Comparative Clinical-Imaging and Histogenetic Analysis Between Astrocytoma IDH-Mutant Grade 4 and Glioblastoma IDH-Wildtype—Is There Really a Worse One?
Abstract
:1. Introduction
2. Materials and Methods
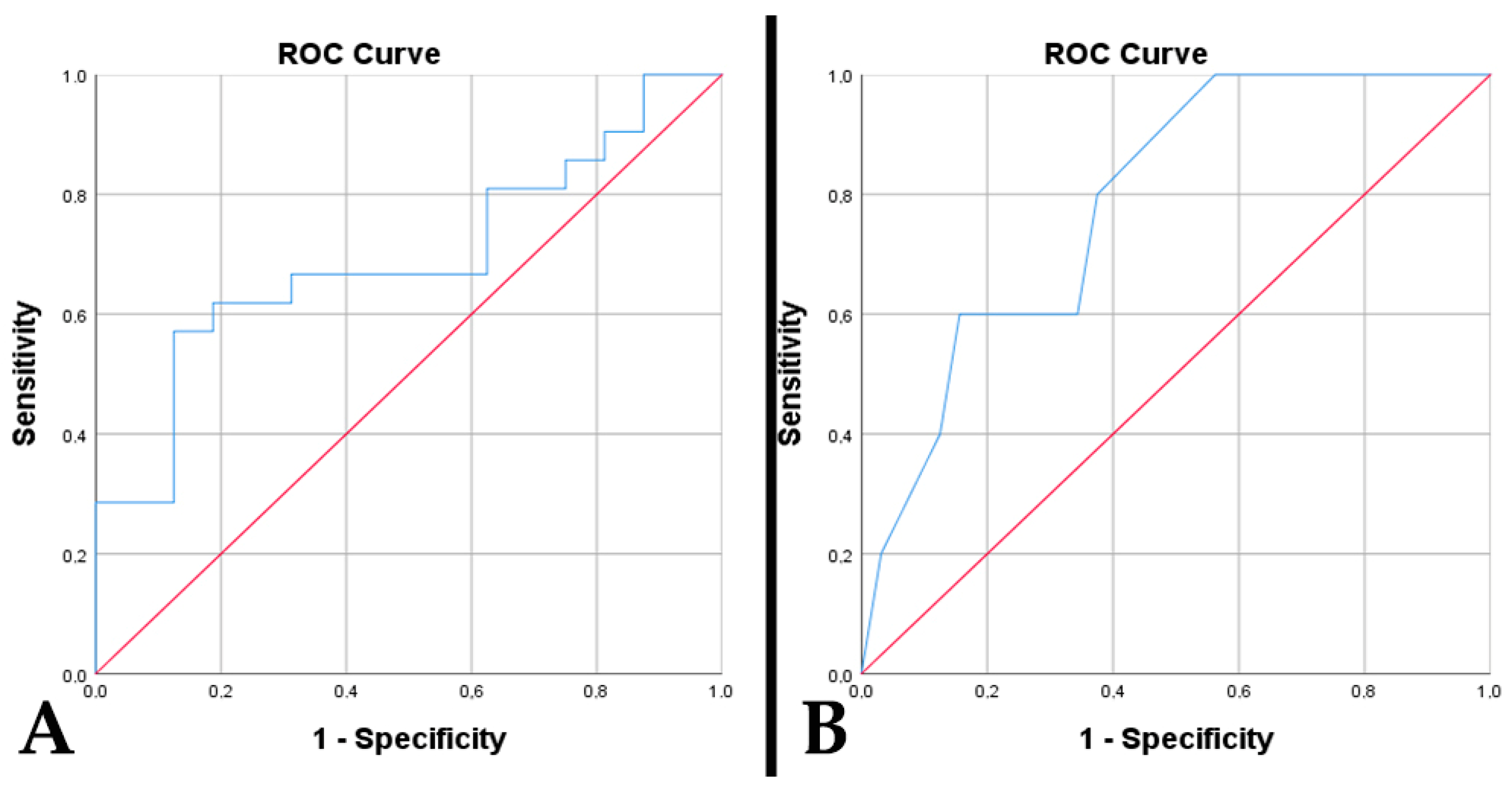
3. Results
3.1. Clinical Features and Survival Outcomes
3.2. Imaging Characteristics and Tumor Metrics
3.3. Histogenetic and Molecular Findings
4. Discussion
4.1. The Integration of Clinical Aspects
4.2. The Integration of Immaging Aspects
4.3. The Involvement of Hypoxic Effects in Morphology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Primers 2024, 10, 33. [Google Scholar] [CrossRef]
- Girardi, F.; Matz, M.; Stiller, C.; You, H.; Marcos Gragera, R.; Valkov, M.Y.; Bulliard, J.L.; De, P.; Morrison, D.; Wanner, M.; et al. Global survival trends for brain tumors, by histology: Analysis of individual records for 556,237 adults diagnosed in 59 countries during 2000–2014 (CONCORD-3). Neuro-Oncol. 2023, 25, 580–592. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Reuss, D.E. Updates on the WHO diagnosis of IDH-mutant glioma. J. Neuro-Oncol. 2023, 162, 461–469. [Google Scholar] [CrossRef]
- Komori, T. Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab. Investig. 2022, 102, 126–133. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Gilard, V.; Tebani, A.; Dabaj, I.; Laquerrière, A.; Fontanilles, M.; Derrey, S.; Marret, S.; Bekri, S. Diagnosis and Management of Glioblastoma: A Comprehensive Perspective. J. Pers. Med. 2021, 11, 258. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, H.; Guo, X.; Chen, W.; Wang, Y.; Liang, T.; Wang, H.; Li, Y.; Jin, S.; Shi, Y.; et al. Clinical features, MRI, molecular alternations, and prognosis of astrocytoma based on WHO 2021 classification of central nervous system tumors: A single-center retrospective study. Cancer Med. 2024, 13, e7369. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; pp. 19–27, 39–54. [Google Scholar]
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro-Oncol. 2017, 19, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro-Oncol. 2024, 26 (Suppl. S6), vi1–vi85. [Google Scholar] [CrossRef]
- Miller, J.J.; Gonzalez Castro, L.N.; McBrayer, S.; Weller, M.; Cloughesy, T.; Portnow, J.; Andronesi, O.; Barnholtz-Sloan, J.S.; Baumert, B.G.; Berger, M.S.; et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro-Oncol. 2023, 25, 4–25. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef] [PubMed]
- Downie, P.A.; Chen, J.J.; Bhatti, M.T.; Melson, A.T.; Van Stavern, G.P.; McClelland, C.M.; Lindgren, B.R.; Sharieff, J.A.; Lee, M.S. Clinical Characteristics of Idiopathic Intracranial Hypertension in Patients Over 50 Years of Age: A multicenter clinical cohort study. Am. J. Ophthalmol. 2021, 224, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Lanza, M.; Filippone, A.; Caffo, M.; Paterniti, I.; Campolo, M.; Colarossi, L.; Sciacca, D.; Lombardo, S.P.; Cuzzocrea, S.; et al. Overview on Common Genes Involved in the Onset of Glioma and on the Role of Migraine as Risk Factor: Predictive Biomarkers or Therapeutic Targets? J. Pers. Med. 2022, 12, 1969. [Google Scholar] [CrossRef]
- Russo, M.; Villani, V.; Taga, A.; Genovese, A.; Terrenato, I.; Manzoni, G.C.; Servadei, F.; Torelli, P.; Pace, A. Headache as a presenting symptom of glioma: A cross-sectional study. Cephalalgia 2018, 38, 730–735. [Google Scholar] [CrossRef]
- Johnson, D.R.; Wefel, J.S. Relationship between cognitive function and prognosis in glioblastoma. CNS Oncol. 2013, 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Di, G.; Yan, Z.; Liu, D.; Liu, Y.; Song, K.; Yang, K.; Hu, X.; Jiang, Z.; Hu, X.; et al. Does epilepsy always indicate worse outcomes? A longitudinal follow-up analysis of 485 glioma patients. World J. Surg. Oncol. 2022, 20, 297. [Google Scholar] [CrossRef]
