Diagnosis of Cholangiocarcinoma: The New Biological and Technological Horizons
Abstract
:1. Introduction
2. Biological Approaches to the Diagnosis and Prognosis of CCA
2.1. Diagnostic and Prognostic Biomarkers for CCA
2.2. Liquid Biopsy for Diagnosis of CCA
2.3. Gut Microbiota and CCA
2.4. Specific Microbial Features as a Diagnostic and Predictive Markers for CCA
2.5. Modulating Gut Microbiota in CCA
3. Non-Biological Approaches to the Diagnosis and Prognosis of CCA
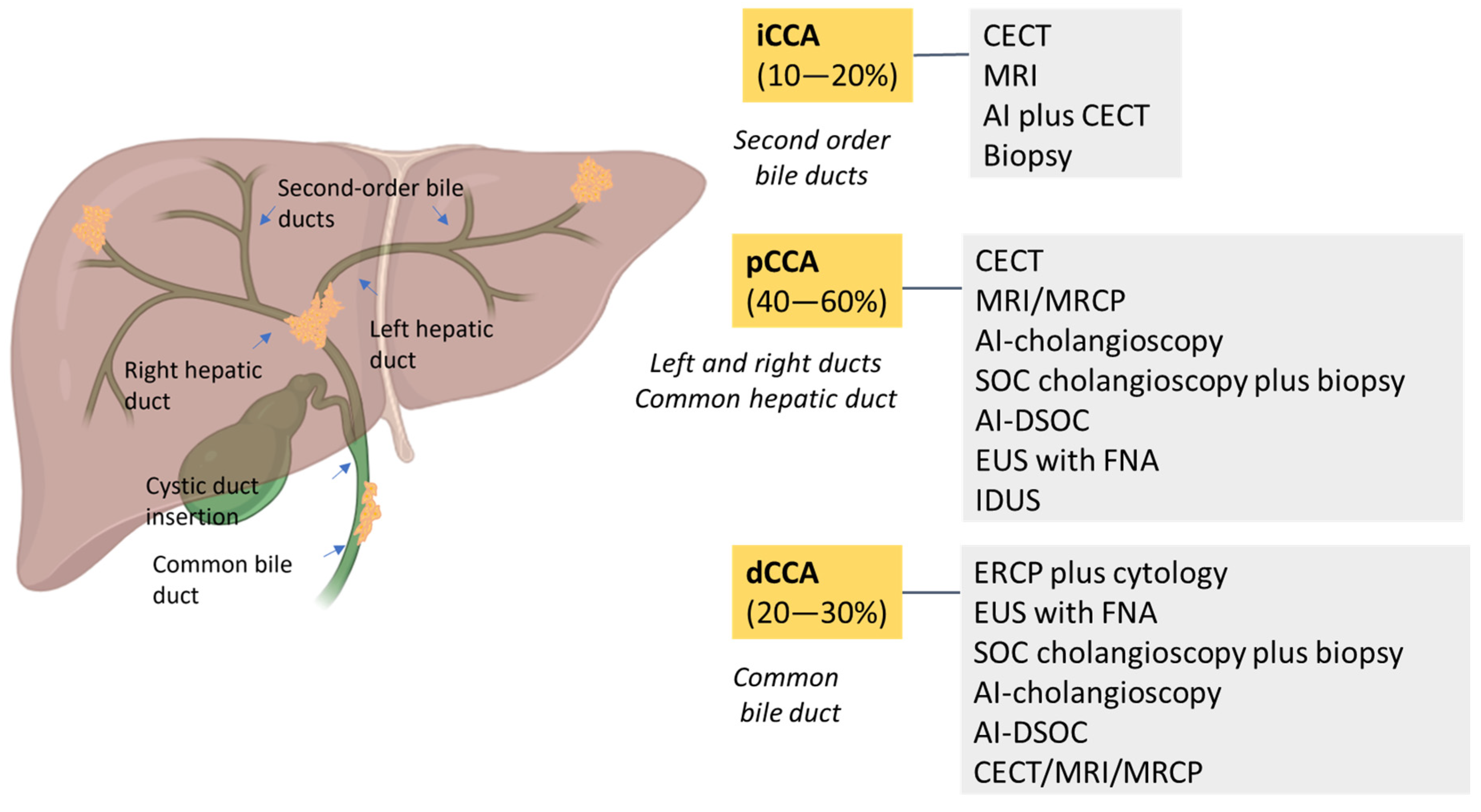
3.1. Endoscopic Diagnosis of CCA
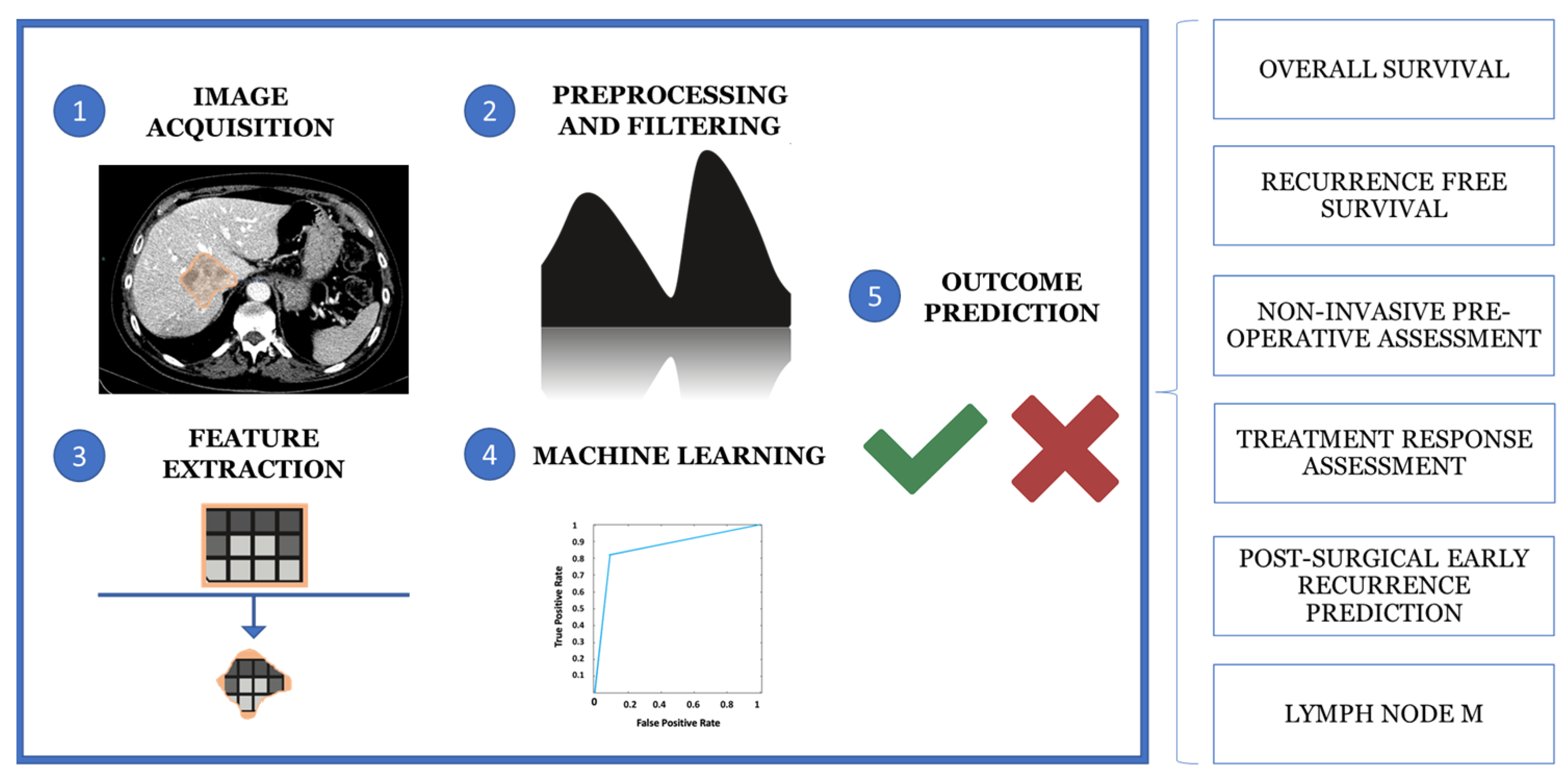
3.2. Radiomics and Radiogenomics in the Diagnosis of CCA
3.3. Artificial Intelligence in Diagnosis of CCA
| METHODS FOR CCA DIAGNOSIS | ACCURACY (%) | SENSITIVITY (%) | SPECIFICITY (%) | REFERENCES |
|---|---|---|---|---|
| BIOLOGICAL APPROACHES | ||||
| Ca 19-9 | 50–90 | 54–98 | Kodali S et al., 2024 [3] Shin DW et al., 2023 [6] | |
| CEA (>5.2 ng/mL) | 68 | 82 | Shin DW et al., 2023 [6] | |
| FISH | 70 | 60–97 | Ilyas SI et al., 2018 [24] Azeem N et al., 2014 [35] | |
| Brush cytology | 27–56 | Dar FS et al., 2024 [13] | ||
| Brush cytology plus biopsy and elevated CA 19.9 | 70.7–88 | 97 | Doong Woo Shin et al., 2023 [6] | |
| Brush cytology plus biopsy and FISH | 70.7–82 | Dar FS et al., 2024 [13] | ||
| Brush cytology and elevated CA 19.9 | 88 | 97 | Shin DW et al., 2023 [6] | |
| NON-BIOLOGICAL APPROACHES | ||||
| AI-cholangioscopy (eCCA) | 89–95 | 81–94.7 | 91–92.1 | Njei B et al., 2023 [20] Saraiva MM et al., 2022 [125] |
| SOC cholangioscopy plus biopsy | 75–82 | 65–72 | 98–99 | Njei B et al., 2023 [20] Dar FS et al., 2024 [13] Azeem N et al., 2014 [35] |
| PTC plus cholangioscopy | 80–92 | Doong Woo Shin et al., 2023 [6] | ||
| AI plus CECT (iCCA) | 80.4–84.6 | Njei B et al., 2023 [20] | ||
| MRI/MRCP | 88–90 | 75–85 | Ilyas SI et al., 2018 [24] Rushbrook SM et al., 2024 [4] | |
| CECT | 86 | 75–89 | 79–80 | Ilyas SI et al., 2018 [24] Rushbrook SM et al., 2024 [4] |
| EUS with FNA (dCCA) | 76–81 | 100 | Ilyas SI et al., 2018 [24] Dar FS et al., 2024 [13] | |
| ERCP plus cytology | 21–70 | 20–100 | Ilyas SI et al., 2018 [24] Azeem N et al., 2014 [35] Kodali S et al., 2024 [3] Rushbrook SM et al., 2024 [4] | |
| EUS with FNA (eCCA) | 43–89 | Ilyas SI et al., 2018 [24] Shin DW et al., 2023 [6] | ||
| IDUS | 91 | 93 | 89.5 | Ilyas SI et al., 2018 [24] |
| SOC plus visual impression | 78–90 | 71.2–82 | Ilyas SI et al., 2018 [24] Mauro A et al., 2023 [30] Rushbrook SM et al., 2024 [4] | |
| SOC plus biopsy | 66 | 97 | Ilyas SI et al., 2018 [24] | |
| DSOC | 94 | 95 | Mauro A et al., 2023 [30] | |
| DSOC plus biopsy | 74 | 98 | Mauro A et al., 2023 [30] | |
| AI-DSOC | 94.9 | 94.7 | 92.1 | Mauro A et al., 2023 [30] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haghbin, H.; Aziz, M. Artificial intelligence and cholangiocarcinoma: Updates and prospects. World J. Clin. Oncol. 2022, 13, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Köhler, B.; Bes, M.; Chan, H.L.; Esteban, J.I.; Piratvisuth, T.; Sukeepaisarnjaroen, W.; Tanwandee, T.; Thongsawat, S.; Mang, A.; Morgenstern, D.; et al. A new biomarker panel for differential diagnosis of cholangiocarcinoma: Results from an exploratory analysis. Int. J. Biol. Markers 2024, 39, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Connor, A.A.; Brombosz, E.W.; Ghobrial, R.M. Update on the Screening, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterol. Hepatol. 2024, 20, 151–158. [Google Scholar]
- Rushbrook, S.M.; Kendall, T.J.; Zen, Y.; Albazaz, R.; Manoharan, P.; Pereira, S.P.; Sturgess, R.; Davidson, B.R.; Malik, H.Z.; Manas, D.; et al. British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma. Gut 2023, 73, 16–46. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Ariyaskul, D.; Kulkraisri, K.; Apiparakoon, T.; Sukcharoen, S.; Chaichuen, O.; Pensuwan, P.; Tiyarattanachai, T.; Rerknimitr, R.; Marukatat, S. Artificial intelligence for ultrasonographic detection and diagnosis of hepatocellular carcinoma and cholangiocarcinoma. Sci. Rep. 2024, 14, 20617. [Google Scholar] [CrossRef]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Catalano, T.; Selvaggi, F.; Esposito, D.L.; Cotellese, R.; Aceto, G.M. Infectious Agents Induce Wnt/β-Catenin Pathway Deregulation in Primary Liver Cancers. Microorganisms 2023, 11, 1632. [Google Scholar] [CrossRef]
