Artificial Intelligence in Psychiatry: A Review of Biological and Behavioral Data Analyses
Abstract
1. Introduction: The Rise of Artificial Intelligence in Psychiatry
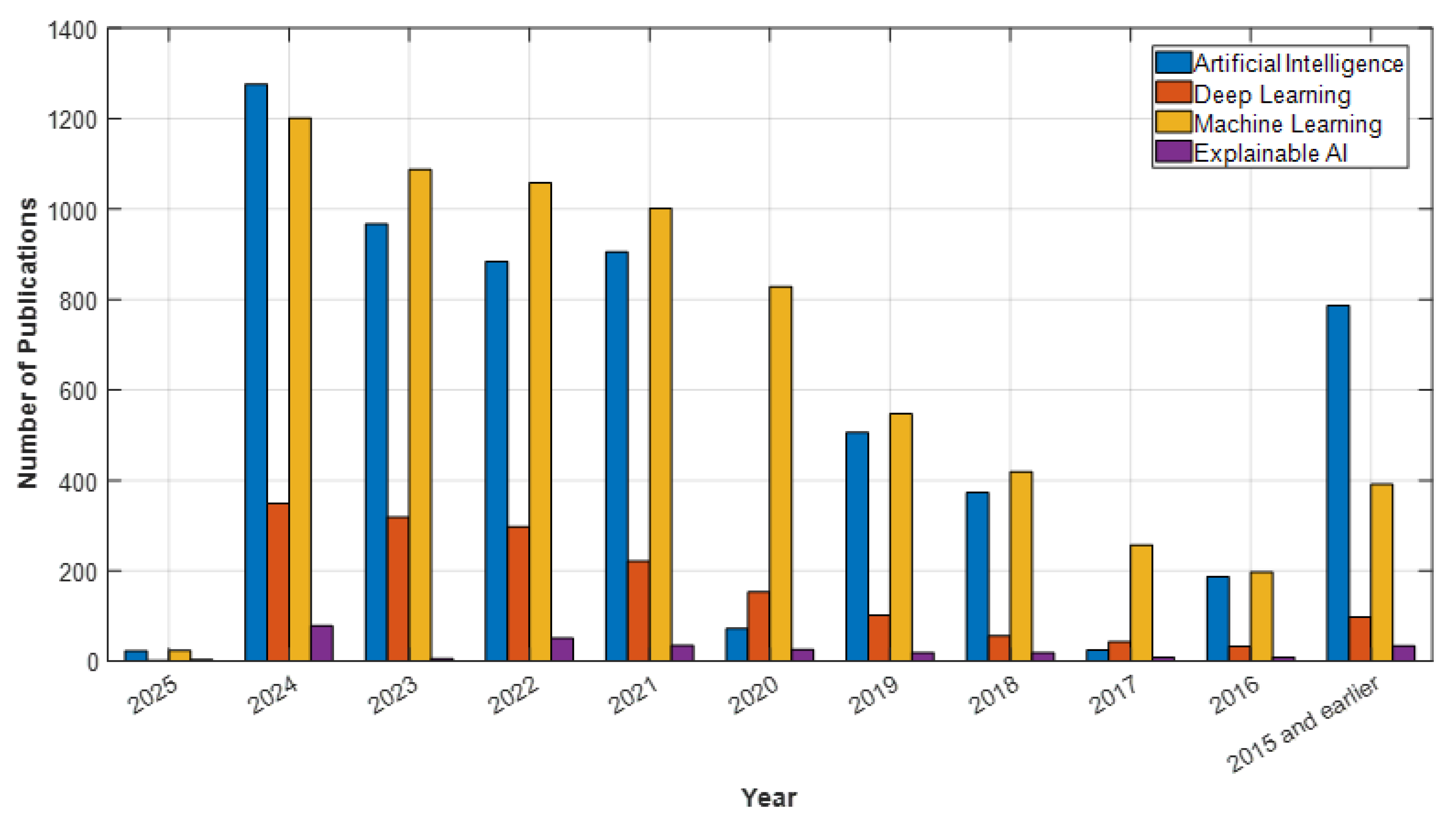
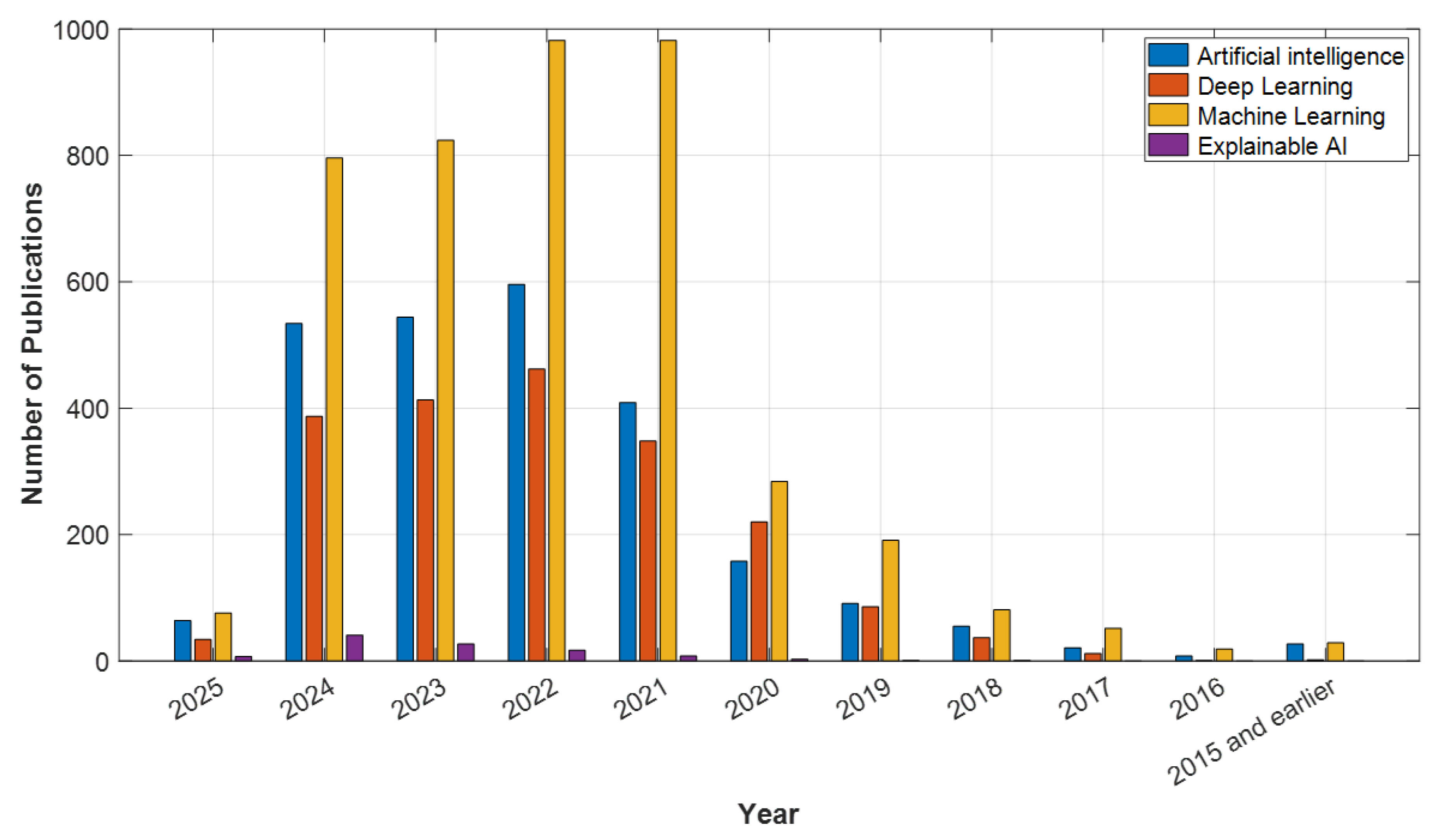
2. Methodology
3. EEG and AI: New Horizons in Brain Wave Analysis
4. ECG and AI: The Link Between Heart Rhythm and Mental Health
5. Speech Analysis and Artificial Intelligence: Detection of Emotional States
6. Blood Tests and AI: New Approaches in Biomarker Analysis
7. Social Media and Psychiatry: A New Frontier in Mental Health Assessment
8. Open-Source Datasets for AI Applications in Psychiatry
9. Recent Advances and Future Trends in AI-Based Psychiatry
10. Challenges and Limitations of AI in Psychiatry
11. Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmerman, M.; Morgan, T.A.; Stanton, K. The severity of psychiatric disorders. World Psychiatry 2018, 17, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.R. An analysis of diagnostic reasoning. I. The domains and disorders of clinical macrobiology. Yale J. Biol. Med. 1973, 46, 212. [Google Scholar]
- National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Jarrahi, M.H. Artificial intelligence and the future of work: Human-AI symbiosis in organizational decision making. Bus. Horiz. 2018, 61, 577–586. [Google Scholar] [CrossRef]
- Liu, G.-D.; Li, Y.-C.; Zhang, W.; Zhang, L. A brief review of artificial intelligence applications and algorithms for psychiatric disorders. Engineering 2020, 6, 462–467. [Google Scholar] [CrossRef]
- Arslan, S.; Kaya, M.K.; Tasci, B.; Kaya, S.; Tasci, G.; Ozsoy, F.; Dogan, S.; Tuncer, T. Attention TurkerNeXt: Investigations into Bipolar Disorder Detection Using OCT Images. Diagnostics 2023, 13, 3422. [Google Scholar] [CrossRef]
- Aminizadeh, S.; Heidari, A.; Dehghan, M.; Toumaj, S.; Rezaei, M.; Navimipour, N.J.; Stroppa, F.; Unal, M. Opportunities and challenges of artificial intelligence and distributed systems to improve the quality of healthcare service. Artif. Intell. Med. 2024, 149, 102779. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 1–17. [Google Scholar] [CrossRef]
- Kendler, K.S. Toward a philosophical structure for psychiatry. Am. J. Psychiatry 2005, 162, 433–440. [Google Scholar] [CrossRef]
- Kessler, R.C.; Wittchen, H.-U.; Abelson, J.; Zhao, S.; Stone, A. Methodological issues in assessing psychiatric disorders with self-reports. In The Science of Self-Report: Implications for Research and Practice; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2000; pp. 229–255. [Google Scholar]
- Jiang, M.; Zhao, Q.; Li, J.; Wang, F.; He, T.; Cheng, X.; Yang, B.X.; Ho, G.W.; Fu, G. A Generic Review of Integrating Artificial Intelligence in Cognitive Behavioral Therapy. arXiv 2024, arXiv:2407.19422. [Google Scholar]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. [Google Scholar]
- Ray, A.; Bhardwaj, A.; Malik, Y.K.; Singh, S.; Gupta, R. Artificial intelligence and Psychiatry: An overview. Asian J. Psychiatry 2022, 70, 103021. [Google Scholar] [CrossRef]
- Sun, J.; Dong, Q.-X.; Wang, S.-W.; Zheng, Y.-B.; Liu, X.-X.; Lu, T.-S.; Yuan, K.; Shi, J.; Hu, B.; Lu, L. Artificial intelligence in psychiatry research, diagnosis, and therapy. Asian J. Psychiatry 2023, 87, 103705. [Google Scholar] [CrossRef] [PubMed]
- Noachtar, S.; Remi, J.; Kaufmann, E. EEG-Update. Klin. Neurophysiol. 2022, 53, 243–252. [Google Scholar] [CrossRef]
- Jafari, M.; Sadeghi, D.; Shoeibi, A.; Alinejad-Rokny, H.; Beheshti, A.; García, D.L.; Chen, Z.; Acharya, U.R.; Gorriz, J.M. Empowering precision medicine: AI-driven schizophrenia diagnosis via EEG signals: A comprehensive review from 2002–2023. Appl. Intell. 2024, 54, 35–79. [Google Scholar] [CrossRef]
- Jafari, M.; Shoeibi, A.; Khodatars, M.; Bagherzadeh, S.; Shalbaf, A.; García, D.L.; Gorriz, J.M.; Acharya, U.R. Emotion recognition in EEG signals using deep learning methods: A review. Comput. Biol. Med. 2023, 165, 107450. [Google Scholar] [CrossRef]
- Metin, S.Z.; Uyulan, Ç.; Farhad, S.; Ergüzel, T.T.; Türk, Ö.; Metin, B.; Çerezci, Ö.; Tarhan, N. Deep learning-based artificial Intelligence can differentiate treatment-resistant and responsive depression cases with high accuracy. Clin. EEG Neurosci. 2024, 56, 119–130. [Google Scholar] [CrossRef]
- Şahin Sadık, E.; Saraoğlu, H.M.; Canbaz Kabay, S.; Tosun, M.; Keskinkılıç, C.; Akdağ, G. Investigation of the effect of rosemary odor on mental workload using EEG: An artificial intelligence approach. Signal Image Video Process. 2022, 16, 497–504. [Google Scholar] [CrossRef]
- Anderer, P.; Roberts, S.; Schlögl, A.; Gruber, G.; Klösch, G.; Herrmann, W.; Rappelsberger, P.; Filz, O.; Barbanoj, M.J.; Dorffner, G. Artifact processing in computerized analysis of sleep EEG—A review. Neuropsychobiology 1999, 40, 150–157. [Google Scholar] [CrossRef]
- Erguzel, T.T.; Ozekes, S.; Sayar, G.H.; Tan, O.; Tarhan, N. A hybrid artificial intelligence method to classify trichotillomania and obsessive compulsive disorder. Neurocomputing 2015, 161, 220–228. [Google Scholar] [CrossRef]
- Earl, E.H.; Goyal, M.; Mishra, S.; Kannan, B.; Mishra, A.; Chowdhury, N.; Mishra, P. EEG based Functional Connectivity in Resting and Emotional States may identify Major Depressive Disorder using Machine Learning. Clin. Neurophysiol. 2024, 164, 130–137. [Google Scholar] [CrossRef]
- Xia, M.; Zhao, X.; Deng, R.; Lu, Z.; Cao, J. EEGNet classification of sleep EEG for individual specialization based on data augmentation. Cogn. Neurodynamics 2024, 18, 1539–1547. [Google Scholar] [CrossRef]
- Madhu, S.; Kumar, P.; Chandra, S. Ensemble Learning based EEG Classification–Investigating the Effects of Combined Yoga and Rajyog Meditation. J. Sci. Ind. Res. 2024, 84, 36–47. [Google Scholar]
- Liu, Y.; Xiang, Z.; Yan, Z.; Jin, J.; Shu, L.; Zhang, L.; Xu, X. CEEMDAN fuzzy entropy based fatigue driving detection using single-channel EEG. Biomed. Signal Process. Control 2024, 95, 106460. [Google Scholar] [CrossRef]
- Corrivetti, G.; Monaco, F.; Vignapiano, A.; Marenna, A.; Palm, K.; Fernández-Arroyo, S.; Frigola-Capell, E.; Leen, V.; Ibarrola, O.; Amil, B. Optimizing and predicting antidepressant efficacy in patients with major depressive disorder using multi-omics analysis and the Opade AI prediction tools. Brain Sci. 2024, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Cambay, V.Y.; Tasci, I.; Tasci, G.; Hajiyeva, R.; Dogan, S.; Tuncer, T. QuadTPat: Quadruple Transition Pattern-based explainable feature engineering model for stress detection using EEG signals. Sci. Rep. 2024, 14, 27320. [Google Scholar] [CrossRef]
- Cerdán-Martínez, V.; López-Segura, P.; Lucia-Mulas, M.J.; Sanz, P.R.; Alonso, T.O. Male and Female Brain Activity During the Screening of a Violent Movie: An EEG Study. J. Creat. Commun. 2024, 19, 259–275. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Li, C.-W.; Hsu, A.-L.; Liu, C.-Y.; Wu, C.W.; Chang, W.-C.; Lin, C.-P. Neurovascular coupling in eye-open-eye-close task and resting state: Spectral correspondence between concurrent EEG and fMRI. NeuroImage 2024, 289, 120535. [Google Scholar] [CrossRef]
- Chen, I.-C.; Chang, C.-L.; Chang, M.-H.; Ko, L.-W. The utility of wearable electroencephalography combined with behavioral measures to establish a practical multi-domain model for facilitating the diagnosis of young children with attention-deficit/hyperactivity disorder. J. Neurodev. Disord. 2024, 16, 62. [Google Scholar] [CrossRef]
- Maschke, C.; O’Byrne, J.; Colombo, M.A.; Boly, M.; Gosseries, O.; Laureys, S.; Rosanova, M.; Jerbi, K.; Blain-Moraes, S. Critical dynamics in spontaneous EEG predict anesthetic-induced loss of consciousness and perturbational complexity. Commun. Biol. 2024, 7, 946. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, Y.W.; Kim, S.; Lee, J.-Y.; Yoo, S.Y.; Jang, J.H.; Choi, J.-S. Resting-state EEG microstate analysis of internet gaming disorder and alcohol use disorder. Dialogues Clin. Neurosci. 2024, 26, 89–102. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, C.; You, L.; Wang, X.; Yuan, Y.; Jeannès, R.L.B.; Shu, H.; Xiang, W. WS-BiLSTM-MA: Wavelet scattering-based BiLSTM with mixed attention block for MDD recognition using multi-channel EEG signals. IEEE Trans. Instrum. Meas. 2024, 74, 6500513. [Google Scholar] [CrossRef]
- Ouyang, A.; Zhang, C.; Adra, N.; Tesh, R.A.; Sun, H.; Lei, D.; Jing, J.; Fan, P.; Paixao, L.; Ganglberger, W. Effects of Aerobic Exercise on Brain Age and Health in Middle-Aged and Older Adults: A Single-Arm Pilot Clinical Trial. Life 2024, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Chang, C.-L.; Huang, I.-W.; Chang, M.-H.; Ko, L.-W. Electrophysiological functional connectivity and complexity reflecting cognitive processing speed heterogeneity in young children with ADHD. Psychiatry Res. 2024, 340, 116100. [Google Scholar] [CrossRef]
- Meinert, E.; Milne-Ives, M.; Sawyer, J.; Boardman, L.; Mitchell, S.; Mclean, B.; Richardson, M.; Shankar, R. Subcutaneous electroencephalography monitoring for people with epilepsy and intellectual disability: Co-production workshops. BJPsych Open 2025, 11, e3. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.-L.; Wu, C.-Y.; Ng, H.-Y.H.; Chuang, C.-H.; Huang, C.-M.; Wu, C.W.; Chao, Y.-P. Classification of mindfulness experiences from gamma-band effective connectivity: Application of machine-learning algorithms on resting, breathing, and body scan. Comput. Methods Programs Biomed. 2024, 257, 108446. [Google Scholar] [CrossRef] [PubMed]
- Gui, A.; Throm, E.; da Costa, P.; Penza, F.; Mayans, M.A.; Jordan-Barros, A.; Haartsen, R.; Leech, R.; Jones, E. Neuroadaptive Bayesian optimisation to study individual differences in infants’ engagement with social cues. Dev. Cogn. Neurosci. 2024, 68, 101401. [Google Scholar] [CrossRef]
- Pandey, P.; McLinden, J.; Rahimi, N.; Kumar, C.; Shao, M.; Spencer, K.; Ostadabbas, S.; Shahriari, Y. fNIRSNET: A multi-view spatio-temporal convolutional neural network fusion for functional near-infrared spectroscopy-based auditory event classification. Eng. Appl. Artif. Intell. 2024, 137, 109256. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, Y.-M.; Shim, M. Differences in Functional Connectivity between Patients with Depression with and without Nonsuicidal Self-injury. Clin. Psychopharmacol. Neurosci. 2023, 22, 451. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, X.; Wen, J.; Li, Y.; Fan, C.; Ma, L.; Wang, H.; Zhang, M.; Zhang, S.; Hu, D. Hypnotherapy modulating early and late event-related potentials components of face processing in social anxiety. Front. Psychiatry 2024, 15, 1449946. [Google Scholar] [CrossRef]
