Prenatal Diagnosis and Prognosis of Abdominal Arteriovenous Fistulae: A Comprehensive Case Series and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Results of the Systematic Review
3.1.1. Postnatal Manifestations
3.1.2. Prenatal and Postnatal Management
3.2. Case Series
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartung, J.; Chaoui, R.; Kalache, K.; Tennstedt, C.; Bollmann, R. Prenatal diagnosis of intrahepatic communications of the umbilical vein with atypical arteries (A-V fistulae) in two cases of trisomy 21 using color Doppler ultrasound. Ultrasound Obstet. Gynecol. 2000, 16, 271–274. [Google Scholar] [CrossRef]
- Demirci, O.; Celayir, A. Prenatal diagnosis and treatment of intrahepatic arteriovenous fistulas: Case reports and the literature review. J. Matern. Fetal Neonatal Med. 2022, 35, 837–845. [Google Scholar] [CrossRef]
- Achiron, R.; Kivilevitch, Z. Fetal umbilical-portal-systemic venous shunt: In-utero classification and clinical significance. Ultrasound Obst. Gynecol. 2016, 47, 739–747. [Google Scholar] [CrossRef]
- Nagy, R.D.; Iliescu, D.G. Prenatal Diagnosis and Outcome of Umbilical-Portal-Systemic Venous Shunts: Experience of a Tertiary Center and Proposal for a New Complex Type. Diagnostics 2022, 12, 873. [Google Scholar] [CrossRef]
- Gembruch, U.; Baschat, A.A.; Gloeckner-Hoffmann, K.; Gortner, L.; Germer, U. Prenatal diagnosis and management of fetuses with liver hemangiomata. Ultrasound Obstet. Gynecol. 2002, 19, 454–460. [Google Scholar] [CrossRef]
- Arnell, H.; Fischler, B. Congenital Vascular Malformations Are Associated With Trisomy 21. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e82. [Google Scholar] [CrossRef]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to do a fetal cardiac scan. Arch. Gynecol. Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef]
- Jouannic, J.M.; Jacquemard, F.; Mirlesse, V.; Capella-Pavlovsky, M.; Fermont, L.; Brunelle, F.; Daffos, F. Fistule artério-veineuse intrahépatique. Diagnostic anténatal, étude physiopathologique et prise en charge néonatale [Intrahepatic arteriovenous fistula. Prenatal diagnosis, physiopathological study and neonatal management]. J. Gynecol. Obstet. Biol. Reprod. 1998, 27, 90–94. [Google Scholar]
- Tseng, J.J.; Chou, M.M.; Lee, Y.H.; Ho, E.S. Prenatal diagnosis of intrahepatic arteriovenous shunts. Ultrasound Obstet. Gynecol. 2000, 15, 441–444. [Google Scholar] [CrossRef]