- Capuozzo, M.; Santorsola, M.; Ferrara, F.; Cinque, C.; Farace, S.; Patrone, R.; Granata, V.; Zovi, A.; Nasti, G.; Ottaiano, A. Intrahepatic cholangiocarcinoma biomarkers: Towards early detection and personalized pharmacological treatments. Mol. Cell Probes 2024, 73, 101951. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chen, C.P.; Wu, C.E. Precision Medicine in Cholangiocarcinoma: Past, Present, and Future. Life 2022, 12, 829. [Google Scholar] [CrossRef]
- Andersen, J.B. Molecular pathogenesis of intrahepatic cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2015, 22, 101–113. [Google Scholar] [CrossRef]
- Squadroni, M.; Tondulli, L.; Gatta, G.; Mosconi, S.; Beretta, G.; Labianca, R. Cholangiocarcinoma. Crit. Rev. Oncol. Hematol. 2017, 116, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Catalano, T.; Cotellese, R.; Aceto, G.M. Targeting Wnt/β-Catenin Pathways in Primary Liver Tumours: From Microenvironment Signaling to Therapeutic Agents. Cancers 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- Dar, F.S.; Abbas, Z.; Ahmed, I.; Atique, M.; Aujla, U.I.; Azeemuddin, M.; Aziz, Z.; Bhatti, A.B.H.; Bangash, T.A.; Butt, A.S.; et al. National guidelines for the diagnosis and treatment of hilar cholangiocarcinoma. World J. Gastroenterol. 2024, 30, 1018–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, C.; Duan, Y.; Heinrich, B.; Rosato, U.; Diggs, L.P.; Ma, L.; Roy, S.; Fu, Q.; Brown, Z.J.; et al. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discov. 2021, 11, 1248–1267. [Google Scholar] [CrossRef]
- Binda, C.; Gibiino, G.; Coluccio, C.; Sbrancia, M.; Dajti, E.; Sinagra, E.; Capurso, G.; Sambri, V.; Cucchetti, A.; Ercolani, G.; et al. Biliary Diseases from the Microbiome Perspective: How Microorganisms Could Change the Approach to Benign and Malignant Diseases. Microorganisms 2022, 10, 312. [Google Scholar] [CrossRef]
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906. [Google Scholar] [CrossRef]
- Gopal, P.; Robert, M.E.; Zhang, X. Cholangiocarcinoma: Pathologic and Molecular Classification in the Era of Precision Medicine. Arch. Pathol. Lab. Med. 2024, 148, 359–370. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; The, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Maker, A.V. Insights on liver-directed surgical innovations, targeted therapies, and immunotherapy for biliary tract cancer: Navigating the new European Society for Medical Oncology (ESMO) Clinical Practice Guidelines. Hepatobiliary Surg. Nutr. 2024, 13, 516–519. [Google Scholar] [CrossRef]
- Njei, B.; Kanmounye, U.S.; Seto, N.; McCarty, T.R.; Mohan, B.P.; Fozo, L.; Navaneethan, U. Artificial intelligence in medical imaging for cholangiocarcinoma diagnosis: A systematic review with scientometric analysis. J. Gastroenterol. Hepatol. 2023, 38, 874–882. [Google Scholar] [CrossRef]
- Ayoub, F.; Othman, M.O. Guidelines on cholangioscopy for indeterminate biliary strictures: One step closer to consensus. Hepatobiliary Surg. Nutr. 2023, 12, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Milluzzo, S.M.; Landi, R.; Perri, V.; Familiar, P.; Boškoski, I.; Pafundi, P.C.; Farina, A.; Ricci, R.; Spada, C.; Costamagna, G.; et al. Diagnostic accuracy and interobserver agreement of cholangioscopy for indeterminate biliary strictures: A single-center experience. Dig. Liver Dis. 2024, 56, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, C.; Chen, X.; Feng, Y.; Feng, J.; Zhang, R.; Ouyang, F.; Li, X.; Tan, Z.; Deng, L.; et al. Noninvasive prediction of perineural invasion in intrahepatic cholangiocarcinoma by clinicoradiological features and computed tomography radiomics based on interpretable machine learning: A multicenter cohort study. Int. J. Surg. 2024, 110, 1039–1051. [Google Scholar] [PubMed]
- Ilyas, S.I.; Eaton, J.; Yang, J.D.; Chandrasekhara, V.; Gores, G.J. Emerging Technologies for the Diagnosis of Perihilar Cholangiocarcinoma. Semin. Liver Dis. 2018, 38, 160–169. [Google Scholar] [CrossRef]
