHSS equation (2001) [7]:
- Discriminant score (2006) [8]:
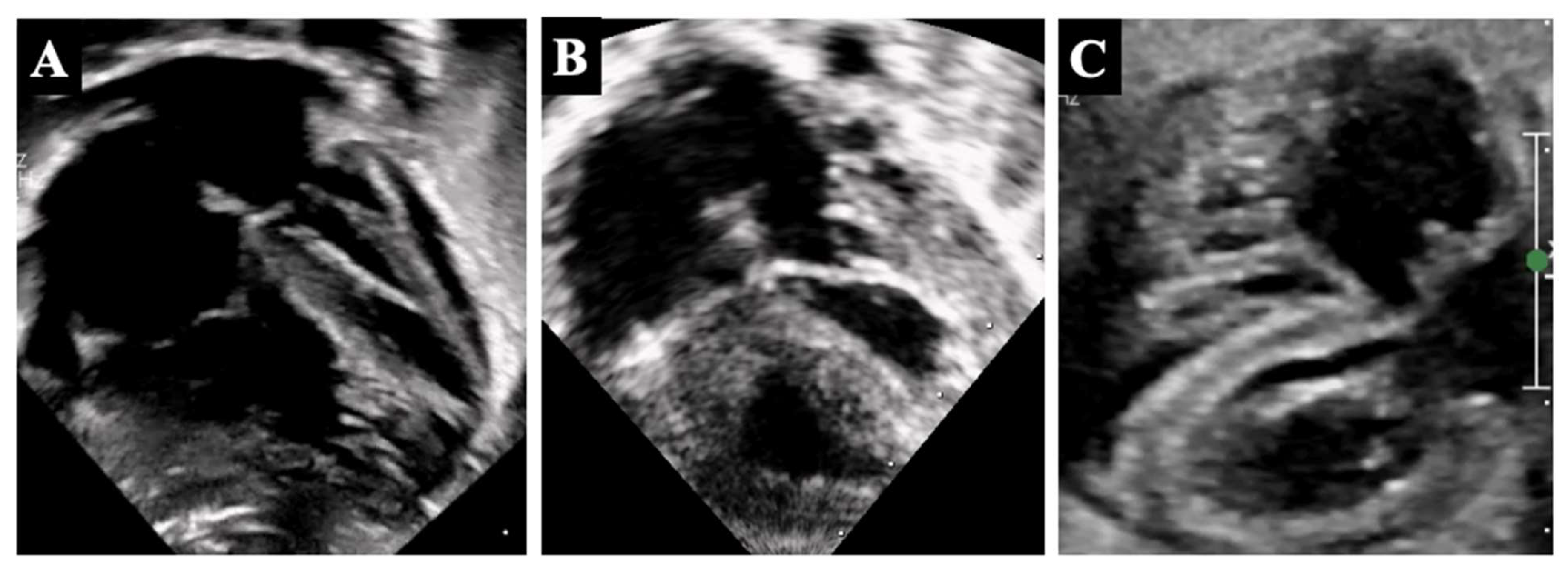
4. Borderline Right Ventricle: Predictors of Biventricular Repair
- The determination of whether the RV can support (or can be rehabilitated to support) a full pulmonary circulation and the systemic venous return;
- The morphologic assessment of the RV (unipartite, bipartite, or tripartite) and the infundibulum (membranous vs. muscular pulmonary atresia);
- The assessment of the TV size, morphology, and function;
5. Surgical Strategies
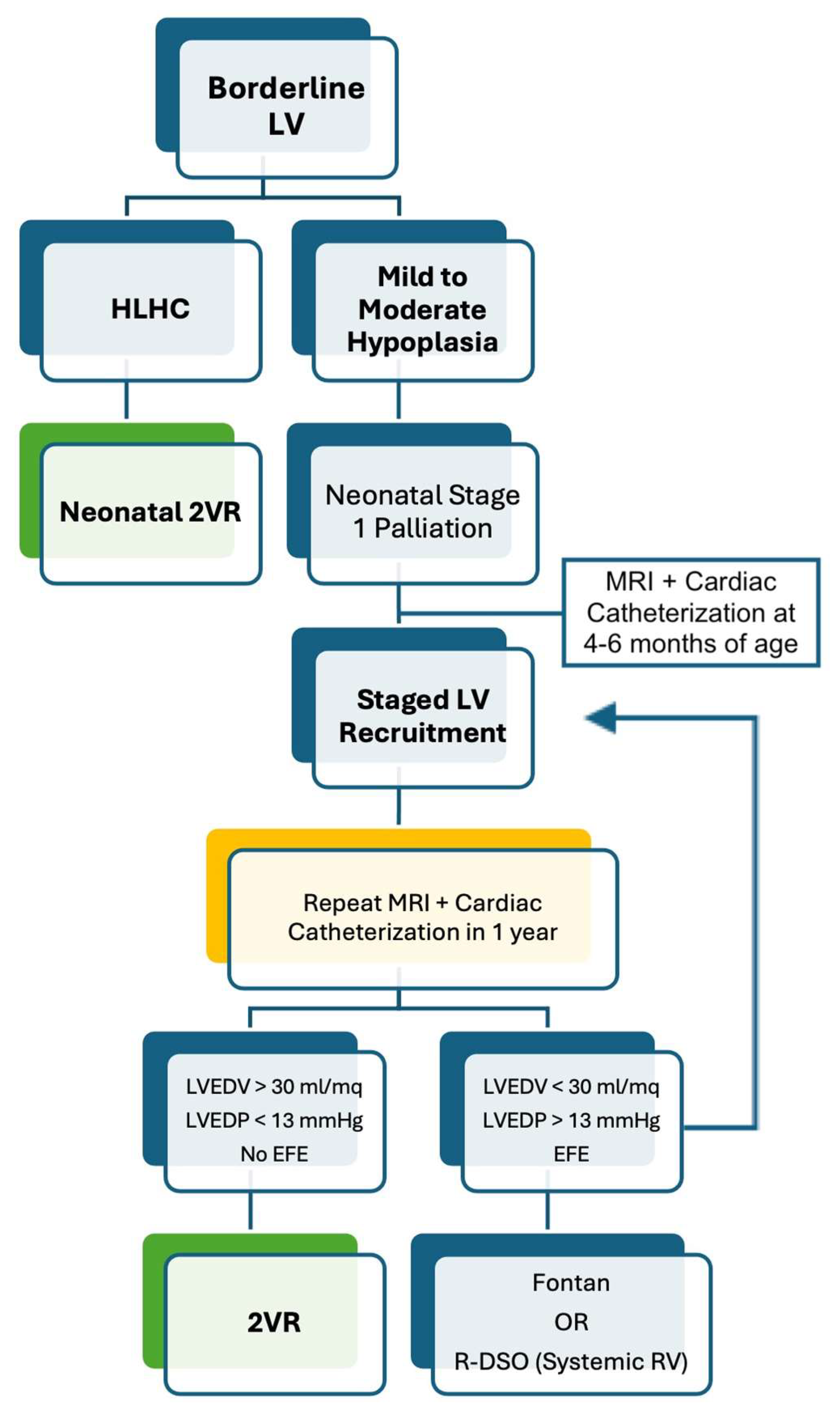
5.1. Borderline Left Ventricle
5.2. Borderline Right Ventricle
6. Post-Surgery Period
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Corno, A.F. Borderline left ventricle. Eur. J. Cardiothorac. Surg. 2005, 27, 67–73. [Google Scholar]
- Kaplinski, M.; Cohen, M.S. Characterising adequacy or inadequacy of the borderline left ventricle: What tools can we use? Cardiol. Young 2015, 25, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.J.; Caldarone, C.A.; McCrindle, B.W. Left ventricular hypoplasia: A spectrum of disease involving the left ventricular outflow tract, aortic valve, and aorta. J. Am. Coll. Cardiol. 2012, 59 (Suppl. S1), S43–S54. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.J.; Sleeper, L.A.; Lu, M.; Teele, S.A.; Breitbart, R.E.; Esch, J.J.; Salvin, J.W.; Kapoor, U.; Oladunjoye, O.; Emani, S.M.; et al. Factors associated with morbidity, mortality, and hemodynamic failure after biventricular conversion in borderline hypoplastic left hearts. J. Thorac. Cardiovasc. Surg. 2023, 166, 933–942.e3. [Google Scholar] [CrossRef]
- Rhodes, L.A.; Colan, S.D.; Perry, S.B.; Jonas, R.A.; Sanders, S.P. Predictors of survival in neonates with critical aortic stenosis. Circulation 1991, 84, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.M.; Mercer-Rosa, L.; Ravishankar, C.; Rome, J.J.; Gaynor, J.W.; Spray, T.L.; Cohen, M.S. Experience with biventricular intervention for neonates with mitral valve abnormalities in the setting of critical left-side heart obstruction. Ann. Thorac. Surg. 2015, 99, 877–883. [Google Scholar] [CrossRef]
- Lofland, G.K.; McCrindle, B.W.; Williams, W.G.; Blackstone, E.H.; Tchervenkov, C.I.; Sittiwangkul, R.; Jonas, R.A. Critical aortic stenosis in the neonate: A multi-institutional study of management, outcomes, and risk factors. Congenital Heart Surgeons Society. J. Thorac. Cardiovasc. Surg. 2001, 121, 10–27. [Google Scholar] [CrossRef]
- Colan, S.D.; McElhinney, D.B.; Crawford, E.C.; Keane, J.F.; Lock, J.E. Validation and re-evaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J. Am. Coll. Cardiol. 2006, 47, 1858–1865. [Google Scholar] [CrossRef]
- Cantinotti, M.; Marchese, P.; Giordano, R.; Franchi, E.; Assanta, N.; Koestenberger, M.; Jani, V.; Duignan, S.; Kutty, S.; McMahon, C.J. Echocardiographic scores for biventricular repair risk prediction of congenital heart disease with borderline left ventricle: A review. Heart Fail. Rev. 2023, 28, 63–76. [Google Scholar]
