Biosensors, Artificial Intelligence Biosensors, False Results and Novel Future Perspectives
Abstract
:1. Introduction
2. The Concept of “Biosensor” in Medicine
2.1. The Concept of “Sensor”
2.2. The Common “Biosensor”
2.3. The Biosensors with Integrated Artificial Intelligence (AI-Enabled Biosensors or AI Biosensors)
2.3.1. The History of Artificial Intelligence
2.3.2. Artificial Intelligence Applications in Biosensors
3. Current Common Types of Diagnostic Biosensors and False Results
3.1. Current Common Biosensors Based on Their Bioreceptors
3.1.1. Enzyme-Based Biosensors
3.1.2. Antibody-Based Biosensors (Immunosensors)
3.1.3. Whole-Cell-Based Biosensors
3.1.4. Nucleic Acid-Based Biosensors
3.2. Current Common Biosensors Based on Their Transducers-Detection System
3.2.1. Electrochemical Biosensors
3.2.1.1. Voltametric/Amperometric Biosensors
3.2.1.2. Impedimetric Biosensors
3.2.1.3. Potentiometric Biosensors
3.2.1.4. Conductometric Biosensors
3.2.2. Optical Biosensors
3.2.2.1. Colorimetric Biosensors
3.2.2.2. Fluorescent Biosensors
3.2.2.3. Optical Fiber Biosensors
3.2.2.4. Surface-Enhanced Raman Scattering Biosensors
3.2.2.5. Photonic Crystal Biosensors
3.2.3. Thermal Biosensors
3.2.4. Acoustic Biosensors
3.2.5. Mechanical Biosensors
3.2.6. Magnetic Biosensors
3.3. Current Common Biosensors Based on Their Technology
3.3.1. SPR Biosensors
3.3.2. Microfluidic Biosensors
3.3.2.1. Paper-Based Biosensors
3.3.2.2. Polymer-Based Biosensors
3.3.3. Nanotechnology-Based Biosensors
3.3.3.1. Metal Nanoparticles
3.3.3.2. Nanostructured Surface-Based Biosensors
3.3.3.3. Quantum Dots-Based Biosensors
3.3.4. Implantable Biosensors
4. Current Common Types of AI in Biosensors in Medicine and False Results
4.1. Current Supervised Learning in AI Biosensors
4.1.1. Classification Algorithms
4.1.2. Regression Algorithms
4.2. Current Unsupervised Learning in AI Biosensors
4.2.1. Clustering Algorithms
4.2.2. Dimensionality Reduction Algorithms
4.3. Current Deep Learning Neutral Networks in AI Biosensors
4.4. Current Reinforced Learning in AI Biosensors
5. The Expert’s Opinion and the Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral. Biol. Craniofac Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Milkov, M.; Georgieva, G.; Stoykov, M.; Enchev, M.; Slivovska, G. Artificial Intelligence in Medicine–The New Reality Nowadays. Int. Bull. Otorhinolaryngol. 2022, 17, 29–31. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Gourgoulianis, K.I. False-Positive and False-Negative COVID-19 Cases: Respiratory Prevention and Management Strategies, Vaccination, and Further Perspectives. Expert. Rev. Respir. Med. 2021, 15, 993–1002. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Dardiotis, E. Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns. Diagnostics 2022, 12, 1555. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K.I. COVID-19 Smart Diagnosis in the Emergency Department: All-in in Practice. Expert. Rev. Respir. Med. 2022, 16, 263–272. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Gourgoulianis, K.I. COVID-19 ‘Asymptomatic’ Patients: An Old Wives’ Tale. Expert. Rev. Respir. Med. 2022, 16, 399–407. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K.I. Societal Criticism towards COVID-19: Assessing the Theory of Self-Diagnosis Contrasted to Medical Diagnosis. Diagnostics 2021, 11, 1777. [Google Scholar] [CrossRef]
- Kotsiou, O.S.; Pantazopoulos, I.; Papagiannis, D.; Fradelos, E.C.; Kanellopoulos, N.; Siachpazidou, D.; Kirgou, P.; Mouliou, D.S.; Kyritsis, A.; Kalantzis, G.; et al. Repeated Antigen-Based Rapid Diagnostic Testing for Estimating the Coronavirus Disease 2019 Prevalence from the Perspective of the Workers’ Vulnerability before and during the Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 1638. [Google Scholar] [CrossRef]
- Mouliou, D.S.; Pantazopoulos, I.; Gourgoulianis, K. COVID-19 Diagnosis in the Emergency Department: Seeing the Tree but Losing the Forest. Emerg. Med. J. 2022, 39, 563. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. Managing Viral Emerging Infectious Diseases via Current Molecular Diagnostics in the Emergency Department: The Tricky Cases. Expert. Rev. Anti-Infect. Ther. 2022, 20, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. The Deceptive COVID-19: Lessons from Common Molecular Diagnostics and a Novel Plan for the Prevention of the Next Pandemic. Diseases 2023, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. John Cunningham Virus and Progressive Multifocal Leukoencephalopathy: A Falsely Played Diagnosis. Diseases 2024, 12, 100. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef]
- Kazemi-Darsanaki, R.; Azizzadeh, A.; Nourbakhsh, M.; Raeisi, G.; AzizollahiAliabadi, M. Biosensors: Functions and Applications. JBTW 2013, 2, 53–61. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.; Jaffa, A.A. Recent Advances in Application of Biosensors in Tissue Engineering. BioMed Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef]
- Patel, S.; Nanda, R.; Sahoo, S.; Mohapatra, E. Biosensors in Health Care: The Milestones Achieved in Their Development towards Lab-on-Chip-Analysis. Biochem. Res. Int. 2016, 2016, 3130469. [Google Scholar] [CrossRef]
- Johnson, J.A. Erika Cremer and the Origins of Gas–Solid Adsorption Chromatography, 1944–1947. In ACS Symposium Series; Mainz, V.V., Strom, E.T., Eds.; American Chemical Society: Washington, DC, USA, 2018; Volume 1311, pp. 183–198. ISBN 9780841233911. [Google Scholar]
- Hughes, W.S. The potential difference between glass and electrolytes in contact with the glass. J. Am. Chem. Soc. 1922, 44, 2860–2867. [Google Scholar] [CrossRef]
- Griffin, E.G.; Nelson, J.M. The influence of certain substances on the activity of invertase. J. Am. Chem. Soc. 1916, 38, 722–730. [Google Scholar] [CrossRef]
- Nelson, J.M.; Griffin, E.G. Adsorption of invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115. [Google Scholar] [CrossRef]
- Erratum to “Dielectrophoretic Registration of Living Cells to a Microelectrode Array” [Biosens. Bioelectron. 19 (2004) 771–780]. Biosens. Bioelectron. 2004, 19, 1763. [CrossRef]
- Guilbault, G.G.; Montalvo, J.G. Urea-Specific Enzyme Electrode. J. Am. Chem. Soc. 1969, 91, 2164–2165. [Google Scholar] [CrossRef]
- Heineman, W.R.; Jensen, W.B. Leland C. Clark Jr. (1918–2005). Biosens. Bioelectron. 2006, 21, 1403–1404. [Google Scholar] [CrossRef]
- Clemens, A.H.; Chang, P.H.; Myers, R.W. The Development of Biostator, a Glucose Controlled Insulin Infusion System (GCIIS). Horm. Metab. Res. 1977, 7, 23–33. [Google Scholar]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-Mediated Enzyme Electrode for Amperometric Determination of Glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A Tutorial Review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajkumar, R.; Bhargava, K. Chapter 1—Introduction to Biosensors. In Biosensors and Bioelectronics; Karunakaran, C., Bhargava, K., Benjamin, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–68. ISBN 9780128031001. [Google Scholar]
- Kline, R. Cybernetics, Automata Studies, and the Dartmouth Conference on Artificial Intelligence. IEEE Ann. Hist. Comput. 2011, 33, 5–16. [Google Scholar] [CrossRef]
- Flynn, C.D.; Chang, D. Artificial Intelligence in Point-of-Care Biosensing: Challenges and Opportunities. Diagnostics 2024, 14, 1100. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Q.; Lu, Y.; Liu, L.; Ji, D.; Li, S.; Liu, Q. Passive and Wireless near Field Communication Tag Sensors for Biochemical Sensing with Smartphone. Sens. Actuators B Chem. 2017, 246, 748–755. [Google Scholar] [CrossRef]
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Skottrup, P.D.; Nicolaisen, M.; Justesen, A.F. Towards On-Site Pathogen Detection Using Antibody-Based Sensors. Biosens. Bioelectron. 2008, 24, 339–348. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezic-Renault, N. Amperometric Enzyme Biosensors: Past, Present and Future. IRBM 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Bradford, V.Q.; Whitaker, R.G. Enzyme Electrode Studies of Glucose Oxidase Modified with a Redox Mediator. Talanta 1991, 38, 57–63. [Google Scholar] [CrossRef]
- Prodromidis, M.I.; Karayannis, M.I. Enzyme Based Amperometric Biosensors for Food Analysis. Electroanalysis 2002, 14, 241–261. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.T.; Oh, Y.; Choi, S.-J.; Kim, I.-D.; Oh, M.-K.; Kim, M. Applications and Advances in Bioelectronic Noses for Odour Sensing. Sensors 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. A Review on Impedimetric Biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Free, A.H.; Adams, E.C.; Kercher, M.L.; Free, H.M.; Cook, M.H. Simple Specific Test for Urine Glucose. Clin. Chem. 1957, 3, 163–168. [Google Scholar] [CrossRef]