- Lima, M.; Lalla, M.; Aquino, A.; Dòmini, M.; Tursini, S.; Ruggeri, G.; Pelusi, G.; Pigna, A.; Tani, G.; Pilu, G.L.; et al. Congenital symptomatic intrahepatic arteriovenous fistulas in newborns: Management of 2 cases with prenatal diagnosis. J. Pediatr. Surg. 2005, 40, e1–e5. [Google Scholar] [CrossRef]
- Mejides, A.A.; Adra, A.M.; O’Sullivan, M.J.; Nicholas, M.C. Prenatal diagnosis and therapy for a fetal hepatic vascular malformation. Obstet. Gynecol. 1995, 85, 850–853. [Google Scholar] [CrossRef]
- Botha, T.; Rasmussen, O.; Carlan, S.J.; Greenbaum, L. Congenital Hepatic Arteriovenous Malformation: Sonographic Findings and Clinical Implications. J. Diagn. Med. Sonogr. 2004, 20, 177–181. [Google Scholar] [CrossRef]
- Douhnai, D.; Tassin, M.; Sibiude, J.; Franchi-Abella, S.; Ackermann, O.; Mandelbrot, L.; Picone, O. Prenatal diagnosis of intrahepatic arteriovenous fistula: Case report and review of the literature. J. Matern. Fetal Neonatal Med. 2019, 32, 2575–2578. [Google Scholar] [CrossRef]
- Gedikbasi, A.; Oztarhan, K.; Sahin, B.; Bingol, B.; Kurugoglu, S.; Senyuz, O.F.; Ceylan, Y. Multidisciplinary approach to congenital multiple arterio-porto-caval malformation: Case report. Am. J. Perinatol. 2008, 25, 265–270. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Q.; Peng, Q.; Zeng, S. Prenatal Diagnosis of Aorta-Porto-Umbilical Vein Fistulas with Left-Sided Inferior Vena Cava. AJP Rep. 2016, 6, e59–e61. [Google Scholar] [CrossRef][Green Version]
- Luks, F.I.; Yazbeck, S.; Brandt, M.L.; Bensoussan, A.L.; Brochu, P.; Blanchard, H. Benign liver tumors in children: A 25-year experience. J. Pediatr. Surg. 1991, 26, 1326–1330. [Google Scholar] [CrossRef]
- Anai, T.; Miyakawa, I.; Ohki, H.; Ogawa, T. Hydrops fetalis caused by fetal Kasabach-Merritt syndrome. Acta Paediatr. Jpn. 1992, 34, 324–327. [Google Scholar] [CrossRef]
- Iliescu, D.G.; Ruican, D.; Nagy, R.; Burada, F. Aorta-umbilical vein fistulae in fetus with trisomy-17 mosaicism. Ultrasound Obstet. Gynecol. 2020, 55, 419–421. [Google Scholar] [CrossRef]
- Gallego, C.; Miralles, M.; Marín, C.; Muyor, P.; González, G.; García-Hidalgo, E. Congenital hepatic shunts. Radiographics 2004, 24, 755–772, Erratum in Radiographics 2004, 24, 1514. [Google Scholar] [CrossRef]
- Chavan, A.; Caselitz, M.; Gratz, K.F.; Lotz, J.; Kirchhoff, T.; Piso, P.; Wagner, S.; Manns, M.; Galanski, M. Hepatic artery embolization for treatment of patients with hereditary hemorrhagic telangiectasia and symptomatic hepatic vascular malformations. Eur. Radiol. 2004, 14, 2079–2085. [Google Scholar] [CrossRef]