- Tuo, G.; Khambadkone, S.; Tann, O.; Kostolny, M.; Derrick, G.; Tsang, V.; Sullivan, I.; Marek, J. Obstructive left heart disease in neonates with a “borderline” left ventricle: Diagnostic challenges to choosing the best outcome. Pediatr. Cardiol. 2013, 34, 1567–1576. [Google Scholar]
- Mart, C.R.; Eckhauser, A.W. Development of an echocardiographic scoring system to predict biventricular repair in neonatal hypoplastic left heart complex. Pediatr. Cardiol. 2014, 35, 1456–1466. [Google Scholar] [CrossRef]
- Plymale, J.M.; Frommelt, P.C.; Nugent, M.; Simpson, P.; Tweddell, J.S.; Shillingford, A.J. The Infant with Aortic Arch Hypoplasia and Small Left Heart Structures: Echocardiographic Indices of Mitral and Aortic Hypoplasia Predicting Successful Biventricular Repair. Pediatr. Cardiol. 2017, 38, 1296–1304. [Google Scholar] [CrossRef]
- Tchervenkov, C.I.; Jacobs, J.P.; Weinberg, P.M.; Aiello, V.D.; Béland, M.J.; Colan, S.D.; Elliott, M.J.; Franklin, R.C.; Gaynor, J.W.; Krogmann, O.N.; et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol. Young 2006, 16, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Jegatheeswaran, A.; Pizarro, C.; Caldarone, C.A.; Cohen, M.S.; Baffa, J.M.; Gremmels, D.B.; Mertens, L.; Morell, V.O.; Williams, W.G.; Blackstone, E.H.; et al. Echocardiographic definition and surgical decision-making in unbalanced atrioventricular septal defect: A Congenital Heart Surgeons’ Society multiinstitutional study. Circulation 2010, 122 (Suppl. S11), S209–S215. [Google Scholar] [CrossRef] [PubMed]
- Overman, D.M.; Baffa, J.M.; Cohen, M.S.; Mertens, L.; Gremmels, D.B.; Jegatheeswaran, A.; McCrindle, B.W.; Blackstone, E.H.; Morell, V.O.; Caldarone, C.; et al. Unbalanced atrioventricular septal defect: Definition and decision making. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 91–96. [Google Scholar] [CrossRef]
- Cohen, M.S.; Jegatheeswaran, A.; Baffa, J.M.; Gremmels, D.B.; Overman, D.M.; Caldarone, C.A.; McCrindle, B.W.; Mertens, L. Echocardiographic features defining right dominant unbalanced atrioventricular septal defect: A multi-institutional Congenital Heart Surgeons’ Society study. Circ. Cardiovasc. Imaging 2013, 6, 508–513. [Google Scholar] [PubMed]
- Andersen, N.D.; Scherba, J.C.; Turek, J.W. Biventricular Conversion in the Borderline Hypoplastic Heart. Curr. Cardiol. Rep. 2020, 22, 115. [Google Scholar]
- Cohen, M.S. Assessing the borderline ventricle in a term infant: Combining imaging and physiology to establish the right course. Curr. Opin. Cardiol. 2018, 33, 95–100. [Google Scholar] [CrossRef]