- Li, L.; Fang, S.; Li, G.; Zhang, K.; Huang, R.; Wang, Y.; Zhang, C.; Li, Y.; Zhang, W.; Zhang, Z.; et al. Glioma-related epilepsy in patients with diffuse high-grade glioma after the 2016 WHO update: Seizure characteristics, risk factors, and clinical outcomes. J Neurosurg. 2021, 136, 67–75. [Google Scholar] [CrossRef]
- Houben, M.P.; Louwman, W.J.; Tijssen, C.C.; Teepen, J.L.; Van Duijn, C.M.; Coebergh, J.W. Hypertension as a risk factor for glioma? Evidence from a population-based study of comorbidity in glioma patients. Ann. Oncol. 2004, 15, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Ali, A.N.; Voloschin, A.D.; Liu, Y.; Curran, W.J., Jr.; Crocker, I.R.; Shu, H.K. Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer 2015, 121, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Pierscianek, D.; Ahmadipour, Y.; Kaier, K.; Darkwah Oppong, M.; Michel, A.; Kebir, S.; Stuschke, M.; Glas, M.; Sure, U.; Jabbarli, R. The SHORT Score for Preoperative Assessment of the Risk for Short-Term Survival in Glioblastoma. World Neurosurg. 2020, 138, e370–e380. [Google Scholar] [CrossRef]
- Aboubechara, J.P.; Aboud, O. Metabolic Risk Factors and Survival in Patients with Glioblastoma. Cancers 2024, 16, 3666. [Google Scholar] [CrossRef]
- Liu, W.; Qdaisat, A.; Yeung, J.; Lopez, G.; Weinberg, J.; Zhou, S.; Cohen, L.; Bruera, E.; Yeung, S.J. The Association Between Common Clinical Characteristics and Postoperative Morbidity and Overall Survival in Patients with Glioblastoma. Oncologist 2019, 24, 529–536. [Google Scholar] [CrossRef]
- Montemurro, N.; Perrini, P.; Rapone, B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health 2020, 17, 8501. [Google Scholar] [CrossRef] [PubMed]
- Hagan, K.; Bhavsar, S.; Arunkumar, R.; Grasu, R.; Dang, A.; Carlson, R.; Cowles, C.; Arnold, B.; Potylchansky, Y.; Rahlfs, T.F.; et al. Association Between Perioperative Hyperglycemia and Survival in Patients with Glioblastoma. J. Neurosurg. Anesth. 2017, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.R.; Grommes, C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol. 2013, 2, 237–246. [Google Scholar] [CrossRef]
- Kocaeli, A.A.; Tekin, C.; Ercelik, M.; Tezcan, G.; Aksoy, S.A.; Kocaeli, H.; Bekar, A.; Taskapilioglu, M.O.; Tolunay, S.; Tunca, B. Concurrent presence of diabetes affects the GLUT3 programming of glucose metabolism in glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8110–8118. [Google Scholar] [CrossRef] [PubMed]
- Valerius, A.R.; Webb, L.M.; Thomsen, A.; Lehrer, E.J.; Breen, W.G.; Campian, J.L.; Riviere-Cazaux, C.; Burns, T.C.; Sener, U. Review of Novel Surgical, Radiation, and Systemic Therapies and Clinical Trials in Glioblastoma. Int. J. Mol. Sci. 2024, 25, 10570. [Google Scholar] [CrossRef]
- Guarnaccia, L.; Marfia, G.; Masseroli, M.M.; Navone, S.E.; Balsamo, M.; Caroli, M.; Valtorta, S.; Moresco, R.M.; Campanella, R.; Garzia, E.; et al. Frontiers in Anti-Cancer Drug Discovery: Challenges and Perspectives of Metformin as Anti-Angiogenic Add-On Therapy in Glioblastoma. Cancers 2021, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Curtin, J.; Tian, F. Advances in Diagnostic Tools and Therapeutic Approaches for Gliomas: A Comprehensive Review. Sensors 2023, 23, 9842. [Google Scholar] [CrossRef]
- Sawlani, V.; Patel, M.D.; Davies, N.; Flintham, R.; Wesolowski, R.; Ughratdar, I.; Pohl, U.; Nagaraju, S.; Petrik, V.; Kay, A.; et al. Multiparametric MRI: Practical approach and pictorial review of a useful tool in the evaluation of brain tumours and tumour-like lesions. Insights Imaging 2020, 11, 84. [Google Scholar] [CrossRef]
- Drake, L.R.; Hillmer, A.T.; Cai, Z. Approaches to PET Imaging of Glioblastoma. Molecules 2020, 25, 568. [Google Scholar] [CrossRef] [PubMed]