- Sanz, V.; de Marcos, S.; Galbán, J. A Reagentless Optical Biosensor Based on the Intrinsic Absorption Properties of Peroxidase. Biosens. Bioelectron. 2007, 22, 956–964. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Hwang, S.; Kwon, O.S.; Lee, C.-S.; Shin, Y.-B.; Ha, T.H.; Kim, M. On-Chip Fluorescence Switching System for Constructing a Rewritable Random Access Data Storage Device. Sci. Rep. 2018, 8, 337. [Google Scholar] [CrossRef]
- Galbán, J.; Andreu, Y.; Sierra, J.F.; De Marcos, S.; Castillo, J.R. Intrinsic Fluorescence of Enzymes and Fluorescence of Chemically Modified Enzymes for Analytical Purposes: A Review. Luminescence 2001, 16, 199–210. [Google Scholar] [CrossRef]
- Blum, L.J.; Coulet, P.R. Luminescent Biosensors. In Biosensors and Their Applications; Yang, V.C., Ngo, T.T., Eds.; Springer: Boston, MA, USA, 2000; pp. 213–223. ISBN 9781461541813. [Google Scholar]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Mishra, G.K.; Sharma, A.; Deshpande, K.; Bhand, S. Flow Injection Analysis Biosensor for Urea Analysis in Urine Using Enzyme Thermistor. Appl. Biochem. Biotechnol. 2014, 174, 998–1009. [Google Scholar] [CrossRef]
- Mattiasson, B.; Borrebaeck, C.; Sanfridson, B.; Mosbach, K. Thermometric Enzyme Linked Immunosorbent Assay: TELISA. Biochim. Biophys. Acta (BBA) Enzymol. 1977, 483, 221–227. [Google Scholar] [CrossRef]
- Abad, J.M.; Pariente, F.; Hernández, L.; Abruña, H.D.; Lorenzo, E. Determination of Organophosphorus and Carbamate Pesticides Using a Piezoelectric Biosensor. Anal. Chem. 1998, 70, 2848–2855. [Google Scholar] [CrossRef]
- Simonian, A.L.; Flounders, A.W.; Wild, J.R. FET-Based Biosensors for The Direct Detection of Organophosphate Neurotoxins. Electroanalysis 2004, 16, 1896–1906. [Google Scholar] [CrossRef]
- Trivedi, V.D.; Mohan, K.; Chappell, T.C.; Mays, Z.J.S.; Nair, N.U. Cheating the Cheater: Suppressing False-Positive Enrichment during Biosensor-Guided Biocatalyst Engineering. ACS Synth. Biol. 2022, 11, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Amine, A. Biosensors Based on Enzyme Inhibition. In Biosensors Based on Aptamers and Enzymes; Gu, M.B., Kim, H.-S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 299–326. ISBN 9783642541438. [Google Scholar]
- Bucur, B.; Munteanu, F.-D.; Marty, J.-L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Biosensors—Recent Advances and Future Challenges. Sensors 2020, 20, 6645. [Google Scholar] [CrossRef]
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, Challenges and Opportunities of Enzyme Immobilization on Porous Silicon for Biosensing Applications. J. Environ. Chem. Eng. 2020, 8, 104266. [Google Scholar] [CrossRef]
- Monteiro, T.; Almeida, M.G. Electrochemical Enzyme Biosensors Revisited: Old Solutions for New Problems. Crit. Rev. Anal. Chem. 2019, 49, 44–66. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants—A Review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Biloivan, O.A.; Dzyadevych, S.V.; Boubriak, O.A.; Soldatkin, A.P.; El’skaya, A.V. Development of Enzyme Biosensor Based on ISFETs for Quantitative Analysis of Serine Proteinases. Electroanalysis 2004, 16, 1883–1889. [Google Scholar] [CrossRef]
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. Antibody Production, Design and Use for Biosensor-Based Applications. Semin. Cell Dev. Biol. 2009, 20, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Yalow, R.S.; Berson, S.A. Assay of Plasma Insulin in Human Subjects by Immunological Methods. Nature 1959, 184, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Holford, T.R.J.; Davis, F.; Higson, S.P.J. Recent Trends in Antibody Based Sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A Short History, Principles, and Types of ELISA, and Our Laboratory Experience with Peptide/Protein Analyses Using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Lindström, P.; Wager, O. IgG Autoantibody to Human Serum Albumin Studied by the ELISA-Technique. Scand. J. Immunol. 1978, 7, 419–425. [Google Scholar] [CrossRef]
- Kato, K.; Hamaguchi, Y.; Okawa, S.; Ishikawa, E.; Kobayashi, K.; Katunuma, N. Use of Rabbit Antibody IgG Bound onto Plain and Aminoalkylsilyl Glass Surface for the Enzyme-Linked Sandwich Immunoassay. J. Biochem. 1977, 82, 261–266. [Google Scholar] [CrossRef]
- Yorde, D.E.; Sasse, E.A.; Wang, T.Y.; Hussa, R.O.; Garancis, J.C. Competitive enzyme-liked immunoassay with use of soluble enzyme/antibody immune complexes for labeling. I. Measurement of human choriogonadotropin. Clin. Chem. 1976, 22, 1372–1377. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef]
- Andryukov, B.G. Six Decades of Lateral Flow Immunoassay: From Determining Metabolic Markers to Diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef]
- Xu, W.; Wang, D.; Li, D.; Liu, C.C. Recent Developments of Electrochemical and Optical Biosensors for Antibody Detection. Int. J. Mol. Sci. 2020, 21, 134. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; Wiley: Chichester, UK, 2012; ISBN 9780470710661. [Google Scholar]
- Bousse, L. Whole Cell Biosensors. Sens. Actuators B Chem. 1996, 34, 270–275. [Google Scholar] [CrossRef]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Mascher, T. The Applied Side of Antimicrobial Peptide-Inducible Promoters from Firmicutes Bacteria: Expression Systems and Whole-Cell Biosensors. Appl. Microbiol. Biotechnol. 2016, 100, 4817–4829. [Google Scholar] [CrossRef]
- Hillger, J.M.; Schoop, J.; Boomsma, D.I.; Eline Slagboom, P.; IJzerman, A.P.; Heitman, L.H. Whole-Cell Biosensor for Label-Free Detection of GPCR-Mediated Drug Responses in Personal Cell Lines. Biosens. Bioelectron. 2015, 74, 233–242. [Google Scholar] [CrossRef]
- Si, R.-W.; Yang, Y.; Yu, Y.-Y.; Han, S.; Zhang, C.-L.; Sun, D.-Z.; Zhai, D.-D.; Liu, X.; Yong, Y.-C. Wiring Bacterial Electron Flow for Sensitive Whole-Cell Amperometric Detection of Riboflavin. Anal. Chem. 2016, 88, 11222–11228. [Google Scholar] [CrossRef]
- Shabani, A.; Zourob, M.; Allain, B.; Marquette, C.A.; Lawrence, M.F.; Mandeville, R. Bacteriophage-Modified Microarrays for the Direct Impedimetric Detection of Bacteria. Anal. Chem. 2008, 80, 9475–9482. [Google Scholar] [CrossRef]
- Moraskie, M.; Roshid, M.H.O.; O’Connor, G.; Dikici, E.; Zingg, J.-M.; Deo, S.; Daunert, S. Microbial Whole-Cell Biosensors: Current Applications, Challenges, and Future Perspectives. Biosens. Bioelectron. 2021, 191, 113359. [Google Scholar] [CrossRef]
- Saini, R.; Hegde, K.; Brar, S.K.; Verma, M. Chapter 13—Advances in Whole Cell-Based Biosensors in Environmental Monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Kaur Brar, S., Hegde, K., Pachapur, V.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. ISBN 9780128146798. [Google Scholar]
- Harms, H.; Wells, M.C.; van der Meer, J.R. Whole-Cell Living Biosensors—Are They Ready for Environmental Application? Appl. Microbiol. Biotechnol. 2006, 70, 273–280. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, S.; Jia, X. Highly Sensitive Whole-Cell Biosensor for Cadmium Detection Based on a Negative Feedback Circuit. Front. Bioeng. Biotechnol. 2021, 9, 799781. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-K.; Ishikawa, F.N.; Zhang, R.; Datar, R.; Cote, R.J.; Thompson, M.E.; Zhou, C. Rapid, Label-Free, Electrical Whole Blood Bioassay Based on Nanobiosensor Systems. ACS Nano 2011, 5, 9883–9891. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Dong, S. Nucleic Acid Biosensors: Recent Advances and Perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, S.; Guo, W.; Li, B.; Yang, Y.; Xie, B.; Li, K.; Zhang, L. Recent Advances on Functional Nucleic-Acid Biosensors. Sensors 2021, 21, 7109. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR Past, Present and Future. BioTechniques 2020, 69, 317–325. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Wang, E. “Fitting” Makes “Sensing” Simple: Label-Free Detection Strategies Based on Nucleic Acid Aptamers. Acc. Chem. Res. 2013, 46, 203–213. [Google Scholar] [CrossRef]
- Ren, J.; Wang, T.; Wang, E.; Wang, J. Versatile G-Quadruplex-Mediated Strategies in Label-Free Biosensors and Logic Systems. Analyst 2015, 140, 2556–2572. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, B.; Wu, L.; Huang, M.; Li, X.; Zhang, H.; Song, J.; Wang, W.; Zhao, G.; Song, Y.; et al. DNA Nanolithography Enables a Highly Ordered Recognition Interface in a Microfluidic Chip for the Efficient Capture and Release of Circulating Tumor Cells. Angew. Chem. Int. Ed. Engl. 2020, 59, 14115–14119. [Google Scholar] [CrossRef]
- Henikoff, S. Unidirectional Digestion with Exonuclease III Creates Targeted Breakpoints for DNA Sequencing. Gene 1984, 28, 351–359. [Google Scholar] [CrossRef]