- Ho, C.-C.; Peng, S.-J.; Yu, Y.-H.; Chu, Y.-R.; Huang, S.-S.; Kuo, P.-H. In perspective of specific symptoms of major depressive disorder: Functional connectivity analysis of electroencephalography and potential biomarkers of treatment response. J. Affect. Disord. 2024, 367, 944–950. [Google Scholar] [CrossRef]
- Çatal, Y.; Keskin, K.; Wolman, A.; Klar, P.; Smith, D.; Northoff, G. Flexibility of intrinsic neural timescales during distinct behavioral states. Commun. Biol. 2024, 7, 1667. [Google Scholar] [CrossRef]
- Moreau, Q.; Brun, F.; Ayrolles, A.; Nadel, J.; Dumas, G. Distinct social behavior and inter-brain connectivity in dyads with autistic individuals. Soc. Neurosci. 2024, 19, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Dal Bò, E.; Cecchetto, C.; Callara, A.L.; Greco, A.; Mura, F.; Vanello, N.; Di Francesco, F.; Scilingo, E.P.; Gentili, C. Emotion perception through the nose: How olfactory emotional cues modulate the perception of neutral facial expressions in affective disorders. Transl. Psychiatry 2024, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, H.; Cao, X.; Li, H.; Fang, X.; Yu, L.; Li, S.; Wu, Z.; Li, C.; Zhang, C. Impaired motor-to-sensory transformation mediates auditory hallucinations. PLoS Biol. 2024, 22, e3002836. [Google Scholar] [CrossRef] [PubMed]
- Thunström, A.O.; Carlsen, H.K.; Ali, L.; Larson, T.; Hellström, A.; Steingrimsson, S. Usability Comparison Among Healthy Participants of an Anthropomorphic Digital Human and a Text-Based Chatbot as a Responder to Questions on Mental Health: Randomized Controlled Trial. JMIR Hum. Factors 2024, 11, e54581. [Google Scholar] [CrossRef]
- Jia, H.; Han, S.; Caiafa, C.F.; Duan, F.; Zhang, Y.; Sun, Z.; Solé-Casals, J. Enabling temporal–spectral decoding in multi-class single-side upper limb classification. Eng. Appl. Artif. Intell. 2024, 133, 108473. [Google Scholar] [CrossRef]
- Habib, A.; Vaniya, S.N.; Khandoker, A.; Karmakar, C. MDDBranchNet: A Deep Learning Model for Detecting Major Depressive Disorder Using ECG Signal. IEEE J. Biomed. Health Inform. 2024, 28, 3798–3809. [Google Scholar] [CrossRef]
- Zang, X.; Li, B.; Zhao, L.; Yan, D.; Yang, L. End-to-end depression recognition based on a one-dimensional convolution neural network model using two-lead ECG signal. J. Med. Biol. Eng. 2022, 42, 225–233. [Google Scholar] [CrossRef]
- Tasci, B.; Tasci, G.; Dogan, S.; Tuncer, T. A novel ternary pattern-based automatic psychiatric disorders classification using ECG signals. Cogn. Neurodynamics 2024, 18, 95–108. [Google Scholar] [CrossRef]
- Abbas, Q.; Celebi, M.E.; AlBalawi, T.; Daadaa, Y. Brain and Heart Rate Variability Patterns Recognition for Depression Classification of Mental Health Disorder. Int. J. Adv. Comput. Sci. Appl. 2024, 15, 838–854. [Google Scholar] [CrossRef]
- Mehata, S.; Bhongade, R.A.; Rangaswamy, R. A Novel Deep Learningbased Model for the Efficient Classification of Electrocardiogram Signals. Cardiometry 2022, 24, 1033–1039. [Google Scholar]
- Hwang, B.; You, J.; Vaessen, T.; Myin-Germeys, I.; Park, C.; Zhang, B.-T. Deep ECGNet: An optimal deep learning framework for monitoring mental stress using ultra short-term ECG signals. Telemed. e-Health 2018, 24, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, X.; Wang, L.; Niu, J.; Zhu, X.; Dai, Z. Stress detection via multimodal multi-temporal-scale fusion: A hybrid of deep learning and handcrafted feature approach. IEEE Sens. J. 2023, 23, 27817–27827. [Google Scholar] [CrossRef]
- Seo, W.; Kim, N.; Kim, S.; Lee, C.; Park, S.-M. Deep ECG-respiration network (DeepER net) for recognizing mental stress. Sensors 2019, 19, 3021. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.M.; Alzaabi, Y.; Khandoker, A.H. Explainable computer-aided detection of obstructive sleep apnea and depression. IEEE Access 2022, 10, 110916–110933. [Google Scholar] [CrossRef]
- Ghosh, S.; Kim, S.; Ijaz, M.F.; Singh, P.K.; Mahmud, M. Classification of mental stress from wearable physiological sensors using image-encoding-based deep neural network. Biosensors 2022, 12, 1153. [Google Scholar] [CrossRef]
- Abedinzadeh Torghabeh, F.; Modaresnia, Y.; Hosseini, S.A. A Pre-Processing-Free Mental State Detection Model Using Noisy Ecg Plots and Deep Transfer Learning. Biomed. Eng. Appl. Basis Commun. 2024, 2450051. [Google Scholar] [CrossRef]
- Shermadurai, P.; Thiyagarajan, K. Classification of Human Mental Stress Levels Using a Deep Learning Approach on the K-EmoCon Multimodal Dataset. Trait. Signal 2024, 41, 2559. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhuang, Y.; Yin, S.; Chen, Z.; Liang, Y. Assessment of Mental Workload Level Based on PPG Signal Fusion Continuous Wavelet Transform and Cardiopulmonary Coupling Technology. Electronics 2024, 13, 1238. [Google Scholar] [CrossRef]
- Chen, R.; Wang, R.; Fei, J.; Huang, L.; Bi, X.; Wang, J. Mental fatigue recognition study based on 1D convolutional neural network and short-term ECG signals. Technol. Health Care 2024, 32, 3409–3422. [Google Scholar] [CrossRef]
- Geethanjali, R.; Valarmathi, A. A Deep Learning based Hybrid Model for Maternal Health Risk Detection and Multifaceted Emotion Analysis in Social Networks. Int. J. Appl. Math. Comput. Sci. 2024, 34, 565–577. [Google Scholar] [CrossRef]
- Awan, A.W.; Taj, I.; Khalid, S.; Usman, S.M.; Imran, A.S.; Akram, M.U. Advancing emotional health assessments: A hybrid deep learning approach using physiological signals for robust emotion recognition. IEEE Access 2024, 12, 141890–141904. [Google Scholar] [CrossRef]
- Tuncer, T.; Baig, A.H.; Aydemir, E.; Kivrak, T.; Tuncer, I.; Tasci, G.; Dogan, S. Cardioish: Lead-Based Feature Extraction for ECG Signals. Diagnostics 2024, 14, 2712. [Google Scholar] [CrossRef] [PubMed]
- Telangore, H.; Sharma, N.; Sharma, M.; Acharya, U.R. A Novel ECG-Based Approach for Classifying Psychiatric Disorders: Leveraging Wavelet Scattering Networks. Med. Eng. Phys. 2024, 135, 104275. [Google Scholar] [CrossRef]
- Sun, M.; Cao, X. A Mental Stress Classification Method Based on Feature Fusion Using Physiological Signals. J. Circuits Syst. Comput. 2024, 33, 2450016. [Google Scholar] [CrossRef]
- Mukherjee, P.; Halder Roy, A. A deep learning-based approach for distinguishing different stress levels of human brain using EEG and pulse rate. Comput. Methods Biomech. Biomed. Eng. 2024, 27, 2303–2324. [Google Scholar] [CrossRef]
- Sangeetha, S.; Immanuel, R.R.; Mathivanan, S.K.; Cho, J.; Easwaramoorthy, S.V. An Empirical Analysis of Multimodal Affective Computing Approaches for Advancing Emotional Intelligence in Artificial Intelligence for Healthcare. IEEE Access 2024, 12, 114416–114434. [Google Scholar] [CrossRef]
- Alzate, M.; Torres, R.; De la Roca, J.; Quintero-Zea, A.; Hernandez, M. Machine Learning Framework for Classifying and Predicting Depressive Behavior Based on PPG and ECG Feature Extraction. Appl. Sci. 2024, 14, 8312. [Google Scholar] [CrossRef]
- Ao, H.; Zhai, E.; Jiang, L.; Yang, K.; Deng, Y.; Guo, X.; Zeng, L.; Yan, Y.; Hao, M.; Song, T. Real-Time Cardiac Abnormality Monitoring and Nursing for Patient Using Electrocardiographic Signals. Cardiology 2025, 150, 25–35. [Google Scholar] [CrossRef]
- Monteith, S.; Glenn, T.; Geddes, J.; Whybrow, P.C.; Achtyes, E.; Bauer, M. Expectations for artificial intelligence (AI) in psychiatry. Curr. Psychiatry Rep. 2022, 24, 709–721. [Google Scholar] [CrossRef]
- Sommer, I.E.; de Boer, J.N. How to reap the benefits of language for psychiatry. Psychiatry Res. 2022, 318, 114932. [Google Scholar] [CrossRef]
- Wilson, B.S.; Tucci, D.L.; Moses, D.A.; Chang, E.F.; Young, N.M.; Zeng, F.-G.; Lesica, N.A.; Bur, A.M.; Kavookjian, H.; Mussatto, C. Harnessing the power of artificial intelligence in otolaryngology and the communication sciences. J. Assoc. Res. Otolaryngol. 2022, 23, 319–349. [Google Scholar] [CrossRef] [PubMed]
- Gosztolya, G.; Balogh, R.; Imre, N.; Egas-Lopez, J.V.; Hoffmann, I.; Vincze, V.; Tóth, L.; Devanand, D.P.; Pákáski, M.; Kálmán, J. Cross-lingual detection of mild cognitive impairment based on temporal parameters of spontaneous speech. Comput. Speech Lang. 2021, 69, 101215. [Google Scholar] [CrossRef]
- Vogel, A.P.; Sobanska, A.; Gupta, A.; Vasco, G.; Grobe-Einsler, M.; Summa, S.; Borel, S. Quantitative Speech Assessment in Ataxia—Consensus Recommendations by the Ataxia Global Initiative Working Group on Digital-Motor Markers. Cerebellum 2024, 23, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Semel, B.M. Listening like a computer: Attentional tensions and mechanized care in psychiatric digital phenotyping. Sci. Technol. Hum. Values 2022, 47, 266–290. [Google Scholar] [CrossRef]
- Kim, A.Y.; Jang, E.H.; Lee, S.-H.; Choi, K.-Y.; Park, J.G.; Shin, H.-C. Automatic depression detection using smartphone-based text-dependent speech signals: Deep convolutional neural network approach. J. Med. Internet Res. 2023, 25, e34474. [Google Scholar] [CrossRef] [PubMed]
- Górriz, J.M.; Álvarez-Illán, I.; Álvarez-Marquina, A.; Arco, J.E.; Atzmueller, M.; Ballarini, F.; Barakova, E.; Bologna, G.; Bonomini, P.; Castellanos-Dominguez, G. Computational approaches to explainable artificial intelligence: Advances in theory, applications and trends. Inf. Fusion 2023, 100, 101945. [Google Scholar] [CrossRef]
- Tan, E.J.; Sommer, I.E.; Palaniyappan, L. Language and psychosis: Tightening the association. Schizophr. Bull. 2023, 49, S83–S85. [Google Scholar] [CrossRef]
- Schuller, B.W.; Amiriparian, S.; Batliner, A.; Gebhard, A.; Gerczuk, M.; Karas, V.; Kathan, A.; Seizer, L.; Löchner, J. Computational charisma—A brick by brick blueprint for building charismatic artificial intelligence. Front. Comput. Sci. 2023, 5, 1135201. [Google Scholar] [CrossRef]
- Hampsey, E.; Meszaros, M.; Skirrow, C.; Strawbridge, R.; Taylor, R.H.; Chok, L.; Aarsland, D.; Al-Chalabi, A.; Chaudhuri, R.; Weston, J. Protocol for rhapsody: A longitudinal observational study examining the feasibility of speech phenotyping for remote assessment of neurodegenerative and psychiatric disorders. BMJ Open 2022, 12, e061193. [Google Scholar] [CrossRef]
- Vetráb, M.; Egas-López, J.V.; Balogh, R.; Imre, N.; Hoffmann, I.; Tóth, L.; Pákáski, M.; Kálmán, J.; Gosztolya, G. Using spectral sequence-to-sequence autoencoders to assess mild cognitive impairment. In Proceedings of the ICASSP 2022—2022 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Singapore, 23–27 May 2022; pp. 6467–6471. [Google Scholar]
- Liu, Y.; Xia, S.; Nie, J.; Wei, P.; Shu, Z.; Chang, J.A.; Jiang, X. aiMSE: Toward an AI-based online mental status examination. IEEE Pervasive Comput. 2022, 21, 46–54. [Google Scholar] [CrossRef]