- Selvaraj, E.A.; Ba-Ssalamah, A.; Poetter-Lang, S.; Ridgway, G.R.; Brady, J.M.; Collier, J.; Culver, E.L.; Bailey, A.; Pavlides, M. A Quantitative Magnetic Resonance Cholangiopancreatography Metric of Intrahepatic Biliary Dilatation Severity Detects High-Risk Primary Sclerosing Cholangitis. Hepatol. Commun. 2022, 6, 795–808. [Google Scholar] [CrossRef]
- Nanashima, A.; Komi, M.; Mavar, M.; Ferreira, C.; O’Donoghue, P.; Goldfinger, M.; Langford, C.; Imamura, N. A 3D Quantitative MRC Modeling Images Detected Case of Intrahepatic Biliary Stricture Diseases. Case Rep. Gastroenterol. 2021, 15, 680–688. [Google Scholar] [CrossRef]
- Akita, M.; Sawada, R.; Komatsu, M.; Suleman, N.; Itoh, T.; Ajiki, T.; Heaton, N.; Fukumoto, T.; Zen, Y. An immunostaining panel of C-reactive protein, N-cadherin, and S100 calcium binding protein P is useful for intrahepatic cholangiocarcinoma subtyping. Hum. Pathol. 2021, 109, 45–52. [Google Scholar] [CrossRef]
- Wakai, T.; Sakata, J.; Katada, T.; Hirose, Y.; Soma, D.; Prasoon, P.; Miura, K.; Kobayashi, T. Surgical management of carcinoma in situ at ductal resection margins in patients with extrahepatic cholangiocarcinoma. Ann. Gastroenterol. Surg. 2018, 2, 359–366. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Mauro, A.; Mazza, S.; Scalvini, D.; Lusetti, F.; Bardone, M.; Quaretti, P.; Cobianchi, L.; Anderloni, A. The Role of Cholangioscopy in Biliary Diseases. Diagnostics 2023, 13, 2933. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Masyuk, A.I.; Masyuk, T.V.; O’Hara, S.P.; LaRusso, N.F. Physiology of cholangiocytes. Compr. Physiol. 2013, 3, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.A.; Morton, L.O.; Jefferson, J.R.; Rozeveld, C.N.; Doskey, L.C.; LaRusso, N.F.; Katzmann, D.J. Polarized human cholangiocytes release distinct populations of apical and basolateral small extracellular vesicles. Mol. Biol. Cell. 2020, 31, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tacke, F.; Guillot, A.; Liu, H. Cholangiokines: Undervalued modulators in the hepatic microenvironment. Front. Immunol. 2023, 14, 1192840. [Google Scholar] [CrossRef]
- Azeem, N.; Gostout, C.J.; Knipschield, M.; Baron, T.H. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest. Endosc. 2014, 79, 773–779. [Google Scholar] [CrossRef]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, D.; Yin, L.; Qiu, Y.; Liu, J.; Yu, W.; Fu, X.; Zhu, B.; Xu, X.; Duan, A.; et al. Bile cell-free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol. Rep. 2019, 42, 549–560. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017, 7, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Boerner, T.; Drill, E.; Pak, L.M.; Nguyen, B.; Sigel, C.S.; Doussot, A.; Shin, P.; Goldman, D.A.; Gonen, M.; Allen, P.J.; et al. Genetic Determinants of Outcome in Intrahepatic Cholangiocarcinoma. Hepatology 2021, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Brandi, G.; Niger, M.; Normanno, N.; Melisi, D. Delphi Panel Members. Diagnosis and treatment of cholangiocarcinoma in Italy: A Delphi consensus statement. Crit. Rev. Oncol. Hematol. 2023, 192, 104146. [Google Scholar] [CrossRef] [PubMed]
- Suttiprapa, S.; Sotillo, J.; Smout, M.; Suyapoh, W.; Chaiyadet, S.; Tripathi, T.; Laha, T.; Loukas, A. Opisthorchis viverrini Proteome and Host-Parasite Interactions. Adv. Parasitol. 2018, 102, 45–72. [Google Scholar]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Agüero, R.; Lacasta, A.; et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef]
- Alsaleh, M.; Leftley, Z.; Barbera, T.A.; Koomson, L.K.; Zabron, A.; Crossey, M.M.E.; Reeves, H.L.; Cramp, M.; Ryder, S.; Greer, S.; et al. Characterisation of the Serum Metabolic Signature of Cholangiocarcinoma in a United Kingdom Cohort. J. Clin. Exp. Hepatol. 2020, 10, 17–29. [Google Scholar] [CrossRef]
- Rompianesi, G.; Di Martino, M.; Gordon-Weeks, A.; Montalti, R.; Troisi, R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J. Gastrointest. Oncol. 2021, 13, 332–350. [Google Scholar] [CrossRef]
- Cao, J.; Hu, J.; Liu, S.; Meric-Bernstam, F.; Abdel-Wahab, R.; Xu, J.; Li, Q.; Yan, M.; Feng, Y.; Lin, J.; et al. Intrahepatic Cholangiocarcinoma: Genomic Heterogeneity Between Eastern and Western Patients. JCO Precis. Oncol. 2020, 4, 557–569. [Google Scholar] [CrossRef]