- Lai, W.W.; Geva, T.; Shirali, G.S.; Frommelt, P.C.; Humes, R.A.; Brook, M.M.; Pignatelli, R.H.; Rychik, J. Task Force of the Pediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography. Guidelines and standards for performance of a pediatric echocardiogram: A report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 1413–1430. [Google Scholar]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiac Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease: Endorsed by The American Heart Association. Circ. Cardiovasc. Imaging 2022, 15, e014415. [Google Scholar]
- Lock, J.; Keane, J.F.; Perry, S.B. Diagnostic and Interventional Catheterization in Congenital Heart Disease, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Cantinotti, M.; McMahon, C.J.; Marchese, P.; Köstenberger, M.; Scalese, M.; Franchi, E.; Santoro, G.; Assanta, N.; Jacquemyn, X.; Kutty, S.; et al. Echocardiographic Parameters for Risk Prediction in Borderline Right Ventricle: Review with Special Emphasis on Pulmonary Atresia with Intact Ventricular Septum and Critical Pulmonary Stenosis. J. Clin. Med. 2023, 12, 4599. [Google Scholar] [CrossRef]
- Daubeney, P.E.; Delany, D.J.; Anderson, R.H.; Sandor, G.G.; Slavik, Z.; Keeton, B.R.; Webber, S.A. United Kingdom and Ireland Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Pulmonary atresia with intact ventricular septum: Range of morphology in a population-based study. J. Am. Coll. Cardiol. 2002, 39, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Bacha, E. Pulmonary Atresia with Intact Ventricular Septum with Borderline Tricuspid Valve: How Small Is Too Small. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2019, 22, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; de Leval, M.R.; Mercanti, C.; Macartney, F.J.; Anderson, R.H. Pulmonary atresia and intact ventricular septum: A revised classification. Circulation 1982, 66, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Burrows, P.E.; Freedom, R.M.; Benson, L.N.; Moes, C.A.; Wilson, G.; Koike, K.; Williams, W.G. Coronary angiography of pulmonary atresia, hypoplastic right ventricle, and ventriculocoronary communications. AJR Am. J. Roentgenol. 1990, 154, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Hanley, F.L.; Sade, R.M.; Blackstone, E.H.; Kirklin, J.W.; Freedom, R.M.; Nanda, N.C. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J. Thorac. Cardiovasc. Surg. 1993, 105, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, D.A.; Blackstone, E.H.; Wells, W.J.; Jonas, R.A.; Pigula, F.A.; Manning, P.B.; Lofland, G.K.; Williams, W.G.; McCrindle, B.W.; Congenital Heart Surgeons Study members. Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J. Thorac. Cardiovasc. Surg. 2004, 127, 1000–1007. [Google Scholar] [CrossRef]
- Alwi, M. Management algorithm in pulmonary atresia with intact ventricular septum. Catheter. Cardiovasc. Interv. 2006, 67, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Kostelka, M.; Sorensen, K.; de Leval, M. Outcome measures for the neonatal management of pulmonary atresia with intact ventricular septum. J. Thorac. Cardiovasc. Surg. 1994, 107, 359–366. [Google Scholar] [CrossRef]