- Stenum, J.; Cherry-Allen, K.M.; Pyles, C.O.; Reetzke, R.D.; Vignos, M.F.; Roemmich, R.T. Applications of pose estimation in human health and performance across the lifespan. Sensors 2021, 21, 7315. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.V.; Vyas, P.; Agrawal, S.; Pachpor, T.A.; Paralikar, V.; Khare, S.P. Peripheral blood-based gene expression studies in schizophrenia: A systematic review. Front. Genet. 2021, 12, 736483. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Abhayapala, T.D.; Samarasinghe, P. A preliminary investigation on frequency dependant cues for human emotions. Acoustics 2022, 4, 460–468. [Google Scholar] [CrossRef]
- Taylor, R.; Hampsey, E.; Mészáros, M.; Skirrow, C.; Strawbridge, R.; Chok, L.; Aarsland, D.; Al-Chalabi, A.; Chaudhuri, K.; Weston, J. Clinical Feasibility of Speech Phenotyping for Remote Assessment of Neurodegenerative and Psychiatric Disorders (RHAPSODY): A study protocol. Eur. Psychiatry 2022, 65, S163. [Google Scholar] [CrossRef]
- Kálmán, J.; Devanand, D.P.; Gosztolya, G.; Balogh, R.; Imre, N.; Tóth, L.; Hoffmann, I.; Kovács, I.; Vincze, V.; Pákáski, M. Temporal speech parameters detect mild cognitive impairment in different languages: Validation and comparison of the Speech-GAP Test® in English and Hungarian. Curr. Alzheimer Res. 2022, 19, 373–386. [Google Scholar] [CrossRef]
- Taptiklis, N.; Su, M.; Barnett, J.H.; Skirrow, C.; Kroll, J.; Cormack, F. Prediction of mental effort derived from an automated vocal biomarker using machine learning in a large-scale remote sample. Front. Artif. Intell. 2023, 6, 1171652. [Google Scholar] [CrossRef]
- Fristed, E.; Skirrow, C.; Meszaros, M.; Lenain, R.; Meepegama, U.; Cappa, S.; Aarsland, D.; Weston, J. A remote speech-based AI system to screen for early Alzheimer’s disease via smartphones. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12366. [Google Scholar] [CrossRef]
- Lu, T.; Yang, J.; Zhang, X.; Guo, Z.; Li, S.; Yang, W.; Chen, Y.; Wu, N. Crossmodal audiovisual emotional integration in depression: An event-related potential study. Front. Psychiatry 2021, 12, 694665. [Google Scholar] [CrossRef]
- Otero, J.F.A.; Caballer, O.S.; Marti-Puig, P.; Sun, Z.; Tanaka, T.; Solé-Casals, J. Preliminary Results on the Generation of Artificial Handwriting Data Using a Decomposition-Recombination Strategy. In Proceedings of the ICASSP 2022—2022 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Singapore, 23–27 May 2022; pp. 1166–1170. [Google Scholar]
- Wu, X.; Cai, Y.; Lian, Z.; Leung, H.-f.; Wang, T. Generating natural language from logic expressions with structural representation. IEEE/ACM Trans. Audio Speech Lang. Process. 2023, 31, 1499–1510. [Google Scholar] [CrossRef]
- Elvevåg, B. Reflections on measuring disordered thoughts as expressed via language. Psychiatry Res. 2023, 322, 115098. [Google Scholar] [CrossRef]
- Barrett-Young, A.; Abraham, W.C.; Cheung, C.Y.; Gale, J.; Hogan, S.; Ireland, D.; Keenan, R.; Knodt, A.R.; Melzer, T.R.; Moffitt, T.E. Associations between thinner retinal neuronal layers and suboptimal brain structural integrity in a middle-aged cohort. Eye Brain 2023, 15, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Liu, S.; Li, X.; Wen, D.; Li, Q.; Tong, Y.; Xu, Y. A decrease in hemodynamic response in the right Postcentral cortex is Associated with Treatment-Resistant Auditory Verbal Hallucinations in Schizophrenia: An NIRS Study. Front. Neurosci. 2022, 16, 865738. [Google Scholar] [CrossRef] [PubMed]
- De Angel, V.; Lewis, S.; Munir, S.; Matcham, F.; Dobson, R.; Hotopf, M. Using digital health tools for the Remote Assessment of Treatment Prognosis in Depression (RAPID): A study protocol for a feasibility study. BMJ Open 2022, 12, e059258. [Google Scholar] [CrossRef] [PubMed]
- Compton, M.T.; Ku, B.S.; Covington, M.A.; Metzger, C.; Hogoboom, A. Lexical diversity and other linguistic measures in schizophrenia: Associations with negative symptoms and neurocognitive performance. J. Nerv. Ment. Dis. 2022, 211, 613–620. [Google Scholar] [CrossRef]
- Rezaii, N.; Hochberg, D.; Quimby, M.; Wong, B.; Brickhouse, M.; Touroutoglou, A.; Dickerson, B.C.; Wolff, P. Artificial intelligence classifies primary progressive aphasia from connected speech. Brain 2024, 147, 3070–3082. [Google Scholar] [CrossRef]
- Pugh, S.L.; Chandler, C.; Cohen, A.S.; Diaz-Asper, C.; Elvevåg, B.; Foltz, P.W. Assessing dimensions of thought disorder with large language models: The tradeoff of accuracy and consistency. Psychiatry Res. 2024, 341, 116119. [Google Scholar] [CrossRef]
- Ahammed, M.; Sheikh, R.; Hossain, F.; Liza, S.M.; Rahman, M.A.; Mahmud, M.; Brown, D.J. Speech Emotion Recognition: An Empirical Analysis of Machine Learning Algorithms Across Diverse Data Sets. In Proceedings of the International Conference on Applied Intelligence and Informatics; Springer: Cham, Switzerland, 2023; pp. 32–46. [Google Scholar]
- Leite, D.; Casalino, G.; Kaczmarek-Majer, K.; Castellano, G. Incremental learning and granular computing from evolving data streams: An application to speech-based bipolar disorder diagnosis. Fuzzy Sets Syst. 2025, 500, 109205. [Google Scholar] [CrossRef]
- Wang, N.; Goel, S.; Ibrahim, S.; Badal, V.D.; Depp, C.; Bilal, E.; Subbalakshmi, K.; Lee, E. Decoding loneliness: Can explainable AI help in understanding language differences in lonely older adults? Psychiatry Res. 2024, 339, 116078. [Google Scholar] [CrossRef]
- Park, D.; Lee, G.; Kim, S.; Seo, T.; Oh, H.; Kim, S.J. Probability-based multi-label classification considering correlation between labels–focusing on DSM-5 depressive disorder diagnostic criteria. IEEE Access 2024, 12, 70289–70296. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, Y.; Dai, A.-J.; Qian, C.; Zhong, B.-L.; Liu, C.-L.; Liu, Z.-T. Speech based suicide risk recognition for crisis intervention hotlines using explainable multi-task learning. J. Affect. Disord. 2025, 370, 392–400. [Google Scholar] [CrossRef]
- Rosi, A.; Rose, S.R.; Murugan, C.A.; Balamurugan, E.; Priya, M.S.; Lalitha, K. Automated Gesture Recognition using Deep Learning Model for Visually Challenged People. In Proceedings of the 2024 International Conference on Advances in Computing, Communication and Applied Informatics (ACCAI), Chennai, India, 9–10 May 2024; pp. 1–6. [Google Scholar]
- Takeshige-Amano, H.; Oyama, G.; Ogawa, M.; Fusegi, K.; Kambe, T.; Shiina, K.; Ueno, S.-i.; Okuzumi, A.; Hatano, T.; Motoi, Y. Digital detection of Alzheimer’s disease using smiles and conversations with a chatbot. Sci. Rep. 2024, 14, 26309. [Google Scholar] [CrossRef] [PubMed]
- Taşcı, B. Multilevel hybrid handcrafted feature extraction based depression recognition method using speech. J. Affect. Disord. 2024, 364, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Karmakar, C.; Tamouza, R.; Tran, T.; Yearwood, J.; Hamdani, N.; Laouamri, H.; Richard, J.-R.; Yolken, R.; Berk, M. Precision psychiatry with immunological and cognitive biomarkers: A multi-domain prediction for the diagnosis of bipolar disorder or schizophrenia using machine learning. Transl. Psychiatry 2020, 10, 162. [Google Scholar] [CrossRef]
- Karaglani, M.; Agorastos, A.; Panagopoulou, M.; Parlapani, E.; Athanasis, P.; Bitsios, P.; Tzitzikou, K.; Theodosiou, T.; Iliopoulos, I.; Bozikas, V.-P. A novel blood-based epigenetic biosignature in first-episode schizophrenia patients through automated machine learning. Transl. Psychiatry 2024, 14, 257. [Google Scholar] [CrossRef]
- Sánchez-Carro, Y.; de la Torre-Luque, A.; Leal-Leturia, I.; Salvat-Pujol, N.; Massaneda, C.; de Arriba-Arnau, A.; Urretavizcaya, M.; Pérez-Solà, V.; Toll, A.; Martínez-Ruiz, A. Importance of immunometabolic markers for the classification of patients with major depressive disorder using machine learning. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 121, 110674. [Google Scholar] [CrossRef] [PubMed]
- Wollenhaupt-Aguiar, B.; Librenza-Garcia, D.; Bristot, G.; Przybylski, L.; Stertz, L.; Kubiachi Burque, R.; Ceresér, K.M.; Spanemberg, L.; Caldieraro, M.A.; Frey, B.N. Differential biomarker signatures in unipolar and bipolar depression: A machine learning approach. Aust. N. Zeal. J. Psychiatry 2020, 54, 393–401. [Google Scholar] [CrossRef]
- Mürner-Lavanchy, I.; Koenig, J.; Reichl, C.; Josi, J.; Cavelti, M.; Kaess, M. The quest for a biological phenotype of adolescent non-suicidal self-injury: A machine-learning approach. Transl. Psychiatry 2024, 14, 56. [Google Scholar] [CrossRef]
- Chen, S.; Chen, G.; Li, Y.; Yue, Y.; Zhu, Z.; Li, L.; Jiang, W.; Shen, Z.; Wang, T.; Hou, Z. Predicting the diagnosis of various mental disorders in a mixed cohort using blood-based multi-protein model: A machine learning approach. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1267–1277. [Google Scholar] [CrossRef]
- Siegel, C.E.; Laska, E.M.; Lin, Z.; Xu, M.; Abu-Amara, D.; Jeffers, M.K.; Qian, M.; Milton, N.; Flory, J.D.; Hammamieh, R. Utilization of machine learning for identifying symptom severity military-related PTSD subtypes and their biological correlates. Transl. Psychiatry 2021, 11, 227. [Google Scholar] [CrossRef]
- Marriott, H.; Kabiljo, R.; Hunt, G.P.; Khleifat, A.A.; Jones, A.; Troakes, C.; Consortium, P.M.A.S.; Consortium, T.S.; Pfaff, A.L.; Quinn, J.P. Unsupervised machine learning identifies distinct ALS molecular subtypes in post-mortem motor cortex and blood expression data. Acta Neuropathol. Commun. 2023, 11, 208. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.-l.; Yu, J.-f.; Weng, J.; Han, B.; Liu, Y.; Tang, X.; Pan, B. Identification of immune-related biomarkers in peripheral blood of schizophrenia using bioinformatic methods and machine learning algorithms. Front. Cell. Neurosci. 2023, 17, 1256184. [Google Scholar] [CrossRef] [PubMed]
- Tasci, B.; Tasci, G.; Ayyildiz, H.; Kamath, A.P.; Barua, P.D.; Tuncer, T.; Dogan, S.; Ciaccio, E.J.; Chakraborty, S.; Acharya, U.R. Automated schizophrenia detection model using blood sample scattergram images and local binary pattern. Multimed. Tools Appl. 2024, 83, 42735–42763. [Google Scholar] [CrossRef]
- Cearns, M.; Opel, N.; Clark, S.; Kaehler, C.; Thalamuthu, A.; Heindel, W.; Winter, T.; Teismann, H.; Minnerup, H.; Dannlowski, U. Predicting rehospitalization within 2 years of initial patient admission for a major depressive episode: A multimodal machine learning approach. Transl. Psychiatry 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Gelir, F.; Akan, T.; Alp, S.; Gecili, E.; Bhuiyan, M.S.; Disbrow, E.A.; Conrad, S.A.; Vanchiere, J.A.; Kevil, C.G.; The Alzheimer’s Disease Neuroimaging Initiative (ADNI) & Mohammad Alfrad Nobel Bhuiyan. Machine Learning Approaches for Predicting Progression to Alzheimer’s Disease in Patients with Mild Cognitive Impairment. J. Med. Biol. Eng. 2024, 1–21. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Schiöth, H.B. Decoding depression: A comprehensive multi-cohort exploration of blood DNA methylation using machine learning and deep learning approaches. Transl. Psychiatry 2024, 14, 287. [Google Scholar] [CrossRef]