- Hazebroek, F.W.; Tibboel, D.; Robben, S.G.; Bergmeyer, J.H.; Molenaar, J.C. Hepatic artery ligation for hepatic vascular tumors with arteriovenous and arterioportal venous shunts in the newborn: Successful management of two cases and review of the literature. J. Pediatr. Surg. 1995, 30, 1127–1130. [Google Scholar] [CrossRef]
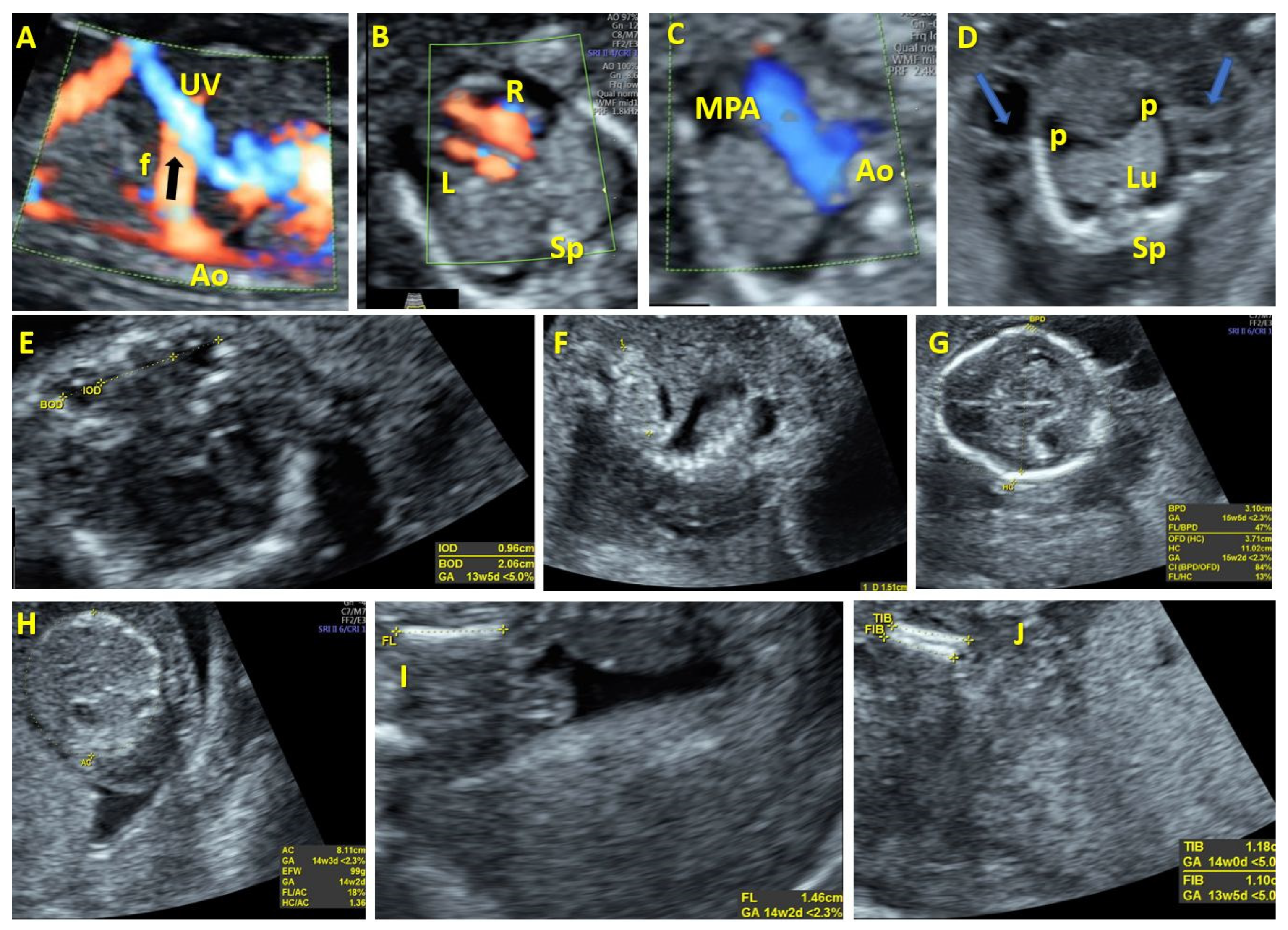
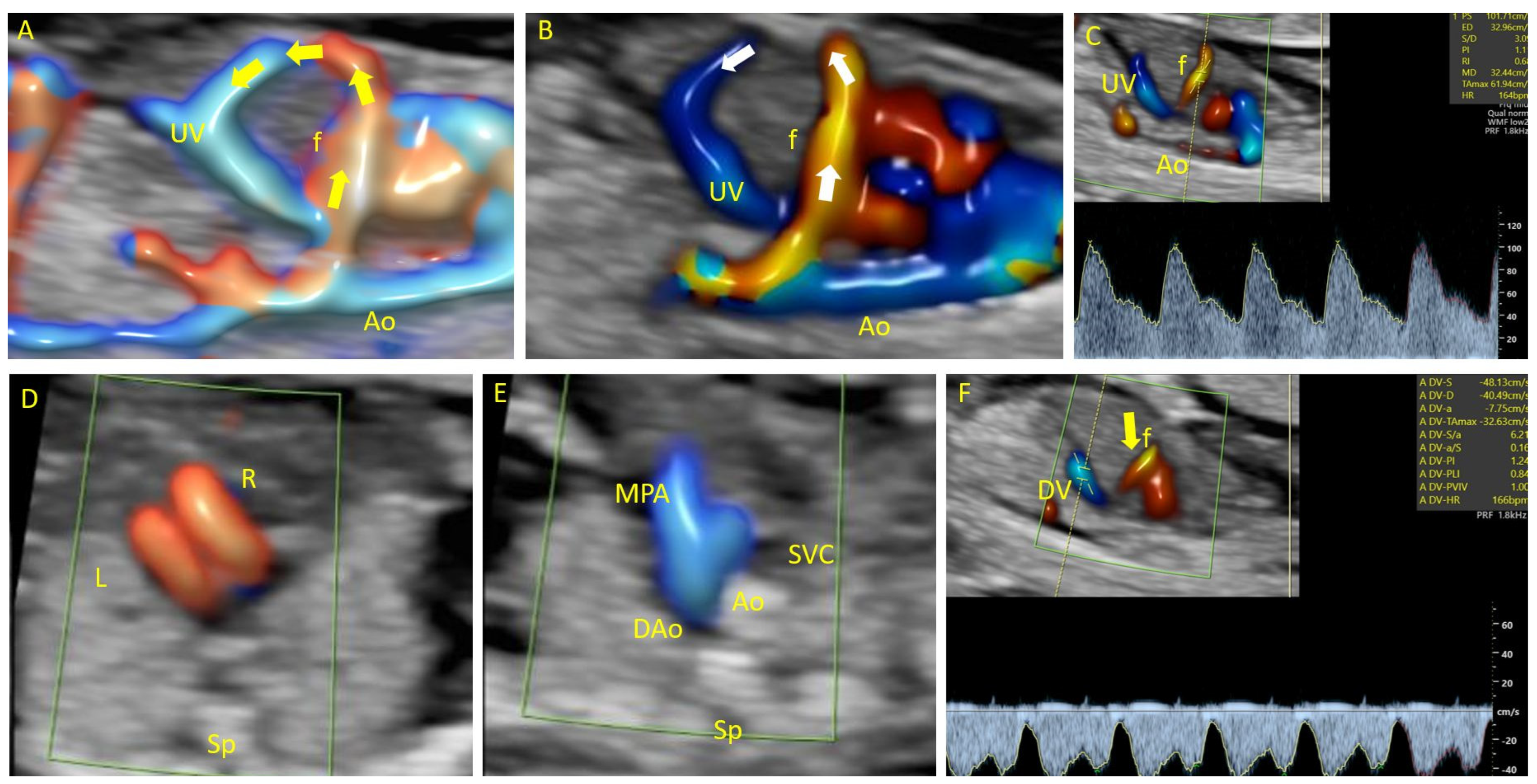
| References | GA | Vascular Anomaly | Associated Anomalies | Type of Fistula | Spectral Analyses | Prenatal Management | Prenatal Outcome | Karyotype | Postnatal Management | Postnatal Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Jouannic et al. [8] 1998 | 30 WG | Vascular hypoechogenic structure in the liver | None | Porto-hepatic | Not evaluated | Follow-up | Stationary | Not evaluated | Embolization | Death due to cardiac failure |
| Tseng et al. [9] 2000 | 35 WG | Prominent portal and hepatic veins | Oligohydramnios cardiomegaly | Hepatic artery-portal vein-middle hepatic vein | Arterial flow | Follow-up | Atable | Not evaluated | Ligature of the left hepatic vein | Patent ductus venosus at 6 months, good at 3 years old |
| Lima et al. [10] 2005 | 25 WG | Suprarenal aortic and left hepatic artery dilatation | Mild cardiomegaly Minimal pericardial effusion | Hepatic artery-hepatic vein | Not evaluated | Follow-up | Stable | Normal | Embolization | Death due to cardiac failure |
| Lima et al. [10] 2005 | 27 WG | Fluid-filled area affecting the upper part of the left hemi abdomen | Severe cardiomegaly | Hepatic artery-hepatic vein | Arterial flow | Follow-up | Stable | Normal | Left hepatectomy | Good at 20 weeks postnatal |
| Mejides et al. [11] 1995 | 29 WG | Anechoic areas within the fetal liver | Cardiomegaly Cardiac failure Moderate pericardial effusion | Hepatic artery-hepatic vein | Arterial flow | Intrauterine corticosteroid therapy Maternal digoxin | Stable | Normal | Prednisone and furosemide | Good |
| Botha et al. [12] 2004 | 34 WG | Echogenic dilated vascular channels within the liver | Cardiomegaly polyhydramnios | Hepatic vein-hepatic artery-right and left internal mammary artery- | Not evaluated | Follow-up | Fetal distress | - | Embolization | Death due to cardiac failure |