- Minich, L.L.; Tani, L.Y.; Ritter, S.; Williams, R.V.; Shaddy, R.E.; Hawkins, J.A. Usefulness of the preoperative tricuspid/mitral valve ratio for predicting outcome in pulmonary atresia with intact ventricular septum. Am. J. Cardiol. 2000, 85, 1325–1328. [Google Scholar] [CrossRef]
- Drighil, A.; Aljufan, M.; Slimi, A.; Yamani, S.; Mathewson, J.; AlFadly, F. Echocardiographic determinants of successful balloon dilation in pulmonary atresia with intact ventricular septum. Eur. J. Echocardiogr. 2010, 11, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.J.; Glatz, A.C.; Qureshi, A.M.; Sachdeva, R.; Maskatia, S.A.; Justino, H.; Goldberg, D.J.; Mozumdar, N.; Whiteside, W.; Rogers, L.S.; et al. Outcomes After Decompression of the Right Ventricle in Infants with Pulmonary Atresia with Intact Ventricular Septum Are Associated with Degree of Tricuspid Regurgitation: Results from the Congenital Catheterization Research Collaborative. Circ. Cardiovasc. Interv. 2017, 10, e004428. [Google Scholar] [CrossRef] [PubMed]
- Maskatia, S.A.; Petit, C.J.; Travers, C.D.; Goldberg, D.J.; Rogers, L.S.; Glatz, A.C.; Qureshi, A.M.; Goldstein, B.H.; Ao, J.; Sachdeva, R. Echocardiographic parameters associated with biventricular circulation and right ventricular growth following right ventricular decompression in patients with pulmonary atresia and intact ventricular septum: Results from a multicenter study. Congenit. Heart Dis. 2018, 13, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.S.; Chau, A.K.T.; Chow, P.C.; Yung, T.C.; Cheung, Y.F.; Lun, K.S. Achieving biventricular circulation in patients with moderate hypoplastic right ventricle in pulmonary atresia intact ventricular septum after transcatheter pulmonary valve perforation. Congenit. Heart Dis. 2018, 13, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Santoro, G.; Gaio, G.; Cappelli, M.B.; Esposito, R.; Marzullo, R.; Di Masi, A.; Palladino, M.T.; Russo, M.G. Novel echocardiographic score to predict duct-dependency after percutaneous relief of critical pulmonary valve stenosis/atresia. Echocardiography 2022, 39, 724–731. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Lock, J.E.; Keane, J.F.; Moran, A.M.; Colan, S.D. Left heart growth, function, and reintervention after balloon aortic valvuloplasty for neonatal aortic stenosis. Circulation 2005, 111, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Tchervenkov, C.I.; Tahta, S.A.; Jutras, L.C.; Béland, M.J. Biventricular repair in neonates with hypoplastic left heart complex. Ann. Thorac. Surg. 1998, 66, 1350–1357. [Google Scholar] [PubMed]
- Chiu, P.; Emani, S. Left Ventricular Recruitment in Patients with Hypoplastic Left Heart Syndrome. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2021, 24, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hammel, J.M.; Duncan, K.F.; Danford, D.A.; Kutty, S. Two-stage biventricular rehabilitation for critical aortic stenosis with severe left ventricular dysfunction. Eur. J. Cardiothorac. Surg. 2013, 43, 143–148. [Google Scholar] [CrossRef]