- Stamate, D.; Kim, M.; Proitsi, P.; Westwood, S.; Baird, A.; Nevado-Holgado, A.; Hye, A.; Bos, I.; Vos, S.J.; Vandenberghe, R. A metabolite-based machine learning approach to diagnose Alzheimer-type dementia in blood: Results from the European Medical Information Framework for Alzheimer disease biomarker discovery cohort. Alzheimers Dement. Transl. Res. Clin. Interv. 2019, 5, 933–938. [Google Scholar] [CrossRef]
- Deike, K.; Decker, A.; Scheyhing, P.; Harten, J.; Zimmermann, N.; Paech, D.; Peters, O.; Freiesleben, S.D.; Schneider, L.-S.; Preis, L. Machine Learning–Based Perivascular Space Volumetry in Alzheimer Disease. Investig. Radiol. 2024, 59, 667–676. [Google Scholar] [CrossRef]
- Dogan, M.V.; Beach, S.R.; Simons, R.L.; Lendasse, A.; Penaluna, B.; Philibert, R.A. Blood-based biomarkers for predicting the risk for five-year incident coronary heart disease in the Framingham Heart Study via machine learning. Genes 2018, 9, 641. [Google Scholar] [CrossRef]
- Lu, A.K.-M.; Lin, J.-J.; Tseng, H.-H.; Wang, X.-Y.; Jang, F.-L.; Chen, P.-S.; Huang, C.-C.; Hsieh, S.; Lin, S.-H. DNA methylation signature aberration as potential biomarkers in treatment-resistant schizophrenia: Constructing a methylation risk score using a machine learning method. J. Psychiatr. Res. 2023, 157, 57–65. [Google Scholar] [CrossRef]
- Chekroud, A.M.; Bondar, J.; Delgadillo, J.; Doherty, G.; Wasil, A.; Fokkema, M.; Cohen, Z.; Belgrave, D.; DeRubeis, R.; Iniesta, R. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry 2021, 20, 154–170. [Google Scholar] [CrossRef]
- Le Glaz, A.; Haralambous, Y.; Kim-Dufor, D.-H.; Lenca, P.; Billot, R.; Ryan, T.C.; Marsh, J.; Devylder, J.; Walter, M.; Berrouiguet, S. Machine learning and natural language processing in mental health: Systematic review. J. Med. Internet Res. 2021, 23, e15708. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; Donat-Vargas, C.; Santoma-Vilaclara, J.; Anta, L.d.; Goena, J.; Sanchez-Bayona, R.; Mora, F.; Ortega, M.A.; Lahera, G.; Rodriguez-Jimenez, R. Assessment of antipsychotic medications on social media: Machine learning study. Front. Psychiatry 2021, 12, 737684. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Cheng, X.; Zhang, J.; Yannam, J.S.; Barnes, A.J.; Koch, J.R.; Hayes, R.; Gimm, G.; Zhao, X.; Purohit, H. Social media data mining of antitobacco campaign messages: Machine learning analysis of facebook posts. J. Med. Internet Res. 2023, 25, e42863. [Google Scholar] [CrossRef]
- Kim, D.; Quan, L.; Seo, M.; Kim, K.; Kim, J.W.; Zhu, Y. Interpretable machine learning-based approaches for understanding suicide risk and protective factors among South Korean females using survey and social media data. Suicide Life-Threat. Behav. 2023, 53, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Bernert, R.A.; Hilberg, A.M.; Melia, R.; Kim, J.P.; Shah, N.H.; Abnousi, F. Artificial intelligence and suicide prevention: A systematic review of machine learning investigations. Int. J. Environ. Res. Public Health 2020, 17, 5929. [Google Scholar] [CrossRef]
- Kaminsky, Z.; McQuaid, R.J.; Hellemans, K.G.; Patterson, Z.R.; Saad, M.; Gabrys, R.L.; Kendzerska, T.; Abizaid, A.; Robillard, R. Machine Learning–Based Suicide Risk Prediction Model for Suicidal Trajectory on Social Media Following Suicidal Mentions: Independent Algorithm Validation. J. Med. Internet Res. 2024, 26, e49927. [Google Scholar] [CrossRef]
- Roy, A.; Nikolitch, K.; McGinn, R.; Jinah, S.; Klement, W.; Kaminsky, Z.A. A machine learning approach predicts future risk to suicidal ideation from social media data. NPJ Digit. Med. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- de Anta, L.; Alvarez-Mon, M.Á.; Pereira-Sanchez, V.; Donat-Vargas, C.C.; Lara-Abelenda, F.J.; Arrieta, M.; Montero-Torres, M.; García-Montero, C.; Fraile-Martínez, Ó.; Mora, F. Assessment of beliefs and attitudes towards benzodiazepines using machine learning based on social media posts: An observational study. BMC Psychiatry 2024, 24, 659. [Google Scholar] [CrossRef]
- Erturk, S.; Hudson, G.; Jansli, S.M.; Morris, D.; Odoi, C.M.; Wilson, E.; Clayton-Turner, A.; Bray, V.; Yourston, G.; Cornwall, A. Codeveloping and Evaluating a Campaign to Reduce Dementia Misconceptions on Twitter: Machine Learning Study. JMIR Infodemiology 2022, 2, e36871. [Google Scholar] [CrossRef]
- Ryu, J.; Sükei, E.; Norbury, A.; Liu, S.H.; Campaña-Montes, J.J.; Baca-Garcia, E.; Artés, A.; Perez-Rodriguez, M.M. Shift in social media app usage during COVID-19 lockdown and clinical anxiety symptoms: Machine learning–based ecological momentary assessment study. JMIR Ment. Health 2021, 8, e30833. [Google Scholar] [CrossRef]
- Lim, S.R.; Ng, Q.X.; Xin, X.; Lim, Y.L.; Boon, E.S.K.; Liew, T.M. Public discourse surrounding suicide during the COVID-19 pandemic: An unsupervised machine learning analysis of Twitter posts over a one-year period. Int. J. Environ. Res. Public Health 2022, 19, 13834. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.W.; Kormilitzin, A.; Hamer-Hunt, J.; McKee, K.R.; Tomasev, N. Defining acceptable data collection and reuse standards for queer artificial intelligence research in mental health: Protocol for the online PARQAIR-MH Delphi study. BMJ Open 2024, 14, e079105. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Torous, J.; De Choudhury, M.; Depp, C.A.; Graham, S.A.; Kim, H.-C.; Paulus, M.P.; Krystal, J.H.; Jeste, D.V. Artificial intelligence for mental health care: Clinical applications, barriers, facilitators, and artificial wisdom. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2021, 6, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Coppersmith, G.; Dickerson, J.; Espy-Wilson, C.; Michel, H.; Resnik, P. Computationally Scalable and Clinically Sound: Laying the Groundwork to Use Machine Learning Techniques for Social Media and Language Data in Predicting Psychiatric Symptoms. Biol. Psychiatry 2022, 91, S50. [Google Scholar] [CrossRef]
- Graham, S.; Depp, C.; Lee, E.E.; Nebeker, C.; Tu, X.; Kim, H.-C.; Jeste, D.V. Artificial intelligence for mental health and mental illnesses: An overview. Curr. Psychiatry Rep. 2019, 21, 1–18. [Google Scholar] [CrossRef]
- Gargari, O.K.; Fatehi, F.; Mohammadi, I.; Firouzabadi, S.R.; Shafiee, A.; Habibi, G. Diagnostic accuracy of large language models in psychiatry. Asian J. Psychiatry 2024, 100, 104168. [Google Scholar] [CrossRef]
- Turner, R.J.; Coenen, F.; Roelofs, F.; Hagoort, K.; Härmä, A.; Grünwald, P.D.; Velders, F.P.; Scheepers, F.E. Information extraction from free text for aiding transdiagnostic psychiatry: Constructing NLP pipelines tailored to clinicians’ needs. BMC Psychiatry 2022, 22, 407. [Google Scholar] [CrossRef]
- Botelle, R.; Bhavsar, V.; Kadra-Scalzo, G.; Mascio, A.; Williams, M.V.; Roberts, A.; Velupillai, S.; Stewart, R. Can natural language processing models extract and classify instances of interpersonal violence in mental healthcare electronic records: An applied evaluative study. BMJ Open 2022, 12, e052911. [Google Scholar] [CrossRef]
- Levis, M.; Levy, J.; Dufort, V.; Gobbel, G.T.; Watts, B.V.; Shiner, B. Leveraging unstructured electronic medical record notes to derive population-specific suicide risk models. Psychiatry Res. 2022, 315, 114703. [Google Scholar] [CrossRef]
- Levis, M.; Levy, J.; Dent, K.R.; Dufort, V.; Gobbel, G.T.; Watts, B.V.; Shiner, B. Leveraging natural language processing to improve electronic health record suicide risk prediction for Veterans Health Administration users. J. Clin. Psychiatry 2023, 84, 47557. [Google Scholar] [CrossRef]
- Tsui, F.R.; Shi, L.; Ruiz, V.; Ryan, N.D.; Biernesser, C.; Iyengar, S.; Walsh, C.G.; Brent, D.A. Natural language processing and machine learning of electronic health records for prediction of first-time suicide attempts. JAMIA Open 2021, 4, ooab011. [Google Scholar] [CrossRef] [PubMed]
- Çabuk, T.; Sevim, N.; Mutlu, E.; Yağcıoğlu, A.E.A.; Koç, A.; Toulopoulou, T. Natural language processing for defining linguistic features in schizophrenia: A sample from Turkish speakers. Schizophr. Res. 2024, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Kizilay, E.; Verim, B.; Demirlek, C.; Dokuyan, Y.; Turan, Y.E.; Kucukakdag, A.; Demir, M.; Cesim, E.; Bora, E. Automated linguistic analysis in speech samples of Turkish-speaking patients with schizophrenia-spectrum disorders. Schizophr. Res. 2024, 267, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zaher, F.; Diallo, M.; Achim, A.M.; Joober, R.; Roy, M.-A.; Demers, M.-F.; Subramanian, P.; Lavigne, K.M.; Lepage, M.; Gonzalez, D. Speech markers to predict and prevent recurrent episodes of psychosis: A narrative overview and emerging opportunities. Schizophr. Res. 2024, 266, 205–215. [Google Scholar] [CrossRef]
- Benger, M.; Wood, D.A.; Kafiabadi, S.; Al Busaidi, A.; Guilhem, E.; Lynch, J.; Townend, M.; Montvila, A.; Siddiqui, J.; Gadapa, N. Factors affecting the labelling accuracy of brain MRI studies relevant for deep learning abnormality detection. Front. Radiol. 2023, 3, 1251825. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chignell, M.; Shan, B.; Sheehan, K.A.; Razak, F.; Verma, A. Boosting delirium identification accuracy with sentiment-based natural language processing: Mixed methods study. JMIR Med. Inform. 2022, 10, e38161. [Google Scholar] [CrossRef]
- Ciampelli, S.; Voppel, A.; De Boer, J.; Koops, S.; Sommer, I. Combining automatic speech recognition with semantic natural language processing in schizophrenia. Psychiatry Res. 2023, 325, 115252. [Google Scholar] [CrossRef]
- Vaci, N.; Liu, Q.; Kormilitzin, A.; De Crescenzo, F.; Kurtulmus, A.; Harvey, J.; O’Dell, B.; Innocent, S.; Tomlinson, A.; Cipriani, A. Natural language processing for structuring clinical text data on depression using UK-CRIS. BMJ Ment. Health 2020, 23, 21–26. [Google Scholar] [CrossRef]
- Kerz, E.; Zanwar, S.; Qiao, Y.; Wiechmann, D. Toward explainable AI (XAI) for mental health detection based on language behavior. Front. Psychiatry 2023, 14, 1219479. [Google Scholar] [CrossRef]
- Sawalha, J.; Yousefnezhad, M.; Shah, Z.; Brown, M.R.; Greenshaw, A.J.; Greiner, R. Detecting presence of PTSD using sentiment analysis from text data. Front. Psychiatry 2022, 12, 811392. [Google Scholar] [CrossRef]
- Acosta, M.J.; Castillo-Sánchez, G.; Garcia-Zapirain, B.; De la Torre Diez, I.; Franco-Martín, M. Sentiment analysis techniques applied to raw-text data from a csq-8 questionnaire about mindfulness in times of covid-19 to improve strategy generation. Int. J. Environ. Res. Public Health 2021, 18, 6408. [Google Scholar] [CrossRef]