- Pellerino, A.; Caccese, M.; Padovan, M.; Cerretti, G.; Lombardi, G. Epidemiology, risk factors, and prognostic factors of gliomas. Clin. Transl. Imaging 2022, 10, 467–475. [Google Scholar] [CrossRef]
- Palpan Flores, A.; Vivancos Sanchez, C.; Roda, J.M.; Cerdán, S.; Barrios, A.J.; Utrilla, C.; Royo, A.; Gandía González, M.L. Assessment of Pre-operative Measurements of Tumor Size by MRI Methods as Survival Predictors in Wild Type IDH Glioblastoma. Front. Oncol. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Porz, N.; Bauer, S.; Pica, A.; Schucht, P.; Beck, J.; Verma, R.K.; Slotboom, J.; Reyes, M.; Wiest, R. Multi-modal glioblastoma segmentation: Man versus machine. PLoS ONE 2014, 9, e96873. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Boulay, J.L.; Thommen, S.; Bucher, H.C.; Stippich, C.; Mariani, L.; Bink, A. Preoperative Two-Dimensional Size of Glioblastoma is Associated with Patient Survival. World Neurosurg. 2018, 115, e448–e463. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, M.; Andratschke, N.; Bendszus, M.; Chalmers, A.J.; Erridge, S.C.; Galldiks, N.; Lagerwaard, F.J.; Navarria, P.; Munck Af Rosenschöld, P.; Ricardi, U.; et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother. Oncol. 2023, 184, 109663. [Google Scholar] [CrossRef]
- Anagnostakis, F.; Kokkorakis, M.; Markouli, M.; Piperi, C. Impact of Solute Carrier Transporters in Glioma Pathology: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 9393. [Google Scholar] [CrossRef]
- Song, S.; Luo, L.; Sun, B.; Sun, D. Roles of glial ion transporters in brain diseases. Glia 2020, 68, 472–494. [Google Scholar] [CrossRef]
- Ohmura, K.; Tomita, H.; Hara, A. Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines 2023, 11, 2731. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Hamed, M.; Schuss, P.; Güresir, E.; Herrlinger, U.; Vatter, H.; Schneider, M. Impact of initial midline shift in glioblastoma on survival. Neurosurg. Rev. 2021, 44, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Shu, T.; Luo, P.; Shao, Y.; Lin, L.; Tu, Z.; Zhu, X.; Wu, L. The peritumoral edema index and related mechanisms influence the prognosis of GBM patients. Front. Oncol. 2024, 14, 1417208. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Seppä, K.; Luostarinen, T.; Malila, N.; Seppälä, M.; Pitkäniemi, J.; Korja, M. Disparities in glioblastoma survival by case volume: A nationwide observational study. J. Neuro-Oncol. 2020, 147, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Putavet, D.A.; de Keizer, P.L.J. Residual Disease in Glioma Recurrence: A Dangerous Liaison with Senescence. Cancers 2021, 13, 1560. [Google Scholar] [CrossRef]
- Meel, M.; Jindal, A.; Kumar, M.; Mathur, K.; Singh, A. IDH1, ATRX, p53, and Ki67 Expression in Glioblastoma patients: Their Clinical and Prognostic Significance-A Prospective Study. Asian J. Neurosurg. 2024, 19, 14–20. [Google Scholar] [CrossRef]
- Lanman, T.A.; Youssef, G.; Huang, R.; Rahman, R.; DeSalvo, M.; Flood, T.; Hassanzadeh, E.; Lang, M.; Lauer, J.; Potter, C.; et al. Ivosidenib for the Treatment of IDH1-mutant Glioma, Grades 2 to 4: Tolerability, Predictors of Response, and Outcomes. Neuro-Oncol. Adv. 2024, vdae227. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Naval-Baudin, P.; Ligero, M.; Pons-Escoda, A.; Bruna, J.; Plans, G.; Calvo, N.; Cos, M.; Majós, C.; Perez-Lopez, R. Precise enhancement quantification in post-operative MRI as an indicator of residual tumor impact is associated with survival in patients with glioblastoma. Sci. Rep. 2021, 11, 695. [Google Scholar] [CrossRef]
- Wykes, V.; Zisakis, A.; Irimia, M.; Ughratdar, I.; Sawlani, V.; Watts, C. Importance and Evidence of Extent of Resection in Glioblastoma. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef]