- Anne, A.; Bonnaudat, C.; Demaille, C.; Wang, K. Enzymatic Redox 3′-End-Labeling of DNA Oligonucleotide Monolayers on Gold Surfaces Using Terminal Deoxynucleotidyl Transferase (TdT)-Mediated Single Base Extension. J. Am. Chem. Soc. 2007, 129, 2734–2735. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanoparticles for Nucleic-Acid-Based Biosensing: Opportunities, Challenges, and Prospects. Anal. Bioanal. Chem. 2019, 411, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, Q.; Yang, Y. Aptamer-Integrated Nucleic Acid Circuits for Biosensing: Classification, Challenges and Perspectives. Biosens. Bioelectron. 2020, 165, 112422. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.N.; Pham, H.T.; Tran, B.N.; Vu, D.-T.; Do, H.H.; Pham, M.T.N.; Nguyen, H.T.A.; Pham, B. Minimizing False Positives in Gold Nanoparticle–Aptamer Biosensors for Enhanced Serotonin Detection. ACS Appl. Nano Mater. 2024, 7, 17697–17706. [Google Scholar] [CrossRef]
- Alkhamis, O.; Canoura, J.; Willis, C.; Wang, L.; Perry, J.; Xiao, Y. Comparison of Aptamer Signaling Mechanisms Reveals Disparities in Sensor Response and Strategies to Eliminate False Signals. J. Am. Chem. Soc. 2023, 145, 12407–12422. [Google Scholar] [CrossRef]
- Yoo, H.; Jo, H.; Soo Oh, S. Detection and beyond: Challenges and Advances in Aptamer-Based Biosensors. Mater. Adv. 2020, 1, 2663–2687. [Google Scholar] [CrossRef]
- Zaouri, N.; Cui, Z.; Sol Peinetti, A.; Lu, Y.; Hong, P.-Y. DNAzyme-Based Biosensor as a Rapid and Accurate Verification Tool to Complement Simultaneous Enzyme-Based Media for E. Coli Detection. Environ. Sci. Water Res. Technol. 2019, 5, 2260–2268. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Liu, S.; Yu, S.; Yu, Z.; Low, S.S. Application and Progress of Chemometrics in Voltammetric Biosensing. Biosensors 2022, 12, 494. [Google Scholar] [CrossRef]
- Sadeghi, J. Amperometric Biosensors. In Encyclopedia of Biophysics; Gordon CK Roberts: Townsville, Australia, 2013; ISBN 9783642167126. [Google Scholar]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical Sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef]
- Grennan, K.; Killard, J.A.; Smyth, R.M. Physical Characterizations of a Screen-Printed Electrode for Use in an Amperometric Biosensor System. Electroanalysis 2001, 13, 745–750. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y. Interdigitated Array Microelectrodes Based Impedance Biosensors for Detection of Bacterial Cells. Biosens. Bioelectron. 2009, 24, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Techniques in Electroanalytical Chemistry, 2nd ed.; rev.expanded.; Kissinger, P.T., Heineman, W.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; ISBN 9780824794453. [Google Scholar]
- UKEssays. (November 2018). AC Voltammetry: Principles, Advantages and Disadvantages. Available online: https://www.ukessays.com/essays/chemistry/ac-voltammetry-principles-advantages-5245.php?vref=1 (accessed on 27 November 2024).
- Kowalczyk, A.; Matysiak-Brynda, E.; Nowicka, A.M. Proteins and Peptides Voltammetry: Trends, Potential, and Limitations. Curr. Opin. Electrochem. 2019, 14, 44–52. [Google Scholar] [CrossRef]
- Wightman, R.M.; Wipf, D.O. Voltammetry At Ultramicroelectrodes. In Electroanalytical Chemistry; CRC Press: Boca Raton, FL, USA, 1988; ISBN 9781003418115. [Google Scholar]
- Stephens, M.M.; Moorhead, E.D. An Examination of the Finite Difference Numerical Approach to the Solution of Electrochemically-Induced Diffusive Transport at Stationary Solid Cylinder Electrodes: Single Sweep Voltammetry of Reversible Systems. J. Electroanal. Chem. Interfacial Electrochem. 1984, 164, 17–26. [Google Scholar] [CrossRef]
- Kolesnichenko, I. Development of a Method for Multisensory Stripping Voltammetry in the Analysis of Medical Preparations. ACS Omega 2023, 8, 40229–40241. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Pessoa, G.D.P.; Dos Santos, A.B.; De Lima-Neto, P.; Correia, A.N. Simultaneous Electrochemical Sensing of Emerging Organic Contaminants in Full-Scale Sewage Treatment Plants. Chem. Eng. J. 2015, 267, 347–354. [Google Scholar] [CrossRef]
- Thiruvottriyur Shanmugam, S.; Van Echelpoel, R.; Boeye, G.; Eliaerts, J.; Samanipour, M.; Ching, H.Y.V.; Florea, A.; Van Doorslaer, S.; Van Durme, F.; Samyn, N.; et al. Towards Developing a Screening Strategy for Ecstasy: Revealing the Electrochemical Profile. ChemElectroChem 2021, 8, 4826–4834. [Google Scholar] [CrossRef]
- Puiu, M.; Zamfir, L.-G.; Danila, G.M.; Papi, F.; Nativi, C.; Mirceski, V.; Bala, C. Label-Free Detection of Target Proteins Using Peptide Molecular Wires as Conductive Supports. Sens. Actuators B Chem. 2021, 345, 130416. [Google Scholar] [CrossRef]
- Etesami, M.; Karoonian, F.S.; Mohamed, N. Electrochemical Deposition of Gold Nanoparticles on Pencil Graphite by Fast Scan Cyclic Voltammetry. J. Chin. Chem. Soc. 2011, 58, 688–693. [Google Scholar] [CrossRef]
- Monroe, D. Amperometric Immunoassay. Crit. Rev. Clin. Lab. Sci. 1990, 28, 1–18. [Google Scholar] [CrossRef]
- Palchetti, I.; Mascini, M. Amperometric Biosensor for Pathogenic Bacteria Detection. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer: New York, NY, USA, 2008; pp. 299–312. ISBN 9780387751139. [Google Scholar]
- Lorenz, W.; Schulze, K.D. Zur Anwendung Der Transformations—Impedanzspektrometrie. J. Electroanal. Chem. Interfacial Electrochem. 1975, 65, 141–153. [Google Scholar] [CrossRef]
- Guan, J.-G.; Miao, Y.-Q.; Zhang, Q.-J. Impedimetric Biosensors. J. Biosci. Bioeng. 2004, 97, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.S.J.; Limson, J.L.; Fogel, R. Certain Methods of Electrode Pretreatment Create Misleading Responses in Impedimetric Aptamer Biosensors. ACS Omega 2019, 4, 5839–5847. [Google Scholar] [CrossRef]
- Štukovnik, Z.; Bren, U. Recent Developments in Electrochemical-Impedimetric Biosensors for Virus Detection. Int. J. Mol. Sci. 2022, 23, 15922. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Biswas, S.; O Kare, S.P.; Biswas, P.; Jana, S.K.; Das, S.; Chaudhury, K. Development of an Impedimetric Immunosensor for Machine Learning-Based Detection of Endometriosis: A Proof of Concept. Sens. Actuators B Chem. 2021, 346, 130460. [Google Scholar] [CrossRef]
- Sitkov, N.; Ryabko, A.; Moshnikov, V.; Aleshin, A.; Kaplun, D.; Zimina, T. Hybrid Impedimetric Biosensors for Express Protein Markers Detection. Micromachines 2024, 15, 181. [Google Scholar] [CrossRef]
- Shimizu, F.M.; de Barros, A.; Braunger, M.L.; Gaal, G.; Riul Jr, A. Information Visualization and Machine Learning Driven Methods for Impedimetric Biosensing. TrAC Trends Anal. Chem. 2023, 165, 117115. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. A Review of Electrochemical Impedance Spectroscopy for Bioanalytical Sensors. Anal. Methods 2022, 14, 4602–4624. [Google Scholar] [CrossRef]
- Poghossian, A.; Schöning, M.J. Capacitive Field-Effect EIS Chemical Sensors and Biosensors: A Status Report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef]
- Alsabbagh, K.; Hornung, T.; Voigt, A.; Sadir, S.; Rajabi, T.; Länge, K. Microfluidic Impedance Biosensor Chips Using Sensing Layers Based on DNA-Based Self-Assembled Monolayers for Label-Free Detection of Proteins. Biosensors 2021, 11, 80. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent Advances in Potentiometric Biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Yunus, S.; Jonas, A.M.; Lakard, B. Potentiometric Biosensors. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1941–1946. ISBN 9783642167126. [Google Scholar]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A Critical Review on the Use of Potentiometric Based Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.H.; Umasankar, Y.; Gonzalez, P.J.; Alfonso, A.; Bhansali, S. Multimodal Technique to Eliminate Humidity Interference for Specific Detection of Ethanol. Biosens. Bioelectron. 2017, 87, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Balevicius, Z.; Lescinskas, R.; Celiesiute, R.; Stirke, A.; Balevicius, S.; Kersulis, S.; Bleizgys, V.; Maciuleviciene, R.; Ramanavicius, A.; Zurauskiene, N. Compact High-Sensitivity Potentiometer for Detection of Low Ion Concentrations in Liquids. Rev. Sci. Instrum. 2018, 89, 044704. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R. Recent Developments in Potentiometric Biosensors for Biomedical Analysis. Anal. Chim. Acta 2007, 599, 7–15. [Google Scholar] [CrossRef]
- Monroe, D. Potentiometric (Bioselective Electrodes) Assay Systems: Utility and Limitations. Crit. Rev. Clin. Lab. Sci. 1989, 27, 109–158. [Google Scholar] [CrossRef]
- Krawczyński vel Krawczyk, T.; Moszczyńska, M.; Trojanowicz, M. Inhibitive Determination of Mercury and Other Metal Ions by Potentiometric Urea Biosensor. Biosens. Bioelectron. 2000, 15, 681–691. [Google Scholar] [CrossRef]