- Kizilay, E.; Arslan, B.; Verim, B.; Demirlek, C.; Demir, M.; Cesim, E.; Eyuboglu, M.S.; Ozbek, S.U.; Sut, E.; Yalincetin, B. Automated linguistic analysis in youth at clinical high risk for psychosis. Schizophr. Res. 2024, 274, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Garcia-Romeu, A.; Johnson, M.W. Predicting changes in substance use following psychedelic experiences: Natural language processing of psychedelic session narratives. Am. J. Drug Alcohol Abus. 2021, 47, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.; Patel, R.; Oliver, D.; Colling, C.; Pritchard, M.; Broadbent, M.; Baldwin, H.; Stahl, D.; Stewart, R.; Fusar-Poli, P. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr. Bull. 2021, 47, 405–414. [Google Scholar] [CrossRef]
- Wu, H.; Hodgson, K.; Dyson, S.; Morley, K.I.; Ibrahim, Z.M.; Iqbal, E.; Stewart, R.; Dobson, R.J.; Sudlow, C. Efficient reuse of natural language processing models for phenotype-mention identification in free-text electronic medical records: A phenotype embedding approach. JMIR Med. Inform. 2019, 7, e14782. [Google Scholar] [CrossRef] [PubMed]
- Horigome, T.; Hino, K.; Toyoshiba, H.; Shindo, N.; Funaki, K.; Eguchi, Y.; Kitazawa, M.; Fujita, T.; Mimura, M.; Kishimoto, T. Identifying neurocognitive disorder using vector representation of free conversation. Sci. Rep. 2022, 12, 12461. [Google Scholar] [CrossRef] [PubMed]
- Viani, N.; Kam, J.; Yin, L.; Bittar, A.; Dutta, R.; Patel, R.; Stewart, R.; Velupillai, S. Temporal information extraction from mental health records to identify duration of untreated psychosis. J. Biomed. Semant. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Oh, I.Y.; Schindler, S.E.; Ghoshal, N.; Lai, A.M.; Payne, P.R.; Gupta, A. Extraction of clinical phenotypes for Alzheimer’s disease dementia from clinical notes using natural language processing. JAMIA Open 2023, 6, ooad014. [Google Scholar] [CrossRef]
- Arslan, B.; Kizilay, E.; Verim, B.; Demirlek, C.; Demir, M.; Cesim, E.; Eyuboglu, M.S.; Ozbek, S.U.; Sut, E.; Yalincetin, B. Computational analysis of linguistic features in speech samples of first-episode bipolar disorder and psychosis. J. Affect. Disord. 2024, 363, 340–347. [Google Scholar] [CrossRef]
- Msosa, Y.J.; Grauslys, A.; Zhou, Y.; Wang, T.; Buchan, I.; Langan, P.; Foster, S.; Walker, M.; Pearson, M.; Folarin, A. Trustworthy Data and AI Environments for Clinical Prediction: Application to Crisis-Risk in People With Depression. IEEE J. Biomed. Health Inform. 2023, 27, 5588–5598. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Iwata, S.; Horikoshi, M.; Sakata, M.; Toyomoto, R.; Luo, Y.; Tajika, A.; Kudo, N.; Aramaki, E. Harnessing AI to Optimize Thought Records and Facilitate Cognitive Restructuring in Smartphone CBT: An Exploratory Study. Cogn. Ther. Res. 2023, 47, 887–893. [Google Scholar] [CrossRef]
- Kosowan, L.; Singer, A.; Zulkernine, F.; Zafari, H.; Nesca, M.; Muthumuni, D. Pan-Canadian Electronic Medical Record Diagnostic and Unstructured Text Data for Capturing PTSD: Retrospective Observational Study. JMIR Med. Inform. 2022, 10, e41312. [Google Scholar] [CrossRef] [PubMed]
- Meerwijk, E.L.; Tamang, S.R.; Finlay, A.K.; Ilgen, M.A.; Reeves, R.M.; Harris, A.H. Suicide theory-guided natural language processing of clinical progress notes to improve prediction of veteran suicide risk: Protocol for a mixed-method study. BMJ Open 2022, 12, e065088. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.; Adekkanattu, P.; Campion, T.R., Jr.; Sholle, E.T.; Myers, A.; Banerjee, S.; Alexopoulos, G.; Wang, Y.; Pathak, J. Using weak supervision and deep learning to classify clinical notes for identification of current suicidal ideation. J. Psychiatr. Res. 2021, 136, 95–102. [Google Scholar] [CrossRef]
- Iqbal, E.; Mallah, R.; Rhodes, D.; Wu, H.; Romero, A.; Chang, N.; Dzahini, O.; Pandey, C.; Broadbent, M.; Stewart, R. ADEPt, a semantically-enriched pipeline for extracting adverse drug events from free-text electronic health records. PLoS ONE 2017, 12, e0187121. [Google Scholar] [CrossRef]


| Study | Methodology | Data Type | Investigated Disease/Condition | Sample Size | Preprocessing Type | Classifier | Limitations | Pros | Cons | Originality Point | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metin et al. [18] | CNN (GoogleNet) and EEG | EEG signals | Treatment-resistant depression (TRD) | 77 in the TRD group, 43 in the non-TRD group, and 40 in the control group | Z-score normalization | GoogleNet CNN | Moderate sample size and retrospective design | High classification accuracy for TRD detection | Moderate sample size and retrospective design | Use of GoogleNet CNN for TRD classification is innovative | TRD–non-TRD classification accuracy: 90.05%; external validation: 73.33% |
| Earl et al. [22] | Random Forest Classifier | EEG and functional connectivity measures | Major depressive disorder (MDD) | 24 in the MDD group and 25 healthy controls | Bandpass filtering (0.5–45 Hz), artifact removal, and ICA | Random Forest | Small sample, need for independent validation, and age and gender differences | High accuracy across emotional states | Small sample size and limited generalizability | Multi-condition EEG analysis for MDD detection | Model 1: 92.3% accuracy (resting state), Model 2: 94.9% accuracy (happy video), Model 3: 89.7% accuracy (sad video); test accuracies: 60%, 80%, and 70%, respectively |
| Xia et al. [23] | Data Augmentation (DCT) and EEGNet | Sleep EEG signals | Sleep pattern analysis | - | Discrete cosine transform (DCT) and normalization | EEGNet (CNN) | Small sample size and need for individual data | Effective use of augmentation for performance improvement | Small sample size and limited generalizability | Individual-specific data augmentation for EEG signal analysis | Model accuracy reached 92.85%. Performance improved with individual-specific classification via data augmentation |
| Madhu et al. [24] | BiLSTM and Ensemble Learning | EEG signals | Psychological stress and meditation effects | 69 students | Spectral analysis | BiLSTM-DT Ensemble | Limited data group and short observation post-meditation | Combines BiLSTM with ensemble methods for improved classification | Small sample size and limited observation duration | Innovative model for meditation-induced stress analysis | BiLSTM-DT model accuracy: 82% |
| Liu et al. [25] | Semi-supervised learning and CEEMDAN Fuzzy Entropy | Single-channel EEG signals | Fatigue detection (drivers) | - | CEEMDAN and bandpass filtering | Fuzzy Entropy + Self-Training | Single-channel EEG usage only | CEEMDAN method effectively improves classification accuracy | Single-channel EEG usage limits generalizability | Application of fuzzy entropy with self-training for EEG-based fatigue detection | RF classifier accuracy: 88.3% |
| Corrivetti et al. [26] | Multi-Omics Analysis and Opade AI Tools | EEG and biological samples | Major depressive disorder (MDD) | 350 patients | Standardization | Random Forest + AI-Assisted Analysis | Multi-center data alignment challenges | Combines EEG and biological data for personalized treatment | Multi-center data alignment challenges | Application of multi-omics analysis for personalized psychiatry | Multi-omics data analysis and personalized treatment prediction |
| Cambay et al. [27] | QuadTPat and kNN | EEG signals | Stress detection | 310 participants | CWNCA (Component analysis) | kNN | Complex model structure | High accuracy for stress detection models | Complex model structure | Innovative CWNCA-based analysis for stress classification | 92.95% accuracy; LOSO cross-validation: 73.63% |
| Cerdan-Martinez et al. [28] | EEG Analysis and Hotelling’s T2 | EEG signals | Watching violent films | 30 students | Bandpass filtering | - | Small sample size | Detailed analysis of gender differences | Small sample size | Unique focus on EEG analysis during violent film exposure | Increased left insula activation in females |
| Kung et al. [29] | EEG-fMRI Spectral Analysis | EEG and fMRI data | Sleep inertia | - | Spectral filtering | - | Small sample size | Demonstrated neurovascular coupling in sleep inertia | Small sample size | Integrated EEG-fMRI spectral analysis | Neurovascular coupling state dependency was demonstrated |
| Chen et al. [30] | Ensemble Model and K-CPT-2 | EEG and behavioral measures | Attention-deficit/hyperactivity disorder (ADHD) | 78 children | Frequency band filtering | Ensemble Model | Small sample size | High accuracy for ADHD detection | Small sample size | Multimodal approach combining EEG and behavioral measures | Ensemble model accuracy: 97.4% |
| Maschke et al. [31] | EEG Dynamic Analysis and PCI | EEG signals | Loss of consciousness (effects of anesthesia) | - | - | - | Controlled experimental conditions | Effective EEG-based prediction of PCI values | Controlled experimental conditions | EEG dynamic properties applied to predict anesthesia outcomes | PCI values were predicted with EEG dynamic properties |
| Kim et al. [32] | Microstate Analysis | EEG signals | Internet gaming disorder and alcohol dependence | 199 participants | Bandpass filtering | - | Single-session data collection | Identified distinct EEG microstate changes in AUD and IGD | Single-session data collection | Microstate analysis for co-occurring disorders (IGD and AUD) | Microstate C duration was different in AUD and IGD patients |
| Zhang et al. [33] | Wavelet Scattering and BiLSTM-MA | Multi-channel EEG | Major depressive disorder (MDD) | 2 datasets | Wavelet transform | BiLSTM-MA | Dataset compatibility issues | Achieved high accuracy for MDD detection | Dataset compatibility issues | Novel use of wavelet scattering for EEG-based depression analysis | 98.8% accuracy; F1-score: 99.81%. |
| Ouyang et al. [34] | EEG and PSG Analysis | EEG and sleep measurements | Brain aging and exercise | 26 participants | Bandpass filtering | - | Small sample size | Observed cognitive improvements post-exercise | Small sample size | EEG and PSG combined to study aging and exercise effects | Cognitive performance improvement after exercise observed |
| Chen et al. [35] | EEG Functional Connectivity Analysis | EEG signals | Attention-deficit/hyperactivity disorder (ADHD) | 78 children | Bandpass filtering | - | Limited age group | ADHD subgroups showed distinct connectivity patterns | Limited to a specific age group | Focused on subgroup analysis of ADHD using EEG functional connectivity | ADHD subgroups showed differences in connectivity models |