- Tavolari, S.; Brandi, G. Mutational Landscape of Cholangiocarcinoma According to Different Etiologies: A Review. Cells 2023, 12, 1216. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Hayes, K.S.; Bancroft, A.J.; Goldrick, M.; Portsmouth, C.; Roberts, I.S.; Grencis, R.K. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 2010, 328, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science. 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sama, I.S.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Molinero, N.; Ruiz, L.; Milani, C.; Gutiérrez-Díaz, I.; Sánchez, B.; Mangifesta, M.; Segura, J.; Cambero, I.; Campelo, A.B.; García-Bernardo, C.M.; et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome 2019, 7, 100. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Z.; Liu, B.; Hou, D.; Liang, Y.; Zhang, J.; Shi, P. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genom. 2013, 14, 669. [Google Scholar] [CrossRef]
- Ye, C.; Dong, C.; Lin, Y.; Shi, H.; Zhou, W. Interplay between the Human Microbiome and Biliary Tract Cancer: Implications for Pathogenesis and Therapy. Microorganisms 2023, 11, 2598. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Liu, Y.; Nie, Y.; Xu, P.; Xia, B.; Tian, F.; Sun, Q. Cholesterol gallstones and bile host diverse bacterial communities with potential to promote the formation of gallstones. Microb. Pathog. 2015, 83–84, 57–63. [Google Scholar] [CrossRef]
- Keren, N.; Konikoff, F.M.; Paitan, Y.; Gabay, G.; Reshef, L.; Naftali, T.; Gophna, U. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ. Microbiol. Rep. 2015, 7, 874–880. [Google Scholar] [CrossRef]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.U.; Schrumpf, E.; Moum , M.; et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017, 66, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Lemoinne, S.; Kemgang, A.; Ben Belkacem, K.; Straube, M.; Jegou, S.; Corpechot, C.; Saint-Antoine, IBD Network; Chazouillères, O.; Housset, C.; Sokol, H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut 2020, 69, 92–102. [Google Scholar] [CrossRef]
- Liwinski, T.; Zenouzi, R.; John, C.; Ehlken, H.; Rühlemann, M.C.; Bang, C.; Groth, S.; Lieb, W.; Kantowski, M.; Andersen, N.; et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 2020, 69, 665–672. [Google Scholar] [CrossRef]
- Saltykova, I.V.; Petrov, V.A.; Logacheva, M.D.; Ivanova, P.G.; Merzlikin, N.V.; Sazonov, A.E.; Ogorodova, L.M.; Brindley, P.J. Biliary Microbiota, Gallstone Disease and Infection with Opisthorchis felineus. PLoS Negl. Trop. Dis. 2016, 10, e0004809. [Google Scholar] [CrossRef]
- Chng, K.R.; Chan, S.H.; Ng, A.H.Q.; Li, C.; Jusakul, A.; Bertrand, D.; Wilm, A.; Choo, S.P.; Tan, D.M.Y.; Lim, K.H.; et al. Tissue Microbiome Profiling Identifies an Enrichment of Specific Enteric Bacteria in Opisthorchis viverrini Associated Cholangiocarcinoma. EBioMedicine 2016, 8, 195–202. [Google Scholar] [CrossRef]
- Soares, K.C.; Kim, Y.; Spolverato, G.; Maithel, S.; Bauer, T.W.; Marques, H.; Sobral, M.; Knoblich, M.; Tran, T.; Aldrighetti, L.; et al. Presentation and Clinical Outcomes of Choledochal Cysts in Children and Adults: A Multi-institutional Analysis. JAMA Surg. 2015, 150, 577–584. [Google Scholar] [CrossRef]
- Ji, J.; Wu, L.; Wei, J.; Wu, J.; Guo, C. The Gut Microbiome and Ferroptosis in MAFLD. J. Clin. Transl. Hepatol. 2022, 11, 174–187. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary Sclerosing Cholangitis—A Comprehensive Review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef]
- Deenonpoe, R.; Mairiang, E.; Mairiang, P.; Pairojkul, C.; Chamgramol, Y.; Rinaldi, G.; Loukas, A.; Brindley, P.J.; Sripa, B. Elevated prevalence of Helicobacter species and virulence factors in opisthorchiasis and associated hepatobiliary disease. Sci. Rep. 2017, 7, 42744. [Google Scholar] [CrossRef] [PubMed]
- Boonyanugomol, W.; Chomvarin, C.; Baik, S.C.; Song, J.Y.; Hahnvajanawong, C.; Kim, K.M.; Cho, M.J.; Lee, W.K.; Kang, H.L.; Rhee, K.H.; et al. Role of cagA-positive Helicobacter pylori on cell proliferation, apoptosis, and inflammation in biliary cells. Dig. Dis. Sci. 2011, 56, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kuroki, T.; Tajima, Y.; Tsuneoka, N.; Kitajima, T.; Matsuzaki, S.; Furui, J.; Kanematsu, T. Comparative analysis of Helicobacter DNAs and biliary pathology in patients with and without hepatobiliary cancer. Carcinogenesis 2002, 23, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Bulajic, M.; Maisonneuve, P.; Schneider-Brachert, W.; Müller, P.; Reischl, U.; Stimec, B.; Lehn, N.; Lowenfels, A.B.; Löhr, M. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer 2002, 95, 1946–1953. [Google Scholar] [CrossRef]