- Fernando, P.U.A.I.; Glasscott, M.W.; Pokrzywinski, K.; Fernando, B.M.; Kosgei, G.K.; Moores, L.C. Analytical Methods Incorporating Molecularly Imprinted Polymers (MIPs) for the Quantification of Microcystins: A Mini-Review. Crit. Rev. Anal. Chem. 2022, 52, 1244–1258. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. Progress in the Development of Intrinsically Conducting Polymer Composites as Biosensors. Macro Chem. Phys. 2019, 220, 1800561. [Google Scholar] [CrossRef]
- Park, R.; Jeon, S.; Jeong, J.; Park, S.-Y.; Han, D.-W.; Hong, S.W. Recent Advances of Point-of-Care Devices Integrated with Molecularly Imprinted Polymers-Based Biosensors: From Biomolecule Sensing Design to Intraoral Fluid Testing. Biosensors 2022, 12, 136. [Google Scholar] [CrossRef]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly Imprinted Polymer-Based Optical Sensors for Pesticides in Foods: Recent Advances and Future Trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Zhu, C.; Hu, L. Recent Advances in Photoelectrochemical Sensing for Food Safety. Anal. Chem. 2024, 96, 8855–8867. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Xu, J.; Liang, T.; Li, M.; Tang, D. Photocurrent-Polarity-Switching Photoelectrochemical Biosensor for Switching Spatial Distance Electroactive Tags. ACS Sens. 2023, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Dzyadevych, S.; Jaffrezic-Renault, N. Conductometric Biosensors. In Biological Identification; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–193. ISBN 9780857095015. [Google Scholar]
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588. [Google Scholar] [CrossRef] [PubMed]
- Soldatkina, O.V.; Soldatkin, O.O.; Velychko, T.P.; Prilipko, V.O.; Kuibida, M.A.; Dzyadevych, S.V. Conductometric Biosensor for Arginine Determination in Pharmaceutics. Bioelectrochemistry 2018, 124, 40–46. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Du, X.; Gui, Y.; He, J.; Xie, F.; Cai, J. Recent Advances in Design and Application of Nanomaterials-Based Colorimetric Biosensors for Agri-Food Safety Analysis. ACS Omega 2023, 8, 46346–46361. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent Advances in the Design of Colorimetric Sensors for Environmental Monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric biosensors for point-of-care virus detections. Mater. Sci. Technol. 2020, 3, 237–349. [Google Scholar] [CrossRef]
- Pohanka, M. Colorimetric Hand-Held Sensors and Biosensors with a Small Digital Camera as Signal Recorder, a Review. Rev. Anal. Chem. 2020, 39, 20–30. [Google Scholar] [CrossRef]
- Gibbs, J. Selecting the Detection System—Colorimetric, Fluorescent, Luminescent Methods. Available online: https://www.labcluster.com/news4_4/ELISA_Episode3.pdf (accessed on 29 November 2024).
- Guo, J.-X.; Pan, L.-M.; Wang, M.-C.; Chen, L.-J.; Zhao, X. Exogenous Interference and Autofluorescence-Free Ratiometric Aptasensor for Detection of OTA Based on Dual-Colored Persistent Luminescence Nanoparticles. Food Chem. 2023, 413, 135611. [Google Scholar] [CrossRef]
- Krishnan, S.; Syed, Z.U.Q. Colorimetric Visual Sensors for Point-of-Needs Testing. Sens. Actuators Rep. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. A Colorimetric Lead Biosensor Using DNAzyme-Directed Assembly of Gold Nanoparticles. J. Am. Chem. Soc. 2003, 125, 6642–6643. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Kim, H.S.; Chung, E.; Lee, D.Y. Nanozyme-Based Colorimetric Biosensor with a Systemic Quantification Algorithm for Noninvasive Glucose Monitoring. Theranostics 2022, 12, 6308–6338. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.C.; Mehta, S.; Zhang, J. Fluorescent Biosensors Illuminate the Spatial Regulation of Cell Signaling across Scales. Biochem. J. 2023, 480, 1693–1717. [Google Scholar] [CrossRef] [PubMed]
- Kyere-Yeboah, K.; Denteh, J.; Liu, K.; Ye, P.; Gao, E.-B. Monitoring Nicotinamide Adenine Dinucleotide and Its Phosphorylated Redox Metabolism Using Genetically Encoded Fluorescent Biosensors. Sens. Bio-Sens. Res. 2019, 26, 100307. [Google Scholar] [CrossRef]
- Kikuchi, K.; Takakusa, H.; Nagano, T. Recent Advances in the Design of Small Molecule-Based FRET Sensors for Cell Biology. TrAC Trends Anal. Chem. 2004, 23, 407–415. [Google Scholar] [CrossRef]
- Li, B.; Yu, Q.; Duan, Y. Fluorescent Labels in Biosensors for Pathogen Detection. Crit. Rev. Biotechnol. 2015, 35, 82–93. [Google Scholar] [CrossRef]
- Ligler, F.S.; Taitt, C.R.; Shriver-Lake, L.C.; Sapsford, K.E.; Shubin, Y.; Golden, J.P. Array Biosensor for Detection of Toxins. Anal. Bioanal. Chem. 2003, 377, 469–477. [Google Scholar] [CrossRef]
- Esfahani Monfared, Y. Overview of Recent Advances in the Design of Plasmonic Fiber-Optic Biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef]
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Recent Development in Optical Fiber Biosensors. Sensors 2007, 7, 797–859. [Google Scholar] [CrossRef]
- Lyu, S.; Wu, Z.; Shi, X.; Wu, Q. Optical Fiber Biosensors for Protein Detection: A Review. Photonics 2022, 9, 987. [Google Scholar] [CrossRef]
- Monk, D.J.; Walt, D.R. Optical Fiber-Based Biosensors. Anal. Bioanal. Chem. 2004, 379, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, V. Fluorescent Probes; Elsevier Science & Technology: San Diego, CA, USA, 2021; ISBN 9780128235430. [Google Scholar]
- Dvorsky, M.; Munalli, D.; Qaseer, M.T.A.; Zoughi, R. Application of Microwave Polarimetry to the Characterization of Fiber Misalignment in Composites. IEEE Open J. Instrum. Meas. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Elsherif, M.; Salih, A.E.; Muñoz, M.G.; Alam, F.; AlQattan, B.; Antonysamy, D.S.; Zaki, M.F.; Yetisen, A.K.; Park, S.; Wilkinson, T.D.; et al. Optical Fiber Sensors: Working Principle, Applications, and Limitations. Adv. Photonics Res. 2022, 3, 2100371. [Google Scholar] [CrossRef]
- Leung, A.; Shankar, P.M.; Mutharasan, R. A Review of Fiber-Optic Biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
- Jin, Y.; Wong, K.H.; Granville, A.M. Developing Localized Surface Plasmon Resonance Biosensor Chips and Fiber Optics via Direct Surface Modification of PMMA Optical Waveguides. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 100–109. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. LI. A Theory of Light-Scattering in Liquids. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1928, 5, 498–512. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; Berlina, A.N.; Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Raman Scattering-Based Biosensing: New Prospects and Opportunities. Biosensors 2021, 11, 512. [Google Scholar] [CrossRef]
- Dumont, E.; De Bleye, C.; Sacré, P.-Y.; Netchacovitch, L.; Hubert, P.; Ziemons, E. From Near-Infrared and Raman to Surface-Enhanced Raman Spectroscopy: Progress, Limitations and Perspectives in Bioanalysis. Bioanalysis 2016, 8, 1077–1103. [Google Scholar] [CrossRef]
- Khetani, A. Photonic Crystal Fiber as a Robust Raman Biosensor; Université d’Ottawa/University of Ottawa: Ottawa, ON, Canada, 2016. [Google Scholar]
- Jahn, I.J.; Grjasnow, A.; John, H.; Weber, K.; Popp, J.; Hauswald, W. Noise Sources and Requirements for Confocal Raman Spectrometers in Biosensor Applications. Sensors 2021, 21, 5067. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; Van Duyne, R.P. Toward a Glucose Biosensor Based on Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Parandin, F.; Heidari, F.; Rahimi, Z.; Olyaee, S. Two-Dimensional Photonic Crystal Biosensors: A Review. Opt. Laser Technol. 2021, 144, 107397. [Google Scholar] [CrossRef]
- Gowdhami, D.; Balaji, V.R.; Murugan, M.; Robinson, S.; Hegde, G. Photonic Crystal Based Biosensors: An Overview. ISSS J. Micro Smart Syst. 2022, 11, 147–167. [Google Scholar] [CrossRef]
- Ayyanar, N.; Sreekanth, K.V.; Raja, G.T.; Rajan, M.S.M. Photonic Crystal Fiber-Based Reconfigurable Biosensor Using Phase Change Material. IEEE Trans. NanoBioscience 2021, 20, 338–344. [Google Scholar] [CrossRef]
- Zhuo, Y. Bioimaging with Photonic Crystal Enhanced Microscopy. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2014. [Google Scholar]
- Zhao, Y.; Zhao, X.; Gu, Z. Photonic Crystals in Bioassays. Adv. Funct. Mater. 2010, 20, 2970–2988. [Google Scholar] [CrossRef]
- Painter, O.; Vučkovič, J.; Scherer, A. Defect Modes of a Two-Dimensional Photonic Crystal in an Optically Thin Dielectric Slab. J. Opt. Soc. Am. B 1999, 16, 275. [Google Scholar] [CrossRef]
- Abadla, M.M.; Elsayed, H.A.; Mehaney, A. Novel Design for the Temperature Sensing Using Annular Photonic Crystals. Silicon 2021, 13, 4737–4745. [Google Scholar] [CrossRef]
- Getahun Kumela, A.; Belay Gemta, A.; Kebede Hordofa, A.; Birhanu, R.; Dagnaw Mekonnen, H.; Sherefedin, U.; Weldegiorgis, K. A Review on Hybridization of Plasmonic and Photonic Crystal Biosensors for Effective Cancer Cell Diagnosis. Nanoscale Adv. 2023, 5, 6382–6399. [Google Scholar] [CrossRef]