- Bozzao, A.; Weber, D.; Crompton, S.; Braz, G.; Csaba, D.; Dhermain, F.; Finocchiaro, G.; Flannery, T.; Kramm, C.; Law, I.; et al. European Cancer Organisation Essential Requirements for Quality Cancer Care: Adult glioma. J. Cancer Policy 2023, 38, 100438. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Brandner, S.; Bertero, L.; Capper, D.; French, P.J.; Figarella-Branger, D.; Giangaspero, F.; Haberler, C.; Hegi, M.E.; Kristensen, B.W.; et al. Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline. Neuro-Oncol. 2023, 25, 1731–1749. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Domènech, M.; Hernández, A.; Plaja, A.; Martínez-Balibrea, E.; Balañà, C. Hypoxia: The Cornerstone of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 12608. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Boyd, N.H.; Tran, A.N.; Bernstock, J.D.; Etminan, T.; Jones, A.B.; Gillespie, G.Y.; Friedman, G.K.; Hjelmeland, A.B. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics 2021, 11, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Annovazzi, L.; Mazzucco, M.; Mellai, M. The origin of circumscribed necroses and perinecrotic niches in glioblastoma multiforme: An additional hypothesis. Integr. Cancer Sci. Ther. 2015, 2, 75–78. [Google Scholar] [CrossRef]
- Markwell, S.M.; Ross, J.L.; Olson, C.L.; Brat, D.J. Necrotic reshaping of the glioma microenvironment drives disease progression. Acta Neuropathol. 2022, 143, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Post, D.E.; Pieper, R.O.; Durden, D.L.; Van Meir, E.G.; Brat, D.J. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005, 65, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Hayati, N.; Tabriz, H.M.; Nazar, E.; Gorji, R. Relationship between phosphatase and tensin homolog (PTEN) expression in high grade glioma and histopathologic findings. Ro. J. Neurol. 2021, 20, 452–457. [Google Scholar] [CrossRef]
- Bisht, P.; Kumar, V.U.; Pandey, R.; Velayutham, R.; Kumar, N. Role of PARP Inhibitors in Glioblastoma and Perceiving Challenges as Well as Strategies for Successful Clinical Development. Front. Pharmacol. 2022, 13, 939570. [Google Scholar] [CrossRef]
- Grimes, D.R.; Jansen, M.; Macauley, R.J.; Scott, J.G.; Basanta, D. Evidence for hypoxia increasing the tempo of evolution in glioblastoma. Br. J. Cancer 2020, 123, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Vilchez, M.L.; Caverzán, M.D.; Milla Sanabria, L.N. Understanding the glioblastoma tumor biology to optimize photodynamic therapy: From molecular to cellular events. J. Neurosci. Res. 2021, 99, 1024–1047. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.H.; Ma, Z.X.; Huang, G.H.; Xu, Q.F.; Xiang, Y.; Li, N.; Sidlauskas, K.; Zhang, E.E.; Lv, S.Q. Downregulation of HIF-1a sensitizes U251 glioma cells to the temozolomide (TMZ) treatment. Exp. Cell Res. 2016, 343, 148–158. [Google Scholar] [CrossRef]
- Butta, S.; Gupta, M.K. Immunohistochemical expression of MGMT in gliomas and its role in ascertaining patient survival. Med. Pharm. Rep. 2021, 94, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—a comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Sahara, N.; Hartanto, R.A.; Yoshuantari, N.; Dananjoyo, K.; Widodo, I.; Malueka, R.G.; Dwianingsih, E.K. Diagnostic Accuracy of Immunohistochemistry in Detecting MGMT Methylation Status in Patients with Glioma. Asian Pac. J. Cancer Prev. 2021, 22, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, M.S.; Ozair, A.; Rudek, M.; Ye, X.; Holdhoff, M.; Lieberman, F.S.; Piotrowski, A.F.; Nabors, B.; Desai, A.; Lesser, G.; et al. A multicenter, phase 1, Adult Brain Tumor Consortium trial of oral terameprocol for patients with recurrent high-grade glioma (GATOR). Cell Rep. Med. 2024, 5, 101630. [Google Scholar] [CrossRef]