| Douhnai et al. [13] 2018 | 26 WG | A tuft of vessel in the left lobe, enlarged left hepatic artery, enlarged left portal vein | Polyhydramnios | Hepatic artery-portal vein | Arterial flow | Follow-up | Stable | - | Follow-up | Good Patent DV |
| Gedikbasi et al. [14] 2008 | 36 WG | Vascular structure/lake | None | Hepatic artery-hepatic vein-superior mesenteric artery | Arterial flow | Follow-up | Stable | - | Right extended hepatectomy, cholecystectomy, local removal of the vascular ectasia/ structure | Good |
| Demirci and Celayir [2] 2020 | 32 WG | Thick multiseptic cystic lobulated contoured mass | None | Hepatic artery-hepatic vein-umbilical vein | Not evaluated | Follow-up | Stable | Not evaluated | Prophylaxis with steroids and Propranolol, followed by right extended hepatectomy | Good at 18 months |
| Demirci and Celayir [2] 2020 | 24 WG | Large celiac artery, enlarged hepatic veins. | Ascites Dilated cardiomegaly | Hepatic artery- hepatic vein | Normal venous pattern | Dexamethasone propranolol | Stable under treatment | Not evaluated | Propranolol | Good at 2 months |
| Hartung et al. [1] 2000 | 14 WG | Atypical vessel | Hydrops Complete atrioventricular canal | Abdominal aorta-umbilical vein | Arterial flow | Follow-up | Stationary | Trisomy 21 | Not applicable | Abortion at 15 WG |
| Hartung et al. [1] 2000 | 31 WG | Dilated umbilical vein joined by hepatic artery | Hydrops Polyhydramnios, suspicious for duodenal atresia | Hepatic artery-umbilical vein | Arterial flow | None | No follow-up | Trisomy 21 | Not applicable | Stillbirth at 36 WG |
| Zhou et al. [15] 2016 | 31 WG | Dilated umbilical vein, vascular structure in the middle of the liver | Cardiomegaly Perimembranous ventricular septal defect, left-sided inferior vena cava | Aorta-portal vein-umbilical vein | Arterial flow | Follow-up | Stable | Normal | Extended right hepatectomy | Good at 3 months |
| Case nr. | GA | Vascular Anomaly | Associated Anomalies | Type of Fistula | Spectral Analyses | Prenatal Management | Prenatal Outcome | Karyotype | Postnatal Management | Postnatal Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | Atypical vessel | Dilation of right heart chambers, early IUGR, hypotelorism, dysplastic kidneys, hydrops fetalis | Aorta-umbilical vein | Arterial flow | Follow-up | Fetal distress | 45X | Not applicable | Abortion at 19 WG |
| 2 | 19 | Intrahepatic arteriovenous complex malformation | Vermian malrotation, partial agenesis of the corpus callosum, frontal bossing, low nasal bridge, dilation of right heart chambers | Aorta-umbilical vein | Arterial flow | Follow-up | Fetal distress | Mosaic trisomy 17 | Not applicable | Abortion at 22 WG |