- Rafiee, E. Photonic Crystal Based Biosensor for Diagnosis of Kidney Failure and Diabetes. Plasmonics 2024, 19, 439–445. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and Applications of Thermal Biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Becker, J.A.; Green, C.B.; Pearson, G.L. Properties and Uses of Thermistors—Thermally Sensitive Resistors. Electr. Eng. 1946, 65, 711–725. [Google Scholar] [CrossRef]
- Wang, Z.; Kimura, M.; Ono, T. Manufacturing and Characterization of Simple Cantilever Thermal Biosensor with Si-Metal Thermocouple Structure for Enzymatic Reaction Detection. Thermochim. Acta 2018, 668, 110–115. [Google Scholar] [CrossRef]
- Yakovleva, M.; Bhand, S.; Danielsson, B. The Enzyme Thermistor—A Realistic Biosensor Concept. A Critical Review. Anal. Chim. Acta 2013, 766, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ye, Q.; Ren, Y.; Gao, B.; Wu, Y.; Zhao, X.; Ling, N.; Chen, M.; Ye, Y.; Wu, Q. Nanomaterial-Mediated Self-Calibrating Biosensors for Ultra-Precise Detection of Food Hazards: Recent Advances and New Horizons. Coord. Chem. Rev. 2025, 522, 216204. [Google Scholar] [CrossRef]
- Ku, Y.-F.; Huang, L.-S.; Yen, Y.-K. A Real-Time Thermal Self-Elimination Method for Static Mode Operated Freestanding Piezoresistive Microcantilever-Based Biosensors. Biosensors 2018, 8, 18. [Google Scholar] [CrossRef]
- Xie, B.; Mecklenburg, M.; Danielsson, B.; Öhman, O.; Norlin, P.; Winquist, F. Development of an Integrated Thermal Biosensor for the Simultaneous Determination of Multiple Analytes. Analyst 1995, 120, 155–160. [Google Scholar] [CrossRef]
- Lindquist, H.D.A. Microbial Biosensors for Recreational and Source Waters. J. Microbiol. Methods 2020, 177, 106059. [Google Scholar] [CrossRef]
- Ramanathan, K.; Jönsson, B.R.; Danielsson, B. Sol–Gel Based Thermal Biosensor for Glucose. Anal. Chim. Acta 2001, 427, 1–10. [Google Scholar] [CrossRef]
- Durmuş, N.G.; Lin, R.L.; Kozberg, M.; Dermici, D.; Khademhosseini, A.; Demirci, U. Acoustic-Based Biosensors. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer US: Boston, MA, USA, 2013; pp. 1–15. ISBN 9783642277580. [Google Scholar]
- Fogel, R.; Limson, J.; Seshia, A.A. Acoustic Biosensors. Essays Biochem. 2016, 60, 101–110. [Google Scholar] [CrossRef]
- Konstantopoulou, A.; Balatli, P. Acoustic Biosensor: A Brief Review. Achaiki Iatriki 2022, 41, 200–202. [Google Scholar]
- Pohanka, M. The Piezoelectric Biosensors: Principles and Applications, a Review. Int. J. Electrochem. Sci. 2017, 12, 496–506. [Google Scholar] [CrossRef]
- Mandal, D.; Indaleeb, M.M.; Younan, A.; Banerjee, S. Piezoelectric Point-of-Care Biosensor for the Detection of SARS-COV-2 (COVID-19) Antibodies. Sens. Bio-Sens. Res. 2022, 37, 100510. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Das, P.K.; Bhethanabotla, V.R. Surface Acoustic Waves in Biosensing Applications. Sens. Actuators Rep. 2021, 3, 100041. [Google Scholar] [CrossRef]
- Ngeh-Ngwainbi, J.; Suleiman, A.A.; Guilbault, G.G. Piezoelectric Crystal Biosensors. Biosens. Bioelectron. 1990, 5, 13–26. [Google Scholar] [CrossRef]
- Ramotti, M. Optimization of Shear Mode Solidly Mounted Resonators Based on Fully-Dielectric Acoustic Reflectors for Biosensing Applications. Bachelor’s Thesis, Politecnico di Torino, Torino, Italy, 2020. [Google Scholar]
- Development of Biosensors for Molecular Analysis—ProQuest. Available online: https://www.proquest.com/openview/f9e89b2f2f5eb06701ea2d3fee21a5a2/1?pq-origsite=gscholar&cbl=2026366 (accessed on 4 February 2025).
- Huang, S.; Gao, Y.; Hu, Y.; Shen, F.; Jin, Z.; Cho, Y. Recent Development of Piezoelectric Biosensors for Physiological Signal Detection and Machine Learning Assisted Cardiovascular Disease Diagnosis. RSC Adv. 2023, 13, 29174–29194. [Google Scholar] [CrossRef]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative Advantages of Mechanical Biosensors. Nat. Nanotech 2011, 6, 203–215. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Sahoo, S.; Konarde, M.S.; Rath, M.R.; Pattanaik, P.P. Chapter 8—Mechanical Biosensors. In Fundamentals of Biosensors in Healthcare; Hasnain, M.S., Nayak, A.K., Aminabhavi, T.M., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 191–210. ISBN 9780443216589. [Google Scholar]
- Fritz, J. Cantilever Biosensors. Analyst 2008, 133, 855. [Google Scholar] [CrossRef]
- Sang, S.; Zhao, Y.; Zhang, W.; Li, P.; Hu, J.; Li, G. Surface Stress-Based Biosensors. Biosens. Bioelectron. 2014, 51, 124–135. [Google Scholar] [CrossRef]
- Cali, K.; Tuccori, E.; Persaud, K.C. Chapter Eighteen—Gravimetric Biosensors. In Methods in Enzymology; Pelosi, P., Knoll, W., Eds.; Odorant Binding and Chemosensory Proteins; Academic Press: Cambridge, MA, USA, 2020; Volume 642, pp. 435–468. [Google Scholar]
- Tamayo, J.; Kosaka, P.M.; Ruz, J.J.; San Paulo, Á.; Calleja, M. Biosensors Based on Nanomechanical Systems. Chem. Soc. Rev. 2013, 42, 1287–1311. [Google Scholar] [CrossRef]
- Alvarez, M.; Lechuga, L.M. Microcantilever-Based Platforms as Biosensing Tools. Analyst 2010, 135, 827. [Google Scholar] [CrossRef]
- SoltanRezaee, M.; Bodaghi, M.; Farrokhabadi, A. A Thermosensitive Electromechanical Model for Detecting Biological Particles. Sci. Rep. 2019, 9, 11706. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, P.M.; Pini, V.; Ruz, J.J.; da Silva, R.A.; González, M.U.; Ramos, D.; Calleja, M.; Tamayo, J. Detection of Cancer Biomarkers in Serum Using a Hybrid Mechanical and Optoplasmonic Nanosensor. Nat. Nanotech 2014, 9, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Ali Tahir, M.; Khan, S.W.; Bajwa, Z.S. Nanosensors for Diagnosis with Optical, Electric and Mechanical Transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W. XIX. On the Electro-Dynamic Qualities of Metals:—Effects of Magnetization on the Electric Conductivity of Nickel and of Iron. Proc. R. Soc. Lond. 1856, 8, 546–550. [Google Scholar] [CrossRef]
- Kriz, C.B.; Rådevik, K.; Kriz, D. Magnetic permeability measurements in bioanalysis and biosensors. Anal. Chem. 1996, 68, 1966–1970. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.; Lee, H.; Weissleder, R. Magnetic Nanoparticle Biosensors. WIREs Nanomed. Nanobiotechnol 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Su, D.; Wu, K.; Saha, R.; Peng, C.; Wang, J.-P. Advances in Magnetoresistive Biosensors. Micromachines 2020, 11, 34. [Google Scholar] [CrossRef]
- Wu, K.; Tonini, D.; Liang, S.; Saha, R.; Chugh, V.K.; Wang, J.-P. Giant Magnetoresistance Biosensors in Biomedical Applications. ACS Appl. Mater. Interfaces 2022, 14, 9945–9969. [Google Scholar] [CrossRef]
- Schrittwieser, S.; Pelaz, B.; Parak, W.J.; Lentijo-Mozo, S.; Soulantica, K.; Dieckhoff, J.; Ludwig, F.; Guenther, A.; Tschöpe, A.; Schotter, J. Homogeneous Biosensing Based on Magnetic Particle Labels. Sensors 2016, 16, 828. [Google Scholar] [CrossRef]
- Jimenez, V.O.; Hwang, K.Y.; Nguyen, D.; Rahman, Y.; Albrecht, C.; Senator, B.; Thiabgoh, O.; Devkota, J.; Bui, V.D.A.; Lam, D.S.; et al. Magnetoimpedance Biosensors and Real-Time Healthcare Monitors: Progress, Opportunities, and Challenges. Biosensors 2022, 12, 517. [Google Scholar] [CrossRef]
- Lin, G.; Makarov, D.; Schmidt, O.G. Magnetic Sensing Platform Technologies for Biomedical Applications. Lab. Chip 2017, 17, 1884–1912. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Gaster, R.S.; Osterfeld, S.J.; Murmann, B.; Wang, S.X. GMR Biosensor Arrays: Correction Techniques for Reproducibility and Enhanced Sensitivity. Biosens. Bioelectron. 2010, 25, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liao, X.; Svedlindh, P.; Strömberg, M.; Wetterskog, E. Ferromagnetic Resonance Biosensor for Homogeneous and Volumetric Detection of DNA. ACS Sens. 2018, 3, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Llandro, J.; Palfreyman, J.J.; Ionescu, A.; Barnes, C.H.W. Magnetic Biosensor Technologies for Medical Applications: A Review. Med. Biol. Eng. Comput. 2010, 48, 977–998. [Google Scholar] [CrossRef]
- Piliarik, M.; Vaisocherová, H.; Homola, J. Surface Plasmon Resonance Biosensing. In Biosensors and Biodetection; Rasooly, A., Herold, K.E., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 65–88. ISBN 9781603275675. [Google Scholar]
- Zhang, Y.; Lai, J.; Wang, C.; Li, Z. Considerations for Improving the Performance of Surface Plasmon Resonance Biosensors. In Proceedings of the 2008 International Conference on BioMedical Engineering and Informatics, Sanya, China, 27–30 May 2008. [Google Scholar] [CrossRef]
- Chiu, N.-F. The Current Status and Future Promise of SPR Biosensors. Biosensors 2022, 12, 933. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Bolduc, O.R.; Pelletier, J.N.; Masson, J.-F. SPR Biosensing in Crude Serum Using Ultralow Fouling Binary Patterned Peptide SAM. Anal. Chem. 2010, 82, 3699–3706. [Google Scholar] [CrossRef]