- Shamsara, J.; Sharif, S.; Afsharnezhad, S.; Lotfi, M.; Raziee, H.R.; Ghaffarzadegan, K.; Moradi, A.; Rahighi, S.; Behravan, J. Association between MGMT promoter hypermethylation and p53 mutation in glioblastoma. Cancer Investig. 2009, 27, 825–829. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Kitange, G.J.; James, C.D.; Plummer, R.; Calvert, H.; Weller, M.; Wick, W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin. Cancer Res. 2008, 14, 2900–2908. [Google Scholar] [CrossRef]
- Alafandi, A.; van Garderen, K.A.; Klein, S.; van der Voort, S.R.; Rizopoulos, D.; Nabors, L.; Stupp, R.; Weller, M.; Gorlia, T.; Tonn, J.C.; et al. Association of pre-radiotherapy tumour burden and overall survival in newly diagnosed glioblastoma adjusted for MGMT promoter methylation status. Eur. J. Cancer 2023, 188, 122–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E. Impact of CDKN2A/B Homozygous Deletion on the Prognosis and Biology of IDH-Mutant Glioma. Biomedicines 2022, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Neradil, J.; Veselska, R. Nestin as a marker of cancer stem cells. Cancer Sci. 2015, 106, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Srinivas, B.H.; Verma, S.K.; Ms, G. Significance of Nestin and CD133 as cancer stem cell markers in diffuse glioma and association with p53 expression and IDH status. Int. J. Clin. Exp. Pathol. 2024, 17, 208–218. [Google Scholar] [CrossRef]
- An, S.; Song, I.H.; Woo, C.G. Diagnostic Value of Nestin Expression in Adult Gliomas. Int. J. Surg. Pathol. 2023, 31, 1014–1020. [Google Scholar] [CrossRef]
- Aiyappa-Maudsley, R.; Chalmers, A.J.; Parsons, J.L. Factors affecting the radiation response in glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac156. [Google Scholar] [CrossRef] [PubMed]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torres, M.D.M.; López-Cerdán, A.; Andreu, Z.; de la Iglesia Vayá, M.; Fuster-Garcia, E.; García-García, F.; García-Gómez, J.M. Vascular differences between IDH-wildtype glioblastoma and astrocytoma IDH-mutant grade 4 at imaging and transcriptomic levels. NMR Biomed. 2023, 36, e5004. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, J.; Liu, Z.; He, M.; Kang, T.; Du, T.; Song, Y.; Fan, Y.; Xu, J. Prognostic role of microvessel density in patients with glioma. Medicine 2019, 98, e14695. [Google Scholar] [CrossRef]
- Sipos, T.C.; Kövecsi, A.; Kocsis, L.; Nagy-Bota, M.; Pap, Z. Evaluation of Microvascular Density in Glioblastomas in Relation to p53 and Ki67 Immunoexpression. Int. J. Mol. Sci. 2024, 25, 6810. [Google Scholar] [CrossRef] [PubMed]
- Hiller-Vallina, S.; Mondejar-Ruescas, L.; Caamaño-Moreno, M.; Cómitre-Mariano, B.; Alcivar-López, D.; Sepulveda, J.M.; Hernández-Laín, A.; Pérez-Núñez, Á.; Segura-Collar, B.; Gargini, R. Sexual-biased necroinflammation is revealed as a predictor of bevacizumab benefit in glioblastoma. Neuro-Oncol. 2024, 26, 1213–1227. [Google Scholar] [CrossRef]
- Castellino, R.C.; Durden, D.L. Mechanisms of disease: The PI3K-Akt-PTEN signaling node—an intercept point for the control of angiogenesis in brain tumors. Nat. Clin. Pract. Neurol. 2007, 3, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Hu, R.; Yang, H.; Liu, J.; Sui, J.; Xiang, X.; Wang, F.; Chu, L.; Song, S. PTEN gene mutations correlate to poor prognosis in glioma patients: A meta-analysis. Onco Targets Ther. 2016, 9, 3485–3492. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, I.A.; Amorim, C.K.; Ferreira, W.A.S.; Sagica, F.; Brito, J.R.; Othman, M.; Meyer, B.; Liehr, T.; de Oliveira, E.H.C. Detection and Correlation of Single and Concomitant TP53, PTEN, and CDKN2A Alterations in Gliomas. Int. J. Mol. Sci. 2019, 20, 2658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, X.; Liu, L.; Li, S.; Li, Y.; Ali, S.; Li, S.; Xiong, J.; Liu, X.; Li, S.; et al. Identification of the Prognostic Signatures of Glioma With Different PTEN Status. Front. Oncol. 2021, 11, 633357. [Google Scholar] [CrossRef]