- Emani, S.M. Management of the Borderline Left Heart and Alternatives to Fontan. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 645–649. [Google Scholar] [CrossRef]
- Greenleaf, C.E.; Salazar, J.D. Biventricular Conversion for Hypoplastic Left Heart Variants: An Update. Children 2022, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; McElhinney, D.B.; Tworetzky, W.; Myers, P.O.; Schroeder, B.; Zurakowski, D.; Pigula, F.A.; Marx, G.R.; Lock, J.E.; del Nido, P.J. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J. Am. Coll. Cardiol. 2012, 60, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Oreto, L.; Mandraffino, G.; Calaciura, R.E.; Poli, D.; Gitto, P.; Saitta, M.B.; Bellanti, E.; Carerj, S.; Zito, C.; Iorio, F.S.; et al. Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair. Children 2023, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Oreto, L.; Guccione, P.; Gitto, P.; Bruno, L.; Zanai, R.; Grasso, N.; Iannace, E.; Zito, C.; Carerj, S.; Agati, S. Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography. Children 2023, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Akintürk, H.; Yörüker, U.; Müller, M.; Schranz, D. Hypoplastic Left Ventricle: Left Ventricular Recruitment with Hybrid Approach. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Scully, B.B.; Feins, E.N.; Tworetzky, W.; Ghelani, S.; Beroukhim, R.; Del Nido, P.J.; Emani, S.M. Early Experience with Reverse Double Switch Operation for the Borderline Left Heart. Semin. Thorac. Cardiovasc. Surg. 2022, 36, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Barretta, J.O.; Grinenco, S.; Osuna, J.M.; Napoli, N.S. Novel use of reverse double switch operation in failed left ventricular recruitment pathway. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac173. [Google Scholar] [CrossRef] [PubMed]
- Delius, R.E.; Rademecker, M.A.; de Leval, M.R.; Elliott, M.J.; Stark, J. Is a high-risk biventricular repair always preferable to conversion to a single ventricle repair? J. Thorac. Cardiovasc. Surg. 1996, 112, 1561–1568. [Google Scholar] [PubMed]
- Shaddy, R.E.; Sturtevant, J.E.; Judd, V.E.; McGough, E.C. Right ventricular growth after transventricular pulmonary valvotomy and central aortopulmonary shunt for pulmonary atresia and intact ventricular septum. Circulation 1990, 82 (Suppl. S5), IV157-63. [Google Scholar] [PubMed]
- Jahangiri, M.; Zurakowski, D.; Bichell, D.; Mayer, J.E.; del Nido, P.J.; Jonas, R.A. Improved results with selective management in pulmonary atresia with intact ventricular septum. J. Thorac. Cardiovasc. Surg. 1999, 118, 1046–1055. [Google Scholar] [CrossRef]