- Murphy, G.; Michel, A.; Taylor, P.R.; Albanes, D.; Weinstein, S.J.; Virtamo, J.; Parisi, D.; Snyder, K.; Butt, J.; McGlynn, K.A.; et al. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology 2014, 60, 1963–1971. [Google Scholar] [CrossRef]
- Chagani, S.; Kwong, L.N. Cholangiocarcinoma Risk Factors Open the Floodgates for Gut Microbes and Immunosuppressive Myeloid Cells. Cancer Discov. 2021, 11, 1014–1015. [Google Scholar] [CrossRef]
- Ketpueak, T.; Thiennimitr, P.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Association of Chronic Opisthorchis Infestation and Microbiota Alteration on Tumorigenesis in Cholangiocarcinoma. Clin. Transl. Gastroenterol. 2020, 12, e00292. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Syal, G.; Fausther, M.; Dranoff, J.A. Advances in Cholangiocyte Immunobiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1077–G1086. [Google Scholar] [CrossRef]
- Deng, T.; Li, J.; He, B.; Chen, B.; Liu, F.; Chen, Z.; Zheng, J.; Shi, Z.; Zhang, T.; Deng, L.; et al. Gut Microbiome Alteration as a Diagnostic Tool and Associated with Inflammatory Response Marker in Primary Liver Cancer. Hepatol. Int. 2022, 16, 99–111. [Google Scholar] [CrossRef]
- Lederer, A.K.; Rasel, H.; Kohnert, E.; Kreutz, C.; Huber, R.; Badr, M.T.; Dellweg, P.K.E.; Bartsch, F.; Lang, H. Gut Microbiota in Diagnosis, Therapy and Prognosis of Cholangiocarcinoma and Gallbladder Carcinoma-A Scoping Review. Microorganisms 2023, 11, 2363. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Czigany, Z.; Heij, L.R.; Luedde, T.; van Dam, R.; Lang, S.A.; Ulmer, T.F.; Hornef, M.W.; Neumann, U.P. Bacterial bile duct colonization in perihilar cholangiocarcinoma and its clinical significance. Sci. Rep. 2021, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, W.; Chen, K.; Lu, C.; Li, X.; Li, Q. Elucidating the causal association between gut microbiota and intrahepatic cholangiocarcinoma through Mendelian randomization analysis. Front. Microbiol. 2023, 14, 1288525. [Google Scholar] [CrossRef] [PubMed]
- Saab, M.; Mestivier, D.; Sohrabi, M.; Rodriguez, C.; Khonsari, M.R.; Faraji, A.; Sobhani, I. Characterization of Biliary Microbiota Dysbiosis in Extrahepatic Cholangiocarcinoma. PLoS ONE 2021, 16, e0247798. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.K.; Min, S.K.; Lee, W.H. 16S rDNA microbiome composition pattern analysis as a diagnostic biomarker for biliary tract cancer. World J. Surg. Oncol. 2020, 18, 19. [Google Scholar] [CrossRef]
- Ketpueak, T.; Sriwichaiin, S.; Suparan, K.; Kerdphoo, S.; Charoentum, C.; Suksombooncharoen, T.; Chewaskulyong, B.; Chattipakorn, N.; Chattipakorn, S. Alteration of gut microbiota composition in patients with cholangiocarcinoma with non-responsiveness to first-line chemotherapy: A pilot study. J. Cin. Oncol. JCO 2023, 41, 4104. [Google Scholar] [CrossRef]
- Khosla, D.; Misra, S.; Chu, P.L.; Guan, P.; Nada, R.; Gupta, R.; Kaewnarin, K.; Ko, T.K.; Heng, H.L.; Srinivasalu, V.K.; et al. Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches. Cancers 2024, 16, 801. [Google Scholar] [CrossRef]
- Chen, Z.T.; Ding, C.C.; Chen, K.L.; Gu, Y.J.; Lu, C.C.; Li, Q.Y. Causal roles of gut microbiota in cholangiocarcinoma etiology suggested by genetic study. World J. Gastrointest. Oncol. 2024, 16, 1319–1333. [Google Scholar] [CrossRef]