- Appay, R.; Dehais, C.; Maurage, C.A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Lu, V.M.; O’Connor, K.P.; Shah, A.H.; Eichberg, D.G.; Luther, E.M.; Komotar, R.J.; Ivan, M.E. The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: A systematic review of the contemporary literature. J. Neuro-Oncol. 2020, 148, 221–229. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, T.G.; Ryu, K.H.; Kim, S.H.; Kim, Y.Z. CDKN2A Homozygous Deletion Is a Stronger Predictor of Outcome than IDH1/2-Mutation in CNS WHO Grade 4 Gliomas. Biomedicines 2024, 12, 2256. [Google Scholar] [CrossRef]
- Wang, J.; Xi, Y.F.; Zhao, Q.; Guo, J.H.; Zhang, Z.; Zhang, M.B.; Chang, J.; Wu, Y.Q.; Su, W. CDKN2A promoter methylation enhances self-renewal of glioblastoma stem cells and confers resistance to carmustine. Mol. Biol. Rep. 2024, 51, 385. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Z.; Wang, Z.F.; Lan, T.; Huang, W.H.; Zhao, Y.H.; Ma, C.; Li, Z.Q. Carmustine as a Supplementary Therapeutic Option for Glioblastoma: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 1036. [Google Scholar] [CrossRef] [PubMed]



| Clinical Aspects | Astrocytoma IDH-Mutant (n = 44) | Glioblastoma IDH-Wildtype (n = 37) | p-Value |
|---|---|---|---|
| Age: | |||
| • Average (min–max) | 57.86 (37–82) | 60.24 (20–81) | 0.232 |
| • Patients <50 years (%) | 34.10 | 13.51 | 0.040 |
| Gender (%): | 0.965 | ||
| • Female | 45.45 | 45.95 | |
| • Male | 54.55 | 54.05 | |
| The onset of symptoms (%): | 0.821 | ||
| • <2 weeks | 51.22 | 48.65 | |
| • >2 weeks | 48.78 | 51.35 | |
| Clinical manifestations (%): | |||
| • Motor impairments/paresis | 65.91 | 59.46 | 0.646 |
| • Headache | 47.73 | 35.14 | 0.269 |
| • Cognitive impairments | 43.18 | 37.84 | 0.656 |
| • Intracranial hypertension | 20.45 | 32.43 | 0.309 |
| • Psychiatric syndromes | 20.45 | 13.51 | 0.558 |
| • Balance and coordination disorders | 15.91 | 21.62 | 0.574 |
| • Epileptic seizures | 13.64 | 21.62 | 0.388 |
| Comorbidities (%): | |||
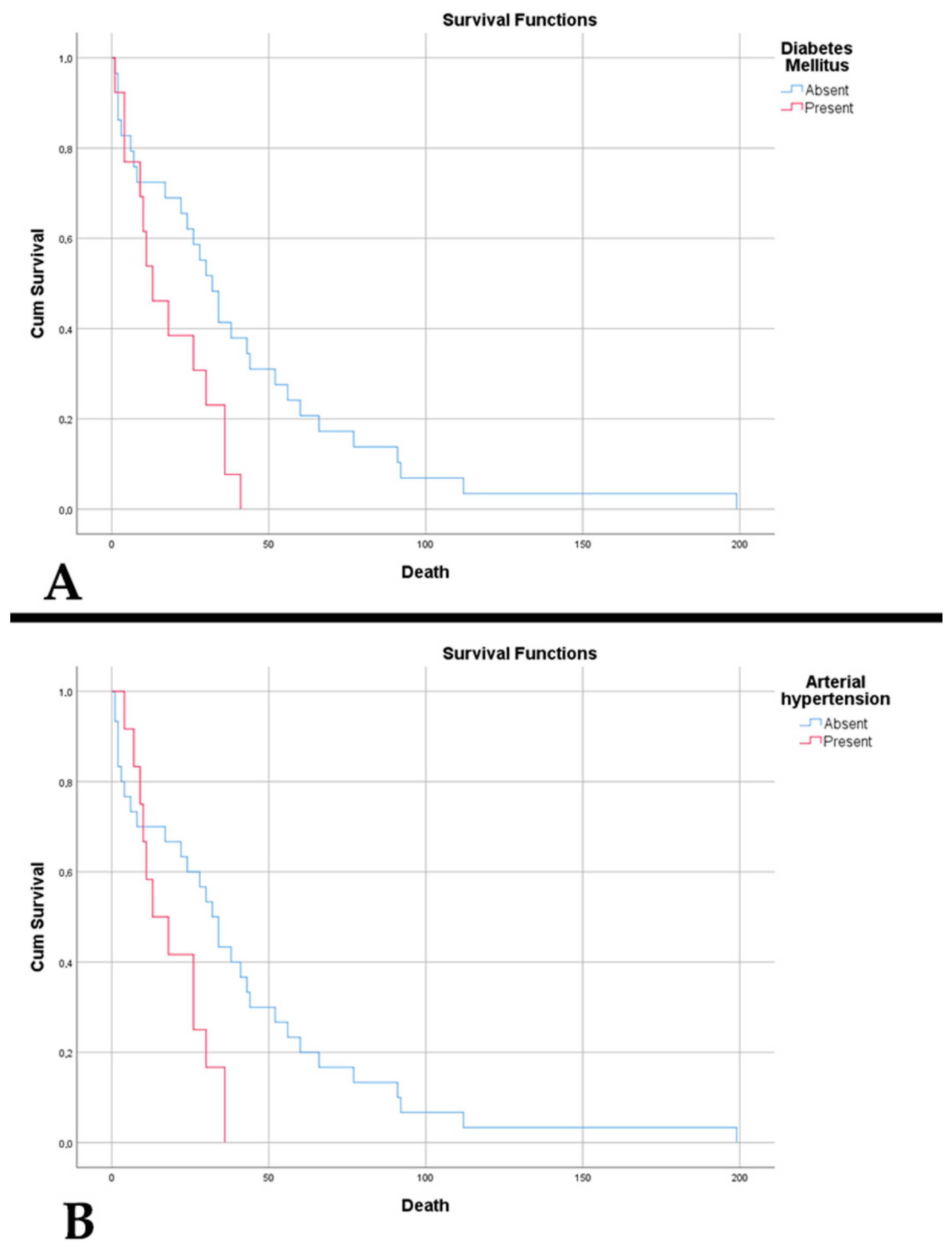
| • Diabetes mellitus | 29.55 | 27.03 | 0.802 |
| • Arterial hypertension | 27.27 | 40.54 | 0.242 |
| • Other tumors | 9.09 | 8.11 | 0.875 |
| • Survival rate (%) | 4.55% | 0% | 0.498 |
| Astrocytoma IDH-Mutant | Glioblastoma IDH-Wildtype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | HR | CI 95% | p-Value | HR | CI 95% | p-Value | |
| Age | 1.035 | 1.005–1.067 | 0.022 | 1.010 | 0.962–1.059 | 0.691 | 1.045 | 1.007–1.084 | 0.019 | 1.061 | 1.001–1.125 | 0.048 |
| Gender | 1.220 | 0.652–2.282 | 0.534 | 1.763 | 0.738–4.207 | 0.202 | 1.312 | 0.644–2.672 | 0.455 | 1.750 | 0.727–4.212 | 0.212 |
| The onset of symptoms (<2 weeks) | 2.038 | 1.044–3.978 | 0.037 | 5.214 | 1.751–15.524 | 0.003 | 1.110 | 0.574–2.145 | 0.757 | 1.313 | 0.525–3.282 | 0.560 |
| Motor impairments/paresis | 0.668 | 0.346–1.290 | 0.230 | 0.404 | 0.109–1.499 | 0.176 | 1.263 | 0.633–2.521 | 0.508 | 0.949 | 0.350–2.574 | 0.918 |
| Headache | 0.630 | 0.329–1.208 | 0.165 | 0.331 | 0.136–0.805 | 0.015 | 1.101 | 0.553–2.191 | 0.785 | 0.707 | 0.237–2.105 | 0.533 |
| Cognitive impairments | 0.667 | 0.350–1.271 | 0.218 | 0.562 | 0.172–1.839 | 0.341 | 0.844 | 0.426–1.673 | 0.627 | 0.447 | 0.147–1.358 | 0.156 |
| Intracranial hypertension | 2.073 | 0.946–4.542 | 0.069 | 1.122 | 0.329–3.824 | 0.854 | 0.898 | 0.447–1.803 | 0.763 | 0.534 | 0.181–1.577 | 0.256 |
| Psychiatric syndromes | 0.794 | 0.375–1.681 | 0.546 | 0.598 | 0.129–2.776 | 0.512 | 0.551 | 0.191–1.593 | 0.271 | 0.456 | 0.118–1.770 | 0.257 |
| Balance and coordination disorders | 2.069 | 0.890–4.807 | 0.091 | 3.586 | 0.824–15.606 | 0.089 | 1.555 | 0.700–3.453 | 0.278 | 1.964 | 0.559–6.899 | 0.292 |
| Epileptic seizures | 0.601 | 0.249–1.448 | 0.256 | 0.504 | 0.119–2.127 | 0.351 | 0.704 | 0.318–1.555 | 0.385 | 0.297 | 0.089–0.995 | 0.049 |