| 3 | 22 | Atypical vessel | Hydrocephalus, corpus callosum hypoplasia | Aorta-umbilical vein | Arterial flow | Follow-up | Stationary | Normal | Not applicable | Abortion at 23 WG |
| 4 | 13 | Atypical vessel | Cystic hygroma PVSA | Aorta-umbilical vein | Arterial flow | Follow-up | Fetal distress | Not performed | Not applicable | Abortion |
| 5 | 13 | Atypical vessel | Bilateral radius agenesis | Aorto-hepatic vein | Not evaluated | Follow-up | Stationary | T18 | Not applicable | Abortion |
| 6 | 13 | Atypical vessel | Megacystis, hand and foot malposition, skeletal malformations, hygroma, hemivertebra, VSD absent CSP | Aorta-umbilical vein | Not evaluated | Follow-up | Fetal distress | Not performed | Not applicable | Abortion |
| 7 | 13 Twin pregnancy | Atypical vessel | Absent | Aorta-umbilical vein | Arterial flow | Follow-up | Fetal distress | T21 | Not applicable | IUD of the affected fetus |
| 8 | 30 | Dilated vascular channels within the liver | Dilation of right heart chambers | Aorto-hepatic vein | Arterial flow | Follow-up | Stationary | Normal | Embolization of the fistulous tract | Death due to cardiac failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, A.; Nagy, R.D.; Constantin, C.; Gheonea, I.A.; Iliescu, D.G. Prenatal Diagnosis and Prognosis of Abdominal Arteriovenous Fistulae: A Comprehensive Case Series and Systematic Review. Diagnostics 2024, 14, 826. https://doi.org/10.3390/diagnostics14080826
Ungureanu A, Nagy RD, Constantin C, Gheonea IA, Iliescu DG. Prenatal Diagnosis and Prognosis of Abdominal Arteriovenous Fistulae: A Comprehensive Case Series and Systematic Review. Diagnostics. 2024; 14(8):826. https://doi.org/10.3390/diagnostics14080826
Chicago/Turabian StyleUngureanu, Anda, Rodica Daniela Nagy, Cristian Constantin, Ioana Andreea Gheonea, and Dominic Gabriel Iliescu. 2024. "Prenatal Diagnosis and Prognosis of Abdominal Arteriovenous Fistulae: A Comprehensive Case Series and Systematic Review" Diagnostics 14, no. 8: 826. https://doi.org/10.3390/diagnostics14080826
APA StyleUngureanu, A., Nagy, R. D., Constantin, C., Gheonea, I. A., & Iliescu, D. G. (2024). Prenatal Diagnosis and Prognosis of Abdominal Arteriovenous Fistulae: A Comprehensive Case Series and Systematic Review. Diagnostics, 14(8), 826. https://doi.org/10.3390/diagnostics14080826