- Gaudreault, J.; Forest-Nault, C.; De Crescenzo, G.; Durocher, Y.; Henry, O. On the Use of Surface Plasmon Resonance-Based Biosensors for Advanced Bioprocess Monitoring. Processes 2021, 9, 1996. [Google Scholar] [CrossRef]
- Naimushin, A.N.; Spinelli, C.B.; Soelberg, S.D.; Mann, T.; Stevens, R.C.; Chinowsky, T.; Kauffman, P.; Yee, S.; Furlong, C.E. Airborne Analyte Detection with an Aircraft-Adapted Surface Plasmon Resonance Sensor System. Sens. Actuators B Chem. 2005, 104, 237–248. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface Plasmon Resonance (SPR) Biosensors for Food Allergen Detection in Food Matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef]
- Yakes, B.J.; Buijs, J.; Elliott, C.T.; Campbell, K. Surface Plasmon Resonance Biosensing: Approaches for Screening and Characterising Antibodies for Food Diagnostics. Talanta 2016, 156–157, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pellacani, P.; Van Beek, T.A.; Zuilhof, H.; Nielen, M.W.F. Surface Characterization and Antifouling Properties of Nanostructured Gold Chips for Imaging Surface Plasmon Resonance Biosensing. Sens. Actuators B Chem. 2015, 209, 505–514. [Google Scholar] [CrossRef]
- Syed Nor, S.N.; Rasanang, N.S.; Karman, S.; Zaman, W.S.W.K.; Harun, S.W.; Arof, H. A Review: Surface Plasmon Resonance-Based Biosensor for Early Screening of SARS-CoV-2 Infection. IEEE Access 2022, 10, 1228–1244. [Google Scholar] [CrossRef]
- Ermini, M.L.; Mariani, S.; Scarano, S.; Minunni, M. Bioanalytical Approaches for the Detection of Single Nucleotide Polymorphisms by Surface Plasmon Resonance Biosensors. Biosens. Bioelectron. 2014, 61, 28–37. [Google Scholar] [CrossRef]
- Giouroudi, I.; Kokkinis, G. Recent Advances in Magnetic Microfluidic Biosensors. Nanomaterials 2017, 7, 171. [Google Scholar] [CrossRef]
- Lafleur, J.P.; Jönsson, A.; Senkbeil, S.; Kutter, J.P. Recent Advances in Lab-on-a-Chip for Biosensing Applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef]
- Choi, S.; Goryll, M.; Sin, L.Y.M.; Wong, P.K.; Chae, J. Microfluidic-Based Biosensors toward Point-of-Care Detection of Nucleic Acids and Proteins. Microfluid. Nanofluid 2011, 10, 231–247. [Google Scholar] [CrossRef]
- Reenen, A.v.; Jong, A.M.d.; Toonder, J.M.J.d.; Prins, J.M.W. Integrated Lab-on-Chip Biosensing Systems Based on Magnetic Particle Actuation—A Comprehensive Review. Lab. A Chip 2014, 14, 1966–1986. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Khan, S.; F Didar, T. Bio-Functionalization of Microfluidic Platforms Made of Thermoplastic Materials: A Review. Anal. Chim. Acta 2022, 1209, 339283. [Google Scholar] [CrossRef]
- Chenani, H.; Saeidi, M.; Rastkhiz, M.A.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and Advances of Hydrogel-Based Wearable Electrochemical Biosensors for Real-Time Monitoring of Biofluids: From Lab to Market. A Review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef]
- Prakash, S.; Pinti, M.; Bhushan, B. Theory, Fabrication and Applications of Microfluidic and Nanofluidic Biosensors. Phil. Trans. R. Soc. A 2012, 370, 2269–2303. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1900451. [Google Scholar] [CrossRef]
- Su, F.; Ozev, S.; Chakrabarty, K. Ensuring the Operational Health of Droplet-Based Microelectrofluidic Biosensor Systems. IEEE Sens. J. 2005, 5, 763–773. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble Metal Nanoparticles in Biosensors: Recent Studies and Applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef]
- Geng, H.; Vilms Pedersen, S.; Ma, Y.; Haghighi, T.; Dai, H.; Howes, P.D.; Stevens, M.M. Noble Metal Nanoparticle Biosensors: From Fundamental Studies toward Point-of-Care Diagnostics. Acc. Chem. Res. 2022, 55, 593–604. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Engelbrekt, C.; Zhang, J.; Ulstrup, J.; Kusk, K.O.; Baun, A. The Challenges of Testing Metal and Metal Oxide Nanoparticles in Algal Bioassays: Titanium Dioxide and Gold Nanoparticles as Case Studies. Nanotoxicology 2013, 7, 1082–1094. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Špačková, B.; Wrobel, P.; Bocková, M.; Homola, J. Optical Biosensors Based on Plasmonic Nanostructures: A Review. Proc. IEEE 2016, 104, 2380–2408. [Google Scholar] [CrossRef]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.I.; Moulton, S.E.; Guijt, R.M.; Greene, G.W.; et al. Antifouling Strategies for Electrochemical Biosensing: Mechanisms and Performance toward Point of Care Based Diagnostic Applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef] [PubMed]
- Yeom, S.-H. Nanostructures in Biosensor-A Review. Front. Biosci. 2011, 16, 997. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.A.; Goggins, S.; Frost, C.G. Ratiometric Electrochemistry: Improving the Robustness, Reproducibility and Reliability of Biosensors. Molecules 2021, 26, 2130. [Google Scholar] [CrossRef]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical Detection of Nucleic Acids, Proteins, Small Molecules and Cells Using a DNA-Nanostructure-Based Universal Biosensing Platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Y. Surface Modifications Technology of Quantum Dots Based Biosensors and Their Medical Applications. Chin. J. Anal. Chem. 2014, 42, 1061–1069. [Google Scholar] [CrossRef]
- Ma, F.; Li, C.; Zhang, C. Development of Quantum Dot-Based Biosensors: Principles and Applications. J. Mater. Chem. B 2018, 6, 6173–6190. [Google Scholar] [CrossRef]
- Das, S.; Mazumdar, H.; Khondakar, K.R.; Mishra, Y.K.; Kaushik, A. Review—Quantum Biosensors: Principles and Applications in Medical Diagnostics. ECS Sens. Plus 2024, 3, 025001. [Google Scholar] [CrossRef]
- Kang, T.; Um, K.; Park, J.; Chang, H.; Lee, D.C.; Kim, C.-K.; Lee, K. Minimizing the Fluorescence Quenching Caused by Uncontrolled Aggregation of CdSe/CdS Core/Shell Quantum Dots for Biosensor Applications. Sens. Actuators B Chem. 2016, 222, 871–878. [Google Scholar] [CrossRef]
- Wegner, K.D.; Hildebrandt, N. Quantum Dots: Bright and Versatile in Vitro and in Vivo Fluorescence Imaging Biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef]
- Hottechamps, J.; Noblet, T.; Brans, A.; Humbert, C.; Dreesen, L. How Quantum Dots Aggregation Enhances Förster Resonant Energy Transfer. ChemPhysChem 2020, 21, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, W.; Chen, J.; Yu, C. A Graphene Quantum Dot-Based Method for the Highly Sensitive and Selective Fluorescence Turn on Detection of Biothiols. Talanta 2014, 119, 538–543. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Ward, C.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; Argyle, D. Implantable Biosensors and Their Contribution to the Future of Precision Medicine. Vet. J. 2018, 239, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.S.; Gifford, R. Biosensors for Real-Time in Vivo Measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef]
- Koschwanez, H.E.; Reichert, W.M. In Vitro, in Vivo and Post Explantation Testing of Glucose-Detecting Biosensors: Current Methods and Recommendations. Biomaterials 2007, 28, 3687–3703. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Tomazos, I.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Emerging Synergy between Nanotechnology and Implantable Biosensors: A Review. Biosens. Bioelectron. 2010, 25, 1553–1565. [Google Scholar] [CrossRef]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S. Biocompatible Material-Based Flexible Biosensors: From Materials Design to Wearable/Implantable Devices and Integrated Sensing Systems. Small 2023, 19, 2207879. [Google Scholar] [CrossRef]
- Scholten, K.; Meng, E. A Review of Implantable Biosensors for Closed-Loop Glucose Control and Other Drug Delivery Applications. Int. J. Pharm. 2018, 544, 319–334. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- Wu, K.Y.; Mina, M.; Carbonneau, M.; Marchand, M.; Tran, S.D. Advancements in Wearable and Implantable Intraocular Pressure Biosensors for Ophthalmology: A Comprehensive Review. Micromachines 2023, 14, 1915. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.; Hassanien, A.E. Wearable and Implantable Wireless Sensor Network Solutions for Healthcare Monitoring. Sensors 2011, 11, 5561–5595. [Google Scholar] [CrossRef] [PubMed]
- Makarovaite, V. Developing Biosensor Technology to Monitor Biofilm Formation on Voice Prosthesis in Throat Cancer Patients Following Total Laryngectomy A dissertation submitted to the University of Kent for the Degree of Doctor of Philosophy in the Faculty of Electronic Engineering. 2019. Available online: https://kar.kent.ac.uk/82054/1/276Makarovaite_Thesis.pdf (accessed on 30 November 2024).