- Elvevi, A.; Laffusa, A.; Gallo, C.; Invernizzi, P.; Massironi, S. Any Role for Microbiota in Cholangiocarcinoma? A Comprehensive Review. Cells 2023, 12, 370. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.; Jin, C.; Lin, Z.; Deng, T.; Xie, X.; Deng, L.; Li, X.; Ma, J.; Ding, X.; et al. A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients with Cholangiocarcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, W.; Zhang, S.; Liu, T.; Gong, L.; Wang, Z.; Zhang, W.; Cui, Y.; Wu, Q.; Li, J.; et al. A Novel Bifidobacterium/Klebsiella Ratio in Characterization Analysis of the Gut and Bile Microbiota of CCA Patients. Microb. Ecol. 2023, 87, 5. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Görrissen, N.; Nguyen, T.T.; Kreutz, C.; Rasel, H.; Bartsch, F.; Lang, H.; Endres, K. Exploring the effects of gut microbiota on cholangiocarcinoma progression by patient-derived organoids. J. Transl. Med. 2025, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Roussel, E.; Brasse-Lagnel, C.; Tuech, J.; Montialoux, H.; Papet, E.; Tortajada, P.; Bekri, S.; Schwarz, L. Influence of Probiotics Administration Before Liver Resection in Patients with Liver Disease: A Randomized Controlled Trial. World J. Surg. 2022, 46, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Kaneko, T.; Murakami, A.; Ueda, M.; Sugimori, K.; Kawana, I.; Maeda, S. New image-enhanced cholangioscopy for the diagnosis of cholangiocarcinoma. Endoscopy 2023, 55, E139–E140. [Google Scholar] [CrossRef]
- Bodalal, Z.; Trebeschi, S.; Beets-Tan, R. Radiomics: A critical step towards integrated healthcare. Insights Imaging 2018, 9, 911–914. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“How-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Belli, A.; Borzillo, V.; Palumbo, P.; Bruno, F.; Grassi, R.; Ottaiano, A.; Nasti, G.; Pilone, V.; et al. Conventional, functional and radiomics assessment for intrahepatic cholangiocarcinoma. Infect. Agent. Cancer 2022, 17, 13, Erratum in Infect. Agent. Cancer 2022, 17, 22. [Google Scholar] [CrossRef]
- Liu, N.; Wu, Y.; Tao, Y.; Zheng, J.; Huang, X.; Yang, L.; Zhang, X. Differentiation of Hepatocellular Carcinoma from Intrahepatic Cholangiocarcinoma through MRI Radiomics. Cancers 2023, 15, 5373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Z.; Cao, L.; Zhang, Z.; Wei, Y.; Zhang, X.; Song, B. Differentiation combined hepatocellular and cholangiocarcinoma from intrahepatic cholangiocarcinoma based on radiomics machine learning. Ann. Transl. Med. 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.C.; Liu, H.Y.; Zhang, Y.M.; Guo, Y.; Yang, S.Y.; Zhang, H.W.; Cui, B.; Zhou, T.M.; Guo, H.X.; Hou, D.W. The diagnostic value of a nomogram based on enhanced CT radiomics for differentiating between intrahepatic cholangiocarcinoma and early hepatic abscess. Front. Mol. Biosci. 2024, 11, 1409060. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, B.; Park, S.Y.; Choi, S.H.; Rhee, H.; Park, J.H.; Cho, E.S.; Yeom, S.K.; Park, S.; Park, M.S.; et al. Preoperative prediction of postsurgical outcomes in mass-forming intrahepatic cholangiocarcinoma based on clinical, radiologic, and radiomics features. Eur. Radiol. 2021, 31, 8638–8648. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, F.; Hou, K.; Wang, F.; Zhou, C.; Huang, P.; Yang, C.; Zeng, M. MR radiomics to predict microvascular invasion status and biological process in combined hepatocellular carcinoma-cholangiocarcinoma. Insights Imaging 2024, 15, 172. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.M.; Su, S.; Wang, W.J.; Fan, L.P.; Shu, J. Machine learning-based Radiomics analysis for differentiation degree and lymphatic node metastasis of extrahepatic cholangiocarcinoma. BMC Cancer 2021, 21, 1268. [Google Scholar] [CrossRef]
- Xu, L.; Yang, P.; Liang, W.; Liu, W.; Wang, W.; Luo, C.; Wang, J.; Peng, Z.; Xing, L.; Huang, M.; et al. A radiomics approach based on support vector machine using MR images for preoperative lymph node status evaluation in intrahepatic cholangiocarcinoma. Theranostics 2019, 9, 5374–5385. [Google Scholar] [CrossRef]
- Lauss, M.; Donia, M.; Svane, I.M.; Jönsson, G. B Cells and Tertiary Lymphoid Structures: Friends or Foes in Cancer Immunotherapy? Clin. Cancer Res. 2022, 28, 1751–1758. [Google Scholar] [CrossRef]
- Ding, G.Y.; Ma, J.Q.; Yun, J.P.; Chen, X.; Ling, Y.; Zhang, S.; Shi, J.Y.; Chang, Y.Q.; Ji, Y.; Wang, X.Y.; et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J. Hepatol. 2022, 76, 608–618. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Yang, Y.; Li, L.; Zhou, Y.; Ouyang, J.; Huang, Z.; Wang, S.; Xie, L.; Ye, F.; et al. A CT-based radiomics approach to predict intra-tumoral tertiary lymphoid structures and recurrence of intrahepatic cholangiocarcinoma. Insights Imaging 2023, 14, 173. [Google Scholar] [CrossRef]