| Meinert et al. [36] | Co-Production Workshop | EEG and survey data | Epilepsy | - | - | - | Lack of participant diversity | Subcutaneous EEG found to be beneficial for epilepsy | Lack of participant diversity | Collaborative approach to assess SubQ EEG potential | SubQ EEG was found to be potentially beneficial |
| Hsu et al. [37] | Gamma Band Effective Connectivity | EEG signals | Mindfulness experiences | - | Gamma band filtering | SVM, Naive Bayes, and Decision Trees | Data scarcity | Decision tree model was highly accurate | Data scarcity | Focused on gamma band connectivity for mindfulness analysis | Decision tree accuracy: 91.7% |
| Gui et al. [38] | Neuroadaptive Bayesian Optimization | EEG signals | Social interaction | 42 infants | Bandpass filtering | - | Limited age group | Observed differential responses to social cues | Limited to infants and small sample size | Neuroadaptive Bayesian optimization for EEG-based social cue analysis | Infants’ responses to social cues differed |
| Pandey et al. [39] | fNIRSNET Model and Multi-View CNN | fNIRS and EEG signals | Auditory event classification | 9 participants | Standardization | CNN | Small sample size | Multimodal analysis combining fNIRS and EEG | Very small sample size | Novel CNN-based model combining fNIRS and EEG for auditory classification | 87.15% accuracy achieved |
| Lee et al. [40] | FC Connectivity Analysis and PLV | EEG signals | Self-harm and depression | 77 MDD patients | PLV analysis | - | Need for clinical validation | Explored differences in EEG functional connectivity | Need for clinical validation | First to study PLV differences in MDD with self-harm | No difference found between NSSI and non-NSSI groups |
| Zhang et al. [41] | Event-Related Potential (ERP) Analysis | EEG signals | Social anxiety disorder (SAD) | 69 SAD patients | ERP component extraction | - | Need for control group | Observed ERP component changes after hypnotherapy | Lack of a control group | Focused on ERP-based analysis of hypnotherapy effects | N170 and LPP reductions observed after hypnotherapy |
| Ho et al. [42] | EEG Functional Connectivity and Graph Theory | EEG signals | Major depressive disorder (MDD) | 54 MDD patients and 39 controls | Bandpass filtering | - | Variety of antidepressants used | Identified EEG functional connectivity differences | Variety of antidepressants used among participants | Graph theory applied to analyze EEG functional connectivity | Delta band EEG FC values found to differ |
| Catal et al. [43] | INT Change Analysis | EEG and calcium imaging | Brain time scales and behavior modulation | - | Bandpass filtering | - | Limited individual difference analysis | Correlated brain intrinsic time scales with behavior | Limited individual difference analysis | Used calcium imaging to investigate brain time scales | Brain intrinsic time scales correlated with behavior modulation |
| Moreau et al. [44] | EEG Hyperscanning | EEG signals | Autism spectrum disorder (ASD) | - | Spectral analysis | - | Small sample and limited interaction variety | Observed leadership behavior differences in ASD and TD | Small sample and limited interaction scenarios | Applied hyperscanning for leadership analysis in ASD | Different leadership behaviors observed in ASD and TD individuals |
| Dal Bo et al. [45] | ERP and Spectral Perturbation Analysis | EEG and ECG signals | Emotional cue effects | 22 individuals (3 groups) | - | - | Small sample size | Analyzed olfactory sensory cue effects on EEG/ECG | Small sample size | Combined ERP and ECG analysis for emotional cues | Olfactory sensory cues showed significant ERSP analysis results |
| Yang et al. [46] | Efference Copy (EC) and CD Analysis | EEG signals | Schizophrenia and auditory hallucinations | - | Bandpass filtering | - | Small sample and schizophrenic patients only | Observed motor-sensory transformation differences | Small sample size and only schizophrenic patients | Efference copy analysis for auditory hallucinations in schizophrenia | Motor-sensory transformation differences observed in AVH and non-AVH groups |
| Thunstroem et al. [47] | Usability Testing | EEG signals | Mental health support tools | 45 participants | - | - | Limited sample and healthy individuals only | Found chatbots more user-friendly than digital human interfaces | Limited sample and healthy individuals only | Usability study on EEG-guided mental health tools | Text-based chatbot was found to be more user-friendly than the digital human interface |
| Jia et al. [48] | TTSNet Model | EEG signals | Brain–computer interface (BC | - | Spectral analysis | CNN | Complex model | Applied multi-classification for BCI tasks | Complex model structure | Novel TTSNet for EEG-based multi-class BCI applications | TTSNet multi-classification accuracy: 45.88% |
| Study | Methodology | Data Type | Investigated Disease/Condition | Sample Size | Preprocessing Type | Classifier | Limitations | Pros | Cons | Originality Point | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Habib et al. [49] | MDDBranchNet and parallel-branch deep learning model | ECG | Major depressive disorder (MDD) | - | Signal segmentation | CNN and ANN | Limited to single-channel ECG for home use | Improved model accuracy for home-based applications | Limited to single-channel ECG for home use | Parallel-branch design tailored for MDD detection in low-resource environments | 70% threshold showed consistent MDD detection; model accuracy increased by 7% |
| Abedinzadeh et al. [59] | Pre-processing-free deep learning with transfer learning | ECG | Mental state classification | - | No preprocessing | VGG16 and GoogLeNet | Limited noise resistance testing | Extremely high accuracy for noisy signals | Limited noise resistance testing | Avoids preprocessing while achieving state-of-the-art performance | 99.35% accuracy for noisy ECG signals; VGG16 showed superior performance |
| Abbas et al. [52] | 2D scalogram + MobileNetV2 + AdaBoost | EEG and ECG | Depression classification | 2 datasets | Scalogram generation | MobileNetV2 and AdaBoost | IoT data transmission stability issues | Robust early detection and high sensitivity and specificity | IoT data transmission stability issues | Combination of scalogram and mobile-optimized deep learning for IoT systems | Sensitivity: 96%, Specificity: 95%, and MCC: 0.96; robust early depression detection |
| Tasci et al. [51] | Ternary pattern-based ANN with majority voting | ECG | Bipolar disorder, depression, and schizophrenia | 3570 beats | Wavelet transform | ANN and Majority Voting | Dataset-specific model limitations | High overall accuracy for psychiatric disorders | Dataset-specific model limitations | Novel ternary pattern-based feature extraction | Overall accuracy: 96.25%; individual lead accuracy ranged from 73.67% to 89.19% |
| Shermadurai et al. [60] | CNN–LSTM hybrid model for multimodal analysis (EEG, ECG, and ACC) | EEG, ECG, and ACC | Stress classification | - | Kruskal–Wallis filtering | SVM, KNN, and RF | High-dimensional data increase computational costs | SVM achieved the best performance for multimodal stress analysis | High-dimensional data increase computational costs | Fusion of CNN and LSTM for multimodal stress classification | SVM achieved the highest performance with 94.58% accuracy |
| Zhang et al. [61] | Continuous wavelet transform (CWT) and cardiopulmonary coupling (CPC) | PPG | Mental workload classification | - | CPC mapping | ResAttNet | Limited to MAUS dataset | Improved over HRV-based methods | Limited to MAUS dataset | Use of CWT and CPC mapping for workload classification | 80.5% accuracy; 6.2% improvement over HRV-based methods |
| Chen et al. [62] | 1D-CNN-based mental fatigue detection | ECG | Mental fatigue | 22 participants | Filtering | 1D-CNN | Small sample size and limited to three time periods | High accuracy for mental fatigue detection | Small sample size and limited temporal data | Compact 1D-CNN design for mental fatigue analysis | Accuracy: 98.44%; F1-score: 98.44% |
| Geethanjali et al. [63] | Multimodal hybrid deep learning model for maternal health risk detection | Text and ECG | Maternal health risk analysis | - | Feature fusion | CNN, LSTM, and Attention | Requires more diverse datasets | High precision and recall for health risk detection | Requires more diverse datasets | Combined textual and ECG data for health risk prediction | Accuracy: 98.4%, precision: 97.6%, and recall: 95.6% |
| Waheed Awan et al. [64] | CNN–vision transformer ensemble with physiological signals (ECG, EEG, and GSR) | EEG, ECG, and GSR | Emotional health assessment | - | Signal segmentation | CNN, Vision Transformer, and SVM | Computationally intensive and long training times | High sensitivity and specificity for emotional assessment | Computationally intensive and long training times | Vision transformer-based ensemble for multimodal emotional health detection | Accuracy: 98.2%, sensitivity: 99.15%, and specificity: 99.53% |
| Sun et al. [67] | Feature fusion model with squeeze-excitation attention mechanism | ECG | Mental stress classification | - | PCA for dimensionality reduction | Voting Classifier | Model complexity increases computational demand | Improved performance over state-of-the-art models | Model complexity increases computational demand | Innovative attention mechanism to enhance feature fusion | Achieved improved accuracy compared to state-of-the-art methods |
| Mukherjee et al. [68] | Attention mechanism-based CNN–TLSTM for stress level detection | EEG and pulse rate | Stress level detection | - | Signal filtering | CNN and TLSTM | Limited validation with additional signals (ECG and GSR) | Robust classification across multiple stress categories | Limited validation with additional signals (ECG and GSR) | Attention-based TLSTM for multi-category stress detection | Average accuracy: 97.86%; robust classification into four stress categories |
| Sangeetha et al. [69] | Multimodal affective computing approach with neural network optimization | EEG, ECG, and EMG | Emotion recognition | - | Filtering and feature extraction | LSTM and CNN | Higher memory usage and training time | Reduced overfitting with neural network optimization | Higher memory usage and training time | Combined EEG, ECG, and EMG signals for emotion recognition | Highest accuracy: 87.83%; regularization reduced overfitting |
| Alzate et al. [70] | PPG and ECG feature extraction with ML framework | ECG and PPG | Depressive behavior detection | 59 participants | Feature extraction | ML Models | Small sample size and requires further refinement | High accuracy for depressive state detection | Small sample size and requires further refinement | Integrated ECG and PPG signals for depression analysis | Accuracy: 92% for depressive state detection |