- Yoshimura, N.; Yamaguchi, M.; Ohashi, H.; Oshima, Y.; Oka, S.; Yoshida, M.; Murakami, H.; Tei, T. Pulmonary atresia with intact ventricular septum: Strategy based on right ventricular morphology. J. Thorac. Cardiovasc. Surg. 2003, 126, 1417–1426. [Google Scholar] [CrossRef]
- Billingsley, A.M.; Laks, H.; Boyce, S.W.; George, B.; Santulli, T.; Williams, R.G. Definitive repair in patients with pulmonary atresia and intact ventricular septum. J. Thorac. Cardiovasc. Surg. 1989, 97, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Muster, A.J.; Zales, V.R.; Ilbawi, M.N.; Backer, C.L.; Duffy, C.E.; Mavroudis, C. Biventricular repair of hypoplastic right ventricle assisted by pulsatile bidirectional cavopulmonary anastomosis. J. Thorac. Cardiovasc. Surg. 1993, 105, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Siddharth, B.; Choudhary, S.K.; Airan, B. One and half ventricle repair: Rationale, indications, and results. Indian J. Thorac. Cardiovasc. Surg. 2018, 34, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Al-Radi, O.; Friedberg, M.K.; Caldarone, C.A.; Coles, J.G.; Oechslin, E.; Williams, W.G.; Van Arsdell, G.S. Superior vena cava to pulmonary artery anastomosis as an adjunct to biventricular repair: 38-year follow-up. Ann. Thorac. Surg. 2009, 87, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Cabrelle, G.; Castaldi, B.; Vedovelli, L.; Gregori, D.; Vida, V.L.; Padalino, M.A. Long-term experience with the one-and-a-half ventricle repair for simple and complex congenital heart defects. Eur. J. Cardiothorac. Surg. 2021, 59, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Banka, P.; Lafranchi, T.; Tworetzky, W.; Del Nido, P.; Emani, S.M. Biventricular conversion after single ventricle palliation in patients with small left heart structures: Short-term outcomes. Ann. Thorac. Surg. 2013, 96, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Di Salvo, G. Role of Cardiovascular Imaging in the Follow-Up of Patients with Fontan Circulation. Children 2022, 9, 1875. [Google Scholar] [CrossRef]
- Mazza, G.A.; Gribaudo, E.; Agnoletti, G. The pathophysiology and complications of Fontan circulation. Acta Biomed. 2021, 92, e2021260. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazza, G.A.; Oreto, L.; Tuo, G.; Sirico, D.; Moscatelli, S.; Meliota, G.; Micari, A.; Guccione, P.; Rinelli, G.; Favilli, S. Borderline Ventricles: From Evaluation to Treatment. Diagnostics 2024, 14, 823. https://doi.org/10.3390/diagnostics14080823
Mazza GA, Oreto L, Tuo G, Sirico D, Moscatelli S, Meliota G, Micari A, Guccione P, Rinelli G, Favilli S. Borderline Ventricles: From Evaluation to Treatment. Diagnostics. 2024; 14(8):823. https://doi.org/10.3390/diagnostics14080823
Chicago/Turabian StyleMazza, Giuseppe Antonio, Lilia Oreto, Giulia Tuo, Domenico Sirico, Sara Moscatelli, Giovanni Meliota, Antonio Micari, Paolo Guccione, Gabriele Rinelli, and Silvia Favilli. 2024. "Borderline Ventricles: From Evaluation to Treatment" Diagnostics 14, no. 8: 823. https://doi.org/10.3390/diagnostics14080823