- Sadot, E.; Simpson, A.L.; Do, R.K.; Gonen, M.; Shia, J.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R. Cholangiocarcinoma: Correlation between Molecular Profiling and Imaging Phenotypes. PLoS ONE 2015, 10, e0132953. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Mähringer-Kunz, A.; Gairing, S.J.; Foerster, F.; Weinmann, A.; Bartsch, F.; Heuft, L.K.; Baumgart, J.; Düber, C.; Hahn, F.; et al. Survival Prediction in Intrahepatic Cholangiocarcinoma: A Proof of Concept Study Using Artificial Intelligence for Risk Assessment. J. Clin. Med. 2021, 10, 2071. [Google Scholar] [CrossRef] [PubMed]
- Tekkeşin, A. Artificial intelligence in healthcare: Past, present and future. Anatol. J. Cardiol. 2019, 22, 8–9. [Google Scholar]
- Parekh, V.; Jacobs, M.A. Radiomics: A new application from established techniques. Expert. Rev. Precis. Med. Drug Dev. 2016, 1, 207–226. [Google Scholar]
- Goyal, H.; Mann, R.; Gandhi, Z.; Perisetti, A.; Zhang, Z.; Sharma, N.; Saligram, S.; Inamdar, S.; Tharian, B. Application of artificial intelligence in pancreaticobiliary diseases. Ther. Adv. Gastrointest. Endosc. 2021, 14, 2631774521993059. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tsuzuki, T.; Akatsuka, J.; Ueki, M.; Morikawa, H.; Numata, Y.; Takahara, T.; Tsuyuki, T.; Tsutsumi, K.; Nakazawa, R.; et al. Automated acquisition of explainable knowledge from unannotated histopathology images. Nat. Commun. 2019, 10, 5642. [Google Scholar] [CrossRef]
- Liang, W.; Xu, L.; Yang, P.; Zhang, L.; Wan, D.; Huang, Q.; Niu, T.; Chen, F. Novel Nomogram for Preoperative Prediction of Early Recurrence in Intrahepatic Cholangiocarcinoma. Front. Oncol. 2018, 8, 360. [Google Scholar] [CrossRef]
- Esteva, A.; Chou, K.; Yeung, S.; Naik, N.; Madani, A.; Mottaghi, A.; Liu, Y.; Topol, E.; Dean, J.; Socher, R. Deep learning-enabled medical computer vision. NPJ Digit. Med. 2021, 4, 5. [Google Scholar] [CrossRef]
- Ji, G.W.; Zhu, F.P.; Zhang, Y.D.; Liu, X.S.; Wu, F.Y.; Wang, K.; Xia, Y.X.; Zhang, Y.D.; Jiang, W.J.; Li, X.C.; et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur. Radiol. 2019, 29, 3725–3735. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Logeswaran, R. Cholangiocarcinoma–An automated preliminary detection system using MLP. J. Med. Syst. 2009, 33, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Meining, A.; Saur, D.; Bajbouj, M.; Becker, V.; Peltier, E.; Höfler, H.; von Weyhern, C.H.; Schmid, R.M.; Prinz, C. In Vivo Histopathology for Detection of Gastrointestinal Neoplasia with a Portable, Confocal Miniprobe: An Examiner Blinded Analysis. Clin. Gastroenterol. Hepatol. 2007, 5, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Pilonis, N.D.; Januszewicz, W.; di Pietro, M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: Technical aspects and clinical applications. Transl. Gastroenterol. Hepatol. 2022, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.M.; Ribeiro, T.; Ferreira, J.P.; Boas, F.V.; Afonso, J.; Santos, A.L.; Parente, M.P.; Jorge, R.N.; Pereira, P.; Macedo, G. Artificial intelligence for automatic diagnosis of biliary stricture malignancy status in single-operator cholangioscopy: A pilot study. Gastrointest. Endosc. 2022, 95, 339–348. [Google Scholar] [CrossRef]
- Stassen, P.M.; Goodchild, G.; de Jonge, P.J.F.; Erler, N.S.; Anderloni, A.; Cennamo, V.; Church, N.I.; Sainz, I.F.-U.; Huggett, M.T.; James, M.W.; et al. Diagnostic accuracy and interobserver agreement of digital single-operator cholangioscopy for indeterminate biliary strictures. Gastrointest. Endosc. 2021, 94, 1059–1068. [Google Scholar] [CrossRef]
- Hassan, C.; Repici, A.; Sharma, P. Incorporating Artificial Intelligence into Gastroenterology Practices. Clin. Gastroenterol. Hepatol. 2023, 21, 1687–1689. [Google Scholar] [CrossRef]
- Gerges, C.; Beyna, T.; Tang, R.S.; Bahin, F.; Lau, J.Y.; van Geenen, E.; Neuhaus, H.; Reddy, D.N.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 91, 1105–1113. [Google Scholar] [CrossRef]