| Tuncer et al. [65] | Cardioish-based explainable feature extraction (XAI) model | ECG | Cardiac disorders | - | INCA and lead transformation | kNN | Lengthy feature extraction process | Over 99% accuracy across datasets | Lengthy feature extraction process | Explainable AI-based approach for cardiac disorder analysis | Over 99% accuracy for both datasets |
| Ao et al. [71] | Real-time cardiac abnormality detection using phase space–time delay | ECG | Cardiac abnormalities | - | Adaptive denoising | Automatic ML | Clinical validation is still needed | Accurate detection of APBs and P-wave peaks | Clinical validation is still needed | Phase space–time delay applied to real-time cardiac abnormality detection | APB detection accuracy: 100%; P-wave peak detection: 98.1% |
| Telangore et al. [66] | Wavelet scattering network (WSN) and machine learning | ECG | Bipolar disorder (BD), depression (DP), and schizophrenia (SZ) | 3570 ECG beats | ECG signals segmented into 1-s epochs from 12-lead channels | Fine K-Nearest Neighbor (FKNN) |
| Excellent accuracy across psychiatric disorders | Dataset imbalances and male participants only | Wavelet scattering for multi-class psychiatric disorder detection | Accuracy: 99.8%, precision: 99–100%, recall: 99–100%, and F1-score: 99–100% (tested with 10-fold cross-validation) |
| Study | Methodology | Data Type | Investigated Disease/Condition | Sample Size | Preprocessing Type | Classifier | Limitations | Pros | Cons | Originality Point | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rezaii et al. [100] | Cross-sectional study and supervised and unsupervised classification | Connected speech samples | Primary progressive aphasia (PPA) | 78 patients | Feature extraction from speech | Custom NLP classifier | Limited to short speech samples | High classification accuracy for PPA variants | Limited to short speech samples | Combination of supervised and unsupervised techniques for PPA classification | 97.9% accuracy in classifying PPA variants |
| Pugh et al. [101] | Model evaluation | Speech samples | Schizophrenia (thought disorder) | 51 participants | Data parameter tuning | Large language models (GPT and Llama) | Trade-off between accuracy and consistency in outputs | High consistency comparable to expert ratings | Trade-off between accuracy and consistency | Application of LLMs for evaluating thought disorders | Consistency comparable to expert ratings; 92% F1-score |
| Ahammed et al. [102] | Empirical analysis | Emotional speech signals | Emotion recognition | RAVDESS, TESS, and SAVEE datasets | MFCC, Chroma, and Mel-spectrogram | SVM | Requires testing on larger datasets | Extremely high accuracy across multiple datasets | Requires testing on larger datasets | Use of diverse spectral features for emotion recognition | Accuracy: 99.82% (TESS) and 98.95% (SAVEE) |
| Leite et al. [103] | Incremental learning approach | Speech streams | Bipolar disorder | 7 months of data | Feature ranking (Pearson–Spearman correlation) | eOGS | High class overlap in psychiatric data | Effective use of incremental learning over time | High class overlap in psychiatric data | Incremental learning for dynamic speech analysis in bipolar disorder | 91.8% accuracy using 8 acoustic features |
| Wang et al. [104] | Proof-of-concept study | Semi-structured interviews | Loneliness in older adults | 97 participants | LIWC feature extraction | Explainable AI (XAI) | Small sample size and gender imbalance | High recall and transparency in prediction models | Small sample size and gender imbalance | First study linking speech to loneliness using XAI | Accuracy: 88.9%, F1-score: 0.8, and recall: 1.0 |
| Park et al. [105] | Multi-label classification | Social media speech | Depressive disorders (DSM-5) | Not specified | Label correlation analysis | Custom classifier | Reliability of online data questioned | Predictions aligned with DSM-5 criteria | Reliability of online data questioned | Multi-label classification for DSM-5-based depression assessment | Predictions based on DSM-5 criteria using speech features |
| Ding et al. [106] | Multi-task learning model | Crisis hotline speech data | Suicide risk assessment | Not specified | Gender-based feature extraction | Deep learning model | Small dataset and ignored multimodal data | High accuracy for identifying crisis speech | Small dataset and ignored multimodal data | Multi-task model for crisis speech analysis | F1-score of 96% in crisis recognition |
| Rosi et al. [107] | Experimental study | Speech and gesture recognition | Assistive tech for the visually impaired | Not specified | Image and audio feature extraction | CNN and OpenCV | Limited real-world testing | Accurate integration of speech and gesture for assistive tech | Limited real-world testing | Application of speech and gesture for assistive technologies | 96.3% accuracy in hand and face recognition |
| Takeshige et al. [108] | ML model | Chatbot conversations | Alzheimer’s disease | 192 participants | Facial and speech feature extraction | ML-based chatbot model | Dependency on chatbot performance | Effective use of chatbots for AD detection | Dependency on chatbot performance | Chatbot-based AD detection with facial and speech features | 94% AUC in distinguishing AD from healthy controls |
| Taşcı et al. [109] | Hybrid feature extraction | Speech audio signals | Depression | MODMA dataset | Wavelet transforms and feature selection | KNN (k-nearest neighbor) | Needs evaluation on broader datasets | High accuracy for depression detection using MODMA dataset | Needs evaluation on broader datasets | Hybrid feature extraction for depression analysis in MODMA dataset | 94.63% accuracy in depression detection |
| Study | Methodology | Data Type | Investigated Disease/Condition | Sample Size | Preprocessing Type | Classifier | Limitations | Pros | Cons | Originality Point | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gargari et al. [143] | Comparative study with LLMs | DSM-5 clinical cases | Psychiatric disorders | 20 cases | RAG (retrieval augmented generation) | GPT-3.5, GPT-4, Aya, and Nemotron | Struggled with some specific disorders | GPT models outperformed other models | Struggled with some specific disorders | Comparison of multiple large language models for psychiatric diagnosis | GPT models outperformed others |
| Pugh et al. [101] | Thought disorder assessment | Speech samples from schizophrenia patients | Thought disorders | 51 participants | Feature extraction | LLM-based models | Inconsistent predictions in some cases | High consistency with expert ratings and strong F1-score | Trade-off between accuracy and consistency in outputs | First application of LLMs for thought disorder assessment | Accuracy vs. consistency trade-off |
| Turner et al. [144] | NLP pipeline for psychiatry | EHR clinical notes | Transdiagnostic psychiatry | 22,170 patients | Semi-rule-based pipeline | NLP-based classification | Limited by HDRS comparison | Comparable consistency with expert ratings | Accuracy vs. consistency trade-off | Use of LLMs for thought disorder assessment | Accuracy 95–99% (F1-score: 0.38–0.86) |
| Benger et al. [152] | Deep learning for MRI labeling | Brain MRI reports | Neurological abnormalities | 5000 MRI reports | Binary and multi-class labeling | Report classifier | Reduced performance in non-expert labeling | High accuracy for binary labels | Reduced performance in non-expert labeling | Novel application of deep learning for MRI report labeling | High accuracy for binary labels |
| Wang et al. [153] | Sentiment-based NLP analysis | Hospital admission records | Delirium detection | 3862 records | Sentiment feature extraction | ML-based model | Retrospective data limitation | Improved AUC with sentiment features | Retrospective data limitation | First integration of sentiment analysis for delirium detection | AUC: 0.930 (with NLP) vs. 0.869 (without NLP) |
| Levis et al. [146] | Suicide risk prediction | VA electronic medical records | Suicide prediction | 5029 cases + controls | Unstructured text notes | ML classification | Lack of ensemble evaluation | Effective in identifying high-risk suicide groups | Lack of ensemble evaluation | Use of unstructured text for suicide risk classification | Top 10% risk group captured 29% of suicides |
| Botelle et al. [145] | Violence text classification | Mental health records | Interpersonal violence | 3771 text fragments | BioBERT fine-tuned | Pretrained transformer | Limited data diversity | High precision and recall for violence classification | Limited data diversity | Application of BioBERT for interpersonal violence detection | Precision: 89–98% and recall: 89–97% |
| Ciampelli et al. [154] | ASR with semantic NLP | Speech transcriptions in schizophrenia | Schizophrenia | Not specified | Word error rate (WER) analysis | Random forest | ASR accuracy impact on NLP results | Accurate schizophrenia classification from speech | ASR accuracy impacts NLP results | Integration of ASR and semantic NLP for schizophrenia analysis | Accuracy: 76.7% (ASR) and 79.8% (manual) |
| Levis et al. [147] | NLP-enhanced risk prediction | Veterans EHR notes | Suicide risk prediction | 4584 cases | Clinical note analysis | ML classification | Model limited to VA data | Improvement in AUC with NLP features | Limited to VA data | Combination of unstructured notes and structured data | AUC: 0.69 (+19% improvement) |
| Vaci et al. [155] | Clinical depression NLP extraction | UK-CRIS EHRs | Depression | Not specified | Active learning-based annotation | Statistical models | Lower performance for auxiliary variables | High accuracy for drug-related information extraction | Lower performance for auxiliary variables | First application of active learning for clinical depression NLP | High accuracy for drug-related information |
| Çabuk et al. [149] | Turkish speech feature analysis | Schizophrenia patient speech samples | Schizophrenia | 76 participants | POS tagging and Word2Vec embeddings | K-means clustering | Language-dependent limitations | High classification accuracy for schizophrenia in Turkish speech | Language-dependent limitations | Application of POS tagging for schizophrenia analysis in Turkish | Accuracy: 86.84% |
| Zaher et al. [151] | Speech marker analysis | Psychosis-related speech data | Psychosis relapse prediction | Not specified | Narrative speech analysis | NLP-based approach | Lacks large-scale validation | Accurate prediction of relapse within 2–4 weeks | Lacks large-scale validation | Use of narrative speech markers for relapse prediction | Predicts relapse within 2–4 weeks |