| Diabetes mellitus | 2.311 | 1.116–4.782 | 0.024 | 2.505 | 0.537–11.690 | 0.243 | 1.735 | 0.821–3.669 | 0.149 | 1.457 | 0.428–4.957 | 0.547 |
| Arterial hypertension | 2.325 | 1.090–4.956 | 0.029 | 0.775 | 0.171–3.510 | 0.740 | 1.246 | 0.634–2.451 | 0.523 | 1.364 | 0.414–4.495 | 0.610 |
| Other tumors | 1.285 | 0.451–3.663 | 0.639 | 2.797 | 0.531–14.731 | 0.225 | 0.708 | 0.214–2.343 | 0.572 | 0.347 | 0.062–1.940 | 0.228 |
| Imaging Aspects | Astrocytoma IDH-Mutant (n = 44) | Glioblastoma IDH-Wildtype (n = 37) | p-Value |
|---|---|---|---|
| Hemisphere (%): | 0.656 | ||
| • Left | 50 | 56.76 | |
| • Right | 50 | 43.24 | |
| Lobe (%): | 0.195 | ||
| • Frontal | 18.18% | 8.11% | |
| • Temporal | 25% | 21.62% | |
| • Parietal | 9.09% | 18.92% | |
| • Occipital | 2.27% | 0% | |
| • Fronto-temporal | 4.55% | 0% | |
| • Fronto-parietal | 18.18% | 5.41% | |
| • Fronto-insular | 2.27% | 8.11% | |
| • Temporo-parietal | 9.09% | 13.51% | |
| • Temporo-occipital | 0% | 5.41% | |
| • Temporo-insular | 2.27% | 8.11% | |
| • Parieto-occipital | 9.09% | 10.81% | |
| The mean of the maximum diameter (min–max) | 50.39 (10–80) | 52.92 (20–87) | 0.612 |
| Average volume (cm3, min–max) | 86.14 (0.90–388.94) | 94.84 (1.22–324.72) | 0.595 |
| Peritumoral edema (%) | 90.91 | 97.30 | 0.369 |
| Midline displacement (mm) | 8.91 | 8.97 | 0.783 |
| Residual tumor present (%) | 77.27 | 75.68 | 0.866 |
| Average resectability rate (%) | 87.67 | 82.88 | 0.380 |
| Residual tumor volume (cm3) | 11.02 | 15.13 | 0.513 |
| Complete treatment (%) | 68.18 | 81.08 | 0.213 |
| Astrocytoma IDH-Mutant | Glioblastoma IDH-Wildtype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | HR | CI 95% | p-Value | HR | CI 95% | p-Value | |
| Hemisphere | 1.581 | 0.838–2.984 | 0.158 | 1.786 | 0.820–3.893 | 0.144 | 1.173 | 0.603–2.283 | 0.637 | 1.434 | 0.605–3.396 | 0.413 |
| Lobes | 1.051 | 0.950–1.163 | 0.331 | 0.937 | 0.822–1.067 | 0.325 | 1.009 | 0.846–1.162 | 0.903 | 1.014 | 0.848–1.214 | 0.876 |
| Maximum diameter | 0.997 | 0.979–1.015 | 0.765 | 1.018 | 0.965–1.075 | 0.509 | 0.984 | 0.962–1.006 | 0.153 | 0.948 | 0.902–0.996 | 0.034 |
| Volume | 0.997 | 0.994–1.001 | 0.179 | 0.980 | 0.966–0.995 | 0.008 | 1.000 | 0.995–1.006 | 0.865 | 1.007 | 0.996–1.019 | 0.231 |
| Peritumoral edema | 1.473 | 0.450–4.825 | 0.522 | 0.633 | 0.136–2.946 | 0.560 | 2.805 | 0.362–21.750 | 0.324 | 8.017 | 0.476–134.968 | 0.148 |
| Midline displacement | 0.998 | 0.948–1.051 | 0.952 | 0.939 | 0.861–1.024 | 0.156 | 0.979 | 0.922–1.040 | 0.492 | 1.043 | 0.966–1.126 | 0.282 |
| Residual tumor present | 2.662 | 1.150–6.160 | 0.022 | 10.225 | 2.076–50.367 | 0.004 | 1.376 | 0.640–2.958 | 0.413 | 1.289 | 0.404–4.109 | 0.668 |
| Resectability rate | 0.866 | 0.817–0.917 | <0.001 | 0.881 | 0.792–0.981 | 0.021 | 0.982 | 0.962–1.003 | 0.094 | 0.995 | 0.948–1.043 | 0.824 |
| Residual tumor volume | 1.060 | 1.034–1.087 | <0.001 | 1.186 | 1.097–1.281 | <0.001 | 1.023 | 1.004–1.042 | 0.018 | 1.023 | 0.980–1.068 | 0.305 |
| Complete treatment | 0.001 | 0.000–1.634 | 0.066 | 0.000 | 0–3.747 × 1068 | 0.876 | 0 | 0–14,472,349.37 | 0.480 | 0 | 0–3.235 × 1079 | 0.887 |
| Histogenetic Aspects | Astrocytoma IDH-Mutant (n = 44) | Glioblastoma IDH-Wildtype (n = 37) | p-Value |
|---|---|---|---|
| Tumor necrosis (%, min–max) | 34.23 (10–69) | 32.49 (7–72) | 0.605 |
| Microvascular density (vessels/mm2, min–max) | 49.95 (22.6–70.8) | 40.91 (20.6–78.6) | 0.010 |
| Ki-67 (%, min-max) | 48.75 (15–90) | 36.70 (4–85) | 0.026 |
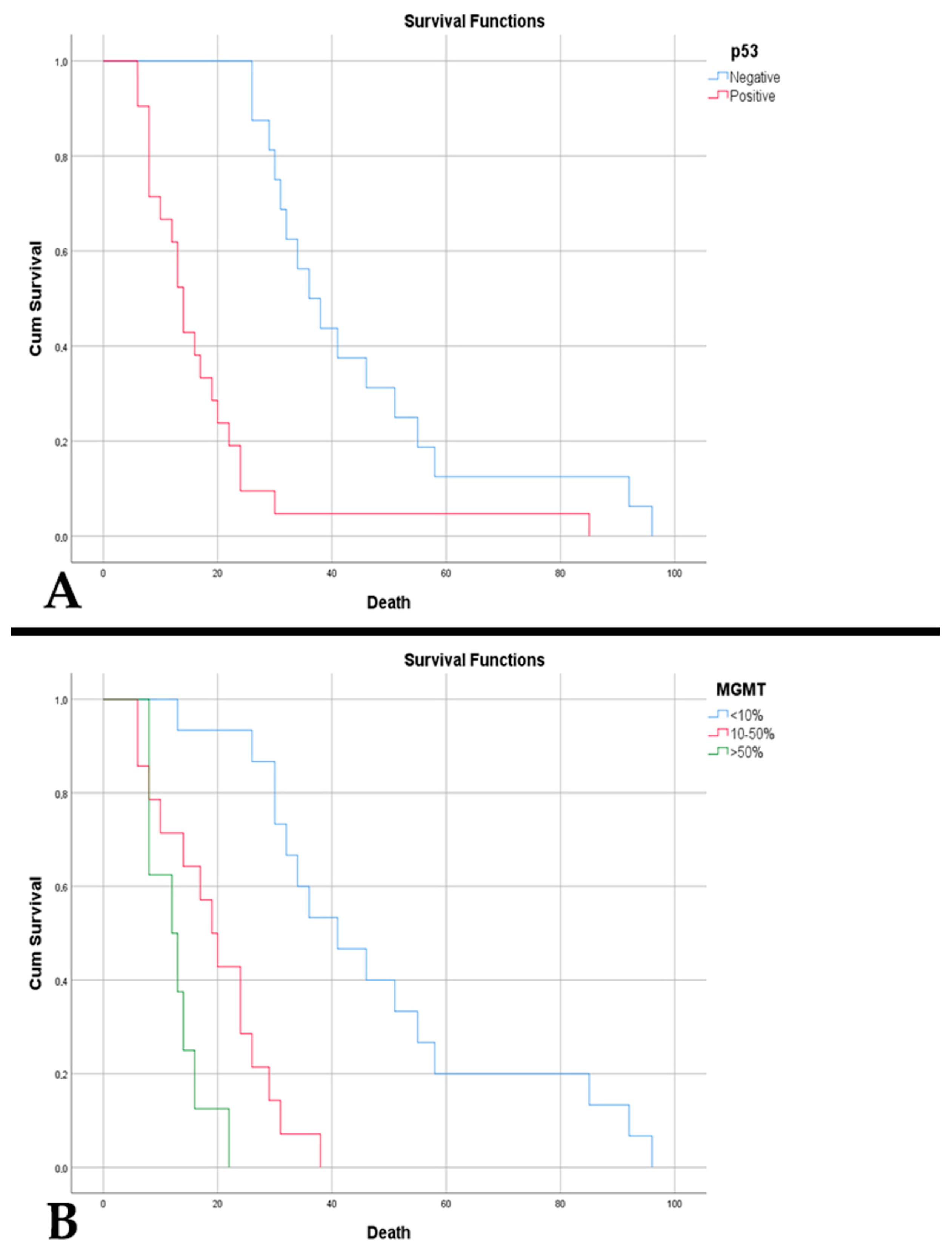
| p53 positive (%) | 79.55 | 56.76 | 0.032 |
| Absent PTEN (%) | 27.27 | 13.51 | 0.174 |
| Nestin (%): | 0.054 | ||
| • Negative | 15.91 | 2.70 | |
| • Weakly positive | 15.91 | 13.51 | |
| • Moderately positive | 15.91 | 13.51 | |
| • Intensely positive | 52.27 | 70.27 | |