- Schackart, K.E.; Yoon, J.-Y. Machine Learning Enhances the Performance of Bioreceptor-Free Biosensors. Sensors 2021, 21, 5519. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Pan, Y.; Kou, W.-B.; Feng, H.; Zhao, Y.; Zhou, K.; Zhong, S. Generalization of a Deep Learning Model for Continuous Glucose Monitoring–Based Hypoglycemia Prediction: Algorithm Development and Validation Study. JMIR Med. Inf. 2024, 12, 56909. [Google Scholar] [CrossRef]
- Anapanani, G. Machine Learning for Biosensors. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2023. 12118. Available online: https://researchrepository.wvu.edu/etd/12118 (accessed on 30 November 2024).
- Yu, J. Academic Performance Prediction Method of Online Education Using Random Forest Algorithm and Artificial Intelligence Methods. EBSCOhost. Available online: https://openurl.ebsco.com/contentitem/doi:10.3991%2Fijet.v16i05.20297?sid=ebsco:plink:crawler&id=ebsco:doi:10.3991%2Fijet.v16i05.20297 (accessed on 9 February 2025).
- Wekalao, J.; Mandela, N.; Selvam, A.K.; Venugopal, S.; Ravi, D.; Pandian, P.; Babu, A.J.; Leon, M.L.; Rashed, A.N.Z. Graphene Metasurface Based Biosensor for COVID-19 Detection in the Terahertz Regime with Machine Learning Optimization Using K-Nearest Neighbours Regression. Plasmonics 2024. [Google Scholar] [CrossRef]
- Wekalao, J.; Mandela, N. Graphene Metasurface-Based Biosensor for Direct Dopamine Detection Utilizing Surface Plasmon Resonance in the Terahertz Regime with Machine Learning Optimization via K-Nearest Neighbors Regression. Plasmonics 2024. [Google Scholar] [CrossRef]
- Kramer, O. K-Nearest Neighbors. In Dimensionality Reduction with Unsupervised Nearest Neighbors; Kramer, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 13–23. ISBN 9783642386527. [Google Scholar]
- Zhang, Z. Introduction to Machine Learning: K-Nearest Neighbors. Ann. Transl. Med. 2016, 4, 218. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Panigrahi, D.; Rewatkar, P.; Haick, H. Role of Machine Learning Assisted Biosensors in Point-of-Care-Testing For Clinical Decisions. ACS Sens. 2024, 9, 4495–4519. [Google Scholar] [CrossRef]
- Kokabi, M.; Tahir, M.N.; Singh, D.; Javanmard, M. Advancing Healthcare: Synergizing Biosensors and Machine Learning for Early Cancer Diagnosis. Biosensors 2023, 13, 884. [Google Scholar] [CrossRef]
- Trishna, T.I.; Emon, S.U.; Ema, R.R.; Sajal, G.I.H.; Kundu, S.; Islam, T. Detection of Hepatitis (A, B, C and E) Viruses Based on Random Forest, K-Nearest and Naïve Bayes Classifier. In Proceedings of the 2019 10th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kanpur, India, 6–8 July 2019; pp. 1–7. [Google Scholar]
- Flach, P.A.; Lachiche, N. Naive Bayesian Classification of Structured Data. Mach. Learn. 2004, 57, 233–269. [Google Scholar] [CrossRef]
- Ren, J.; Lee, S.D.; Chen, X.; Kao, B.; Cheng, R.; Cheung, D. Naive Bayes Classification of Uncertain Data. In Proceedings of the 2009 Ninth IEEE International Conference on Data Mining, Miami Beach, FL, USA, 6–9 December 2009; pp. 944–949. [Google Scholar]
- Zhang, M.-L.; Peña, J.M.; Robles, V. Feature Selection for Multi-Label Naive Bayes Classification. Inf. Sci. 2009, 179, 3218–3229. [Google Scholar] [CrossRef]
- Probst, P.; Boulesteix, A.-L. To Tune or Not to Tune the Number of Trees in Random Forest. J. Mach. Learn. Res. 2018, 18, 1–18. [Google Scholar]
- Hooker, G.; Mentch, L. Bootstrap Bias Corrections for Ensemble Methods. Stat. Comput. 2018, 28, 77–86. [Google Scholar] [CrossRef]
- Barreñada, L.; Dhiman, P.; Timmerman, D.; Boulesteix, A.-L.; Calster, B.V. Understanding Overfitting in Random Forest for Probability Estimation: A Visualization and Simulation Study. Diagn. Progn. Res. 2024, 8, 14. [Google Scholar] [CrossRef]
- Hasan, M.A.M.; Nasser, M.; Ahmad, S.; Molla, K.I. Feature Selection for Intrusion Detection Using Random Forest. JIS 2016, 7, 129–140. [Google Scholar] [CrossRef]
- Khoshgoftaar, T.M.; Golawala, M.; Hulse, J.V. An Empirical Study of Learning from Imbalanced Data Using Random Forest. In Proceedings of the 19th IEEE International Conference on Tools with Artificial Intelligence (ICTAI 2007), Patras, Greece, 29–31 October 2007; Volume 2, pp. 310–317. [Google Scholar]
- Toloşi, L.; Lengauer, T. Classification with Correlated Features: Unreliability of Feature Ranking and Solutions. Bioinformatics 2011, 27, 1986–1994. [Google Scholar] [CrossRef]
- Rhodes, C.; Morari, M. The False Nearest Neighbors Algorithm: An Overview. Comput. Chem. Eng. 1997, 21, S1149–S1154. [Google Scholar] [CrossRef]
- Casanova, G.; Englmeier, E.; Houle, M.E.; Kröger, P.; Nett, M.; Schubert, E.; Zimek, A. Dimensional Testing for Reverse k -Nearest Neighbor Search. Proc. VLDB Endow. 2017, 10, 769–780. [Google Scholar] [CrossRef]
- Steenwijk, M.D.; Pouwels, P.J.W.; Daams, M.; van Dalen, J.W.; Caan, M.W.A.; Richard, E.; Barkhof, F.; Vrenken, H. Accurate White Matter Lesion Segmentation by k Nearest Neighbor Classification with Tissue Type Priors (kNN-TTPs). NeuroImage Clin. 2013, 3, 462–469. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, Q.; Dezert, J. A New Belief-Based K-Nearest Neighbor Classification Method. Pattern Recognit. 2013, 46, 834–844. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Li, P.; Zhu, F. Outlier Detection Algorithm Based on K-Nearest Neighbors-Local Outlier Factor. J. Algorithms Comput. Technol. 2022, 16, 17483026221078111. [Google Scholar] [CrossRef]
- Alizad Rahvar, A.R.; Ardakani, M. Boundary Effect Correction in k -Nearest-Neighbor Estimation. Phys. Rev. E 2011, 83, 051121. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, I.; Kalutarage, H. Naive Bayes: Applications, Variations and Vulnerabilities: A Review of Literature with Code Snippets for Implementation. Soft Comput. 2021, 25, 2277–2293. [Google Scholar] [CrossRef]
- Zhang, H. Exploring conditions for the optimality of naïve Bayes. Int. J. Patt. Recogn. Artif. Intell. 2005, 19, 183–198. [Google Scholar] [CrossRef]
- Wang, X.-Z.; He, Y.-L.; Wang, D.D. Non-Naive Bayesian Classifiers for Classification Problems with Continuous Attributes. IEEE Trans. Cybern. 2014, 44, 21–39. [Google Scholar] [CrossRef]
- Rennie, J.D.M.; Shih, L.; Teevan, J.; Karger, D.R. Tackling the Poor Assumptions of Naive Bayes Text Classifiers. In Proceedings of the Twentieth International Conference on International Conference on Machine Learning, Washington, DC, USA, 21–24 August 2003; AAAI Press: Menlo Park, CA, USA; pp. 616–623. [Google Scholar]
- Lowd, D.; Domingos, P. Naive Bayes Models for Probability Estimation. In Proceedings of the 22nd International Conference on Machine Learning, Bonn, Germany, 7–11 August 2005; Association for Computing Machinery: New York, NY, USA; pp. 529–536. [Google Scholar]
- Thabtah, F.; Hammoud, S.; Kamalov, F.; Gonsalves, A. Data Imbalance in Classification: Experimental Evaluation. Inf. Sci. 2020, 513, 429–441. [Google Scholar] [CrossRef]
- Sykes, A.O. An Introduction to Regression Analysis; Coase-Sandor Institute for Law & Economics Working Paper No. 20; University of Chicago Law School: Chicago, IL, USA, 1993. [Google Scholar]
- Alexopoulos, E.C. Introduction to Multivariate Regression Analysis. Hippokratia 2010, 14, 23–28. [Google Scholar]
- Leatherbarrow, R.J. Using Linear and Non-Linear Regression to Fit Biochemical Data. Trends Biochem. Sci. 1990, 15, 455–458. [Google Scholar] [CrossRef]
- Zhou, H.; Li, L. Regularized Matrix Regression. J. R. Stat. Soc. Ser. B Stat. Methodol. 2014, 76, 463–483. [Google Scholar] [CrossRef]