| Arslan et al. [150] | Turkish SSD speech analysis | Turkish speech samples | Schizophrenia spectrum disorders | 82 participants | SBERT-based features | Random forest | Limited generalizability across languages | Effective differentiation of SSD cases | Limited generalizability across languages | First use of SBERT-based features for SSD in Turkish speech | Mean accuracy: 86.8% |
| Kerz et al. [156] | Explainable AI (XAI) for mental health | Social media data | Mental health detection | Public datasets | Feature ablation and LIME explanations | BiLSTM and transformer | Trade-off between accuracy and interpretability | High interpretability of mental health prediction | Trade-off between accuracy and interpretability | Application of XAI for feature analysis in mental health models | Detailed feature interpretability |
| Sawalha et al. [157] | Sentiment analysis of PTSD data | AVEC-19 corpus speech data | PTSD detection | 275 participants | Sentiment feature extraction | ML model | Small dataset | High accuracy for sentiment-based PTSD detection | Small dataset | Sentiment analysis for PTSD detection in AVEC-19 corpus | Balanced accuracy: 80.4% |
| Acosta et al. [158] | Sentiment analysis for mindfulness | CSQ-8 questionnaire texts | Mindfulness during COVID-19 | 154 responses | Transfer learning | Neural networks | Limited dataset size | High accuracy for mindfulness detection | Limited dataset size | First use of sentiment analysis for mindfulness assessment | Accuracy: 93.02% (first set) and 90.53% (second set) |
| Kizilay et al. [159] | Clinical high-risk for psychosis | CHR-P and HC speech samples | Clinical high-risk psychosis | 107 participants | POS and semantic analysis | ML-based model | Small sample size | Effective differentiation between CHR-P and HC groups | Small sample size | Semantic and POS-based analysis for CHR-P detection | Accuracy: 79.6% and AUC: 0.86 |
| Cox et al. [160] | Psychedelic therapy narrative analysis | Social media user narratives | Substance use reduction | 1141 participants | Topic modeling | ML algorithms | Self-reported narrative bias | Identifies potential impact of therapy from narratives | Bias due to self-reported narratives | Use of topic modeling for analyzing psychedelic therapy narratives | Prediction accuracy: 65% |
| Irving et al. [161] | Psychosis risk prediction | South London and Maudsley EHRs | Psychosis risk | 92,151 patients | LASSO-regularized Cox regression | ML risk calculator | Dataset limited to SLaM NHS Trust | High AUC for predicting psychosis risk | Dataset limited to SLaM NHS Trust | Large-scale application of LASSO-regularized regression | Harrell’s C: 0.85 |
| Wu et al. [162] | Phenotype-mention identification | Free-text medical records | Phenotype embedding tasks | Not specified | Embedding-based adaptation | NLP-based pipeline | Model retraining required in new settings | High accuracy for phenotype extraction | Requires retraining for different datasets | Use of embedding-based methods for phenotype-mention identification | Accuracy: 93–97% |
| Horigome et al. [163] | Dementia detection through free conversation | Dementia patient conversations | Dementia diagnosis | 432 datasets | Morphological analysis | ML classifier | Specific to outpatient clinic setting | Accurate dementia detection in outpatient settings | Specific to outpatient clinic settings | First application of conversational data for dementia detection | Accuracy: 90%, sensitivity: 88.1%, and specificity: 91.6% |
| Viani et al. [164] | Temporal extraction for psychosis | Mental health records | Duration of untreated psychosis | Not specified | Rule-based time expression | NLP pipeline | Complex annotation challenges | Accurate time normalization for psychosis diagnosis | Complex annotation challenges | Rule-based temporal extraction for psychosis diagnosis | Normalization accuracy: 71–86% |
| Tsui et al. [148] | Suicide attempt prediction | EHR structured + unstructured data | First-time suicide attempts | 45,238 patients | NLP and ML feature extraction | Logistic regression and CNN | Historical data bias | High AUC for suicide prediction | Historical data bias | Combination of structured and unstructured data for suicide risk | AUC: 0.932 |
| Oh et al. [165] | AD phenotype extraction | Alzheimer’s clinical notes | Alzheimer’s disease dementia | Not specified | Manual annotation for training | NLP pipeline | Limited to unstructured notes | High F1-scores for phenotype extraction | Limited to unstructured notes | Use of NLP pipelines for Alzheimer’s phenotype extraction | F1-score: 0.65–0.99 |
| Arslan et al. [166] | Bipolar vs. psychosis speech analysis | Turkish speech samples | Bipolar disorder and psychosis | 143 participants | Semantic similarity + POS analysis | ML classifier | Cross-sectional design | Effective differentiation of bipolar vs. psychosis groups | Cross-sectional design | Semantic and POS-based analysis for bipolar and psychosis detection | Accuracy: 82.45% (FEP vs. HC) |
| Msosa et al. [167] | Mental health crisis prediction | Mersey Care EHR | Depression-related crises | Large EHR dataset | MedCAT and BioYODIE annotations | LSTM and random forest | Psychiatrist feedback integration needed | High AUC for mental health crisis prediction | Psychiatrist feedback integration needed | Integration of MedCAT and ML for mental health crisis prediction | AUC: 0.901 (training) and 0.810 (test) |
| Furukawa et al. [168] | Cognitive restructuring in CBT | Japanese iCBT records | Depression | 4369 records | T5-based NLP model | NLP-based prediction | Limited to Japanese records | High accuracy for restructuring prediction | Limited to Japanese records | Use of NLP for cognitive restructuring analysis in CBT | Accuracy: 73.5% |
| Kosowan et al. [169] | PTSD case identification | Pan-Canadian EMR | Post-traumatic stress disorder | 12,104 patients | EMR free-text + diagnostic fields | NLP case definition | Diagnostic code variation by jurisdiction | High accuracy for PTSD identification | Diagnostic code variation by jurisdiction | First application of NLP case definition for PTSD in EMR data | Accuracy: 93.6% |
| Meerwijk et al. [170] | 3ST suicide theory-guided NLP | Veteran progress notes | Veteran suicide risk | Not applicable | Domain-specific ontology | NLP-enhanced risk model | Ontology development challenges | Improved predictive accuracy | Ontology development challenges | Domain-specific ontology integration for suicide risk prediction | Improved predictive accuracy |
| Cusick et al. [171] | Weak supervision for suicidal ideation | Clinical notes | Suicidal ideation detection | 17,978 notes | Rule-based weak supervision | CNN | Manual review for validation | High accuracy for suicidal ideation detection | Manual review for validation | Rule-based weak supervision for clinical suicide risk classification | Accuracy: 94% and F1-score: 0.82 |
| Iqbal et al. [172] | ADE extraction pipeline | Psychiatric EHRs | Adverse drug events | 264k patients | Semantic rule-based approach | ADE annotation pipeline | Contextual ambiguity challenges | High F1-score for ADE extraction | Contextual ambiguity challenges | Semantic rule-based pipeline for adverse drug event identification | F1-score: 0.83 |
| Dataset Name | Features | Data Type | Total Data Count | Access Link |
|---|---|---|---|---|
| MODMA (Multimodal Depression Analysis Dataset) | EEG (128-electrode and 3-electrode) and audio data | Multimodal (EEG and audio) | 53 EEG (128-electrode), 55 EEG (3-electrode), and 52 audio Participants | http://modma.lzu.edu.cn/data/index/ |
| DAIC-WOZ (Distress Analysis Interview Corpus) | Audio recordings, text, interview dialogues, and video | Audio, text, and video | 621 interviews (face-to-face, teleconference, Wizard-of-Oz, and automated) | https://dcapswoz.ict.usc.edu/ |
| IEMOCAP (Interactive Emotional Dyadic Motion Capture Database) | Audio, video, facial, and motion data | Multimodal (audio, video, and motion capture) | ~12 h of 5 sessions, with 10 actors | https://sail.usc.edu/iemocap/ |
| DEAP (Database for Emotion Analysis using Physiological Signals) | EEG, physiological signals, and facial expressions | EEG, physiological (GSR and EMG), and video | 32 participants and 40 video/trials | https://www.eecs.qmul.ac.uk/mmv/datasets/deap/ |
| SEWA (Automatic Sentiment Analysis in the Wild) | Video, audio, and text | Multimodal (video, audio, and text) | 538 clips | http://sewaproject.eu/ |
| Sentiment140 | Tweet text | Text | 1.6 million tweets | http://help.sentiment140.com/home |
| eRisk Dataset | Social media posts (text) | Text | 800+ users and millions of posts | https://erisk.irlab.org/ |
| AVEC 2019 (Audio/Visual Emotion Challenge) | Audio, video, and text (depression and emotion analysis) | Multimodal (audio, video, and text) | DAIC-WOZ, SEWA, and USoM corpora | http://avec2019.dbvis.de/ |
| ABIDE I (Autism Brain Imaging Data Exchange) | Resting-state fMRI, structural MRI, and phenotypic data | Neuroimaging (fMRI and MRI) | 1112 subjects (539 ASD and 573 control; ages 7–64) | http://fcon_1000.projects.nitrc.org/indi/abide/ |
| COBRE (Center for Biomedical Research Excellence) | Resting fMRI, structural MRI, and phenotypic data | Neuroimaging (fMRI and MRI) | 72 schizophrenia patients and 75 controls | https://fcon_1000.projects.nitrc.org/indi/retro/cobre.html |
| Psychiatry-ECG Dataset | ECG signals/beats for bipolar, depression, schizophrenia, and control groups | ECG signals | 198 participants (62 bipolar, 17 depression, and 119 schizophrenia) | https://www.kaggle.com/datasets/buraktaci/Psychiatry-ECG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baydili, İ.; Tasci, B.; Tasci, G. Artificial Intelligence in Psychiatry: A Review of Biological and Behavioral Data Analyses. Diagnostics 2025, 15, 434. https://doi.org/10.3390/diagnostics15040434
Baydili İ, Tasci B, Tasci G. Artificial Intelligence in Psychiatry: A Review of Biological and Behavioral Data Analyses. Diagnostics. 2025; 15(4):434. https://doi.org/10.3390/diagnostics15040434
Chicago/Turabian StyleBaydili, İsmail, Burak Tasci, and Gülay Tasci. 2025. "Artificial Intelligence in Psychiatry: A Review of Biological and Behavioral Data Analyses" Diagnostics 15, no. 4: 434. https://doi.org/10.3390/diagnostics15040434
APA StyleBaydili, İ., Tasci, B., & Tasci, G. (2025). Artificial Intelligence in Psychiatry: A Review of Biological and Behavioral Data Analyses. Diagnostics, 15(4), 434. https://doi.org/10.3390/diagnostics15040434