| MGMT (%): | 0.350 | ||
| • >50% | 36.36 | 21.62 | |
| • 10–50% | 34.09 | 37.84 | |
| • <10% | 29.55 | 40.54 | |
| CDKN2A (%): | 0.221 | ||
| • Normal | 47.73 | 64.86 | |
| • Deletion | 45.45 | 13.70 | |
| • Amplification | 6.82 | 2.74 |
| Astrocytoma IDH-Mutant | Glioblastoma IDH-Wildtype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | CI95% | p-Value | HR | CI95% | p-Value | HR | CI95% | p-Value | HR | CI95% | p-Value | |
| Tumor necrosis | 1.097 | 1.063–1.132 | <0.001 | 1.058 | 0.998–1.122 | 0.060 | 1.007 | 0.989–1.025 | 0.435 | 1.035 | 0.984–1.088 | 0.181 |
| Microvascular density | 1.096 | 1.057–1.137 | <0.001 | 1.092 | 1.022–1.167 | 0.009 | 1.009 | 0.993–1.026 | 0.264 | 0.934 | 0.872–0.999 | 0.046 |
| Ki-67 | 0.999 | 0.978–1.011 | 0.859 | 0.961 | 0.925–0.997 | 0.038 | 1.003 | 0.989–1.017 | 0.679 | 0.989 | 0.960–1.019 | 0.463 |
| p53 positive | 6.962 | 2.035–23.820 | 0.002 | 13.244 | 2.042–85.880 | 0.007 | 4.680 | 2.239–9.781 | <0.001 | 20.459 | 2.823–148.271 | 0.003 |
| PTEN retained | 0 | 0–19.374 | 0.154 | 0 | 0–1.116 × 1085 | 0.889 | 0.030 | 0.004–0.213 | <0.001 | 0.022 | 0.002–0.287 | 0.004 |
| Nestin | ||||||||||||
| • Weakly positive | 1.362 | 0.415–4.475 | 0.610 | 3.935 | 0.862–17.969 | 0.077 | 0.720 | 0.096–5.389 | 0.749 | 8.333 | 0.320–217.183 | 0.202 |
| • Moderately positive | 1.724 | 0.535–5.550 | 0.361 | 2.087 | 0.596–7.309 | 0.250 | 3.953 | 1.350–11.574 | 0.012 | 15.140 | 1.910–120.041 | 0.010 |
| • Intensely positive | 1.204 | 0.446–3.255 | 0.714 | 2.460 | 0.665–9.096 | 0.177 | 0.449 | 0.151–1.335 | 0.150 | 0.309 | 0.053–1.787 | 0.189 |
| MGMT | ||||||||||||
| • 10–50% | 0.599 | 0.267–1.339 | 0.212 | 2.993 | 0.852–10.511 | 0.087 | 6.765 | 2.579–17.741 | <0.001 | 8.672 | 2.055–36.600 | 0.003 |
| • >50% | 1.410 | 0.629–3.158 | 0.404 | 1.187 | 0.146–9.642 | 0.873 | 20.573 | 5.745–73.672 | <0.001 | 28.678 | 3.591–229.058 | 0.002 |
| CDKN2A | ||||||||||||
| • Deletion | 2.612 | 1.278–5.339 | 0.009 | 1.648 | 0.525–5.177 | 0.392 | 1.833 | 0.860–3.910 | 0.117 | 2.842 | 0.274–29.420 | 0.381 |
| • Amplification | 6.793 | 1.757–26.271 | 0.005 | 1.533 | 0.194–12.125 | 0.686 | 2.482 | 0.726–8.486 | 0.147 | 67.028 | 2.841–1581.559 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orasanu, C.I.; Aschie, M.; Deacu, M.; Bosoteanu, M.; Vamesu, S.; Enciu, M.; Cozaru, G.C.; Mitroi, A.F.; Ghitoi, S.A.; Cretu, A.M.; et al. Comparative Clinical-Imaging and Histogenetic Analysis Between Astrocytoma IDH-Mutant Grade 4 and Glioblastoma IDH-Wildtype—Is There Really a Worse One? Diagnostics 2025, 15, 438. https://doi.org/10.3390/diagnostics15040438
Orasanu CI, Aschie M, Deacu M, Bosoteanu M, Vamesu S, Enciu M, Cozaru GC, Mitroi AF, Ghitoi SA, Cretu AM, et al. Comparative Clinical-Imaging and Histogenetic Analysis Between Astrocytoma IDH-Mutant Grade 4 and Glioblastoma IDH-Wildtype—Is There Really a Worse One? Diagnostics. 2025; 15(4):438. https://doi.org/10.3390/diagnostics15040438
Chicago/Turabian StyleOrasanu, Cristian Ionut, Mariana Aschie, Mariana Deacu, Madalina Bosoteanu, Sorin Vamesu, Manuela Enciu, Georgeta Camelia Cozaru, Anca Florentina Mitroi, Sinziana Andra Ghitoi, Ana Maria Cretu, and et al. 2025. "Comparative Clinical-Imaging and Histogenetic Analysis Between Astrocytoma IDH-Mutant Grade 4 and Glioblastoma IDH-Wildtype—Is There Really a Worse One?" Diagnostics 15, no. 4: 438. https://doi.org/10.3390/diagnostics15040438
APA StyleOrasanu, C. I., Aschie, M., Deacu, M., Bosoteanu, M., Vamesu, S., Enciu, M., Cozaru, G. C., Mitroi, A. F., Ghitoi, S. A., Cretu, A. M., Ursica, O. A., & Voda, R. I. (2025). Comparative Clinical-Imaging and Histogenetic Analysis Between Astrocytoma IDH-Mutant Grade 4 and Glioblastoma IDH-Wildtype—Is There Really a Worse One? Diagnostics, 15(4), 438. https://doi.org/10.3390/diagnostics15040438