- Robles-Medranda, C.; Baquerizo-Burgos, J.; Alcivar-Vasquez, J.; Kahaleh, M.; Raijman, I.; Kunda, R.; Puga-Tejada, M.; Egas-Izquierdo, M.; Arevalo-Mora, M.; Mendez, J.C.; et al. Artificial intelligence for diagnosing neoplasia on digital cholangioscopy: Development and multicenter validation of a convolutional neural network model. Endoscopy 2023, 55, 719–727. [Google Scholar] [CrossRef]
- Marya, N.B.; Powers, P.D.; Petersen, B.T.; Law, R.; Storm, A.; Abusaleh, R.R.; Rau, P.; Stead, C.; Levy, M.J.; Martin, J.; et al. Identification of patients with malignant biliary strictures using a cholangioscopy-based deep learning artificial intelligence (with video). Gastrointest. Endosc. 2023, 97, 268–278. [Google Scholar] [CrossRef]
- Alaimo, L.; Lima, H.A.; Moazzam, Z.; Endo, Y.; Yang, J.; Ruzzenente, A.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; et al. Development and Validation of a Machine-Learning Model to Predict Early Recurrence of Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2023, 30, 5406–5415. [Google Scholar] [CrossRef]
- Sirica, A.E.; Strazzabosco, M.; Cadamuro, M. Intrahepatic cholangiocarcinoma: Morpho-molecular pathology, tumor reactive microenvironment, and malignant progression. Adv. Cancer Res. 2021, 49, 321.e387. [Google Scholar]
- Serifis, N.; Tsilimigras, D.I.; Cloonan, D.J.; Pawlik, T.M. Challenges and opportunities for treating intrahepatic cholangiocarcinoma. Hepat. Med. 2021, 2, 93.e104. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Hyer, J.M.; Paredes, A.Z.; Diaz, A.; Moris, D.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; et al. A Novel Classification of Intrahepatic Cholangiocarcinoma Phenotypes Using Machine Learning Techniques: An International Multi-Institutional Analysis. Ann. Surg. Oncol. 2020, 27, 5224–5232. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.V.; Aquina, C.T.; Pawlik, T.M. When a patient regrets having undergone a carefully and jointly considered treatment plan, how should her physician respond? AMA J. Ethics 2020, 22, 352.e357. [Google Scholar]
- Fiz, F.; Masci, C.; Costa, G.; Sollini, M.; Chiti, A.; Ieva, F.; Torzilli, G.; Viganò, L. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3387–3400. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, X.; Zhou, W.; Yuan, G.; Hu, D.; Shen, Y.; Xie, Q.; Zhang, Q.; Kuang, D.; Hu, X.; et al. DWI-based radiomic signature: Potential role for individualized adjuvant chemotherapy in intrahepatic cholangiocarcinoma after partial hepatectomy. Insights Imaging 2022, 13, 37. [Google Scholar] [CrossRef]

| Microbiota | Perihilar CCA | Intrahepatic CCA | Extrahepatic CCA |
|---|---|---|---|
| Bile | E. faecalis E. faecium E. cloacae E. coli Pyramidobacter Klebsiella Bacteroides Enterococcus | Firmicutes Bacteroidetes Actinobacteria Verrucomicrobia | Bacteroides Geobacillus Meiothermus Anoxybacillus |
| Blood | Ruminococcaceae Oribacterium Oxalobacter Peptostreptococcus Aggregabacter Burkholderia Caballeronia Paraburkholderia Faecalibacterium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaggi, F.; Lopetuso, L.R.; delli Pizzi, A.; Melchiorre, E.; Murgiano, M.; Taraschi, A.L.; Cotellese, R.; Diana, M.; Vivarelli, M.; Mocchegiani, F.; et al. Diagnosis of Cholangiocarcinoma: The New Biological and Technological Horizons. Diagnostics 2025, 15, 1011. https://doi.org/10.3390/diagnostics15081011
Selvaggi F, Lopetuso LR, delli Pizzi A, Melchiorre E, Murgiano M, Taraschi AL, Cotellese R, Diana M, Vivarelli M, Mocchegiani F, et al. Diagnosis of Cholangiocarcinoma: The New Biological and Technological Horizons. Diagnostics. 2025; 15(8):1011. https://doi.org/10.3390/diagnostics15081011
Chicago/Turabian StyleSelvaggi, Federico, Loris Riccardo Lopetuso, Andrea delli Pizzi, Eugenia Melchiorre, Marco Murgiano, Alessio Lino Taraschi, Roberto Cotellese, Michele Diana, Marco Vivarelli, Federico Mocchegiani, and et al. 2025. "Diagnosis of Cholangiocarcinoma: The New Biological and Technological Horizons" Diagnostics 15, no. 8: 1011. https://doi.org/10.3390/diagnostics15081011
APA StyleSelvaggi, F., Lopetuso, L. R., delli Pizzi, A., Melchiorre, E., Murgiano, M., Taraschi, A. L., Cotellese, R., Diana, M., Vivarelli, M., Mocchegiani, F., Catalano, T., & Aceto, G. M. (2025). Diagnosis of Cholangiocarcinoma: The New Biological and Technological Horizons. Diagnostics, 15(8), 1011. https://doi.org/10.3390/diagnostics15081011