- Wakefield, J. Bayesian and Frequentist Regression Methods; Springer Series in Statistics; Springer: New York, NY, USA, 2013; ISBN 9781441909244. [Google Scholar]
- Reddy, T.A.; Henze, G.P. Parametric and Non-Parametric Regression Methods. In Applied Data Analysis and Modeling for Energy Engineers and Scientists; Reddy, T.A., Henze, G.P., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 355–407. ISBN 9783031348693. [Google Scholar]
- Stoltzfus, J.C. Logistic Regression: A Brief Primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Alabsi, B.A.; Wekalao, J.; Dhivya, R.; Kouki, M.; Almawgani, A.H.M.; Patel, S.K. Machine Learning Optimized Optical Surface Plasmon Resonance Biosensor Using Locally Weighted Linear Regression for Rapid and Accurate Detection of Tuberculosis Biomarkers. Plasmonics 2025. [Google Scholar] [CrossRef]
- Dhandapani, G.; Wekalao, J.; Patel, S.K.; Al-zahrani, F.A. Design and Analysis of a Highly Sensitive Terahertz Biosensor Using Graphene Metasurfaces and Surface Plasmon Resonance for Protein Detection with AI-Assisted Locally Weighted Linear Regression for Behavior Prediction. Plasmonics 2024. [Google Scholar] [CrossRef]
- Wekalao, J.; Patel, S.K.; Khalifa, S.B.; Chebaane, S.; Armghan, A.; Saidani, T. Optical-Based Aqueous Solution Detection by Graphene Metasurface Surface Plasmon Resonance Biosensor with Behavior Prediction Using Polynomial Regression. Plasmonics 2024. [Google Scholar] [CrossRef]
- Patel, S.K.; Surve, J.; Parmar, J.; Natesan, A.; Katkar, V. Graphene-Based Metasurface Refractive Index Biosensor for Hemoglobin Detection: Machine Learning Assisted Optimization. IEEE Trans. Nanobioscience 2023, 22, 430–437. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, A.; Goel, A. Maharaja Agrasen Institute of Technology/Guru Gobind Singh Indraprastha University Review of Regression Analysis Models. IJERT 2017, V6, IJERTV6IS080060. [Google Scholar] [CrossRef]
- Slinker, B.K.; Glantz, S.A. Multiple Regression for Physiological Data Analysis: The Problem of Multicollinearity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1985, 249, R1–R12. [Google Scholar] [CrossRef]
- Kapoor, S.; Narayanan, A. Leakage and the Reproducibility Crisis in Machine-Learning-Based Science. Patterns 2023, 4, 100804. [Google Scholar] [CrossRef]
- Li, K.-C.; Duan, N. Regression Analysis Under Link Violation. Ann. Stat. 1989, 17, 1009–1052. [Google Scholar] [CrossRef]
- Long, J.S.; Ervin, L.H. Using Heteroscedasticity Consistent Standard Errors in the Linear Regression Model. Am. Stat. 2000, 54, 217–224. [Google Scholar] [CrossRef]
- Purdon, P.L.; Weisskoff, R.M. Effect of Temporal Autocorrelation Due to Physiological Noise and Stimulus Paradigm on Voxel-Level False-Positive Rates in fMRI. Hum. Brain Mapp. 1998, 6, 239–249. [Google Scholar] [CrossRef]
- Spruill, M.C. Good Designs for Polynomial Extrapolation. J. Stat. Plan. Inference 1990, 26, 149–159. [Google Scholar] [CrossRef]
- Follum, J.; Pierre, J.W. Initial Results in the Detection and Estimation of Forced Oscillations in Power Systems. In Proceedings of the 2013 North American Power Symposium (NAPS), Manhattan, KS, USA, 22–24 September 2013; pp. 1–6. [Google Scholar]
- Meinshausen, N.; Meier, L.; Bühlmann, P. P-Values for High-Dimensional Regression. J. Am. Stat. Assoc. 2009, 104, 1671–1681. [Google Scholar] [CrossRef]
- Westad, F.; Marini, F. Variable Selection and Redundancy in Multivariate Regression Models. Front. Anal. Sci. 2022, 2, 897605. [Google Scholar] [CrossRef]
- Shi, L.; Westerhuis, J.A.; Rosén, J.; Landberg, R.; Brunius, C. Variable Selection and Validation in Multivariate Modelling. Bioinformatics 2019, 35, 972–980. [Google Scholar] [CrossRef]
- Fu, Z.; Parikh, C.R.; Zhou, B. Penalized Variable Selection in Competing Risks Regression. Lifetime Data Anal. 2017, 23, 353–376. [Google Scholar] [CrossRef]
- McNeish, D.M. Using Lasso for Predictor Selection and to Assuage Overfitting: A Method Long Overlooked in Behavioral Sciences. Multivar. Behav. Res. 2015, 50, 471–484. [Google Scholar] [CrossRef]
- Pannu, J.; Billor, N. Robust Group-Lasso for Functional Regression Model. Commun. Stat. Simul. Comput. 2015, 46, 3356–3374. [Google Scholar] [CrossRef]
- Holla, L.; Kavitha, K.S. An Improved Fake News Detection Model Using Hybrid Time Frequency-Inverse Document Frequency for Feature Extraction and AdaBoost Ensemble Model as a Classifier. JAIT 2024, 15, 202–211. [Google Scholar] [CrossRef]
- Arpit, D.; Wu, S.; Natarajan, P.; Prasad, R.; Natarajan, P. Ridge Regression Based Classifiers for Large Scale Class Imbalanced Datasets. In Proceedings of the 2013 IEEE Workshop on Applications of Computer Vision (WACV), Clearwater Beach, FL, USA, 15–17 January 2013; pp. 267–274. [Google Scholar]
- Nikolaenko, V.; Weinsberg, U.; Ioannidis, S.; Joye, M.; Boneh, D.; Taft, N. Privacy-Preserving Ridge Regression on Hundreds of Millions of Records. In Proceedings of the 2013 IEEE Symposium on Security and Privacy, Berkeley, CA, USA, 19–22 May 2013; pp. 334–348. [Google Scholar]
- Smith, G.; Campbell, F. A Critique of Some Ridge Regression Methods. J. Am. Stat. Assoc. 1980, 75, 74–81. [Google Scholar] [CrossRef]
- Contreras, P.; Murtagh, F. Hierarchical Clustering. In Handbook of Cluster Analysis; Chapman and Hall/CRC: Boca Raton, FL, USA, 2015; ISBN 9780429185472. [Google Scholar]
- Borro, M.; Gentile, G.; Stigliano, A.; Misiti, S.; Toscano, V.; Simmaco, M. Proteomic Analysis of Peripheral T Lymphocytes, Suitable Circulating Biosensors of Strictly Related Diseases. Clin. Exp. Immunol. 2007, 150, 494–501. [Google Scholar] [CrossRef]
- Sherrill, D.M.; Moy, M.L.; Reilly, J.J.; Bonato, P. Using Hierarchical Clustering Methods to Classify Motor Activities of COPD Patients from Wearable Sensor Data. J. Neuroeng. Rehabil. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Cluster Analysis and K-Means Clustering: An Introduction. In Advances in K-Means Clustering: A Data Mining Thinking; Wu, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–16. ISBN 9783642298073. [Google Scholar]
- Li, Y.; Cui, Z.; Wang, Z.; Shi, L.; Zhuo, J.; Yan, S.; Ji, Y.; Wang, Y.; Zhang, D.; Wang, J. Machine-Learning-Assisted Aggregation-Induced Emissive Nanosilicon-Based Sensor Array for Point-of-Care Identification of Multiple Foodborne Pathogens. Anal. Chem. 2024, 96, 6588–6598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Long, A.; Han, W. Electrochemical Biosensor with Machine Learning. In Electrochemical Biosensors for Whole Blood Analysis; Xia, F., Li, H., Li, S., Lou, X., Eds.; Springer Nature: Singapore, 2023; pp. 253–267. ISBN 9789819956449. [Google Scholar]
- Kasa, S.R.; Rajan, V. Avoiding Inferior Clusterings with Misspecified Gaussian Mixture Models. Sci. Rep. 2023, 13, 19164. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.; Kushwaha, D.S. Clustering Cloud Workloads: K-Means vs Gaussian Mixture Model. Procedia Comput. Sci. 2020, 171, 158–167. [Google Scholar] [CrossRef]
- Mozgovoy, V. Longitudinal Estimation of Stress-Related States through Bio-Sensor Data. Appl. Comput. Inform. 2021. ahead-of-print. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; Mesen-Porras, S.; Quiros-Fernandez, I.; Kop-Montero, M.; Rojas-Cespedes, A.; Quiros, S.; Siles, F.; Mora, R. Sphingolipid Pathway as a Biosensor of Cancer Chemosensitivity: A Proof of Principle. Uniciencia 2022, 36, 1–15. [Google Scholar] [CrossRef]
- Carvalho, P.R.; Munita, C.S.; Lapolli, A.L. Validity Studies among Hierarchical Methods of Cluster Analysis Using Cophenetic Correlation Coefficient. Braz. J. Radiat. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Salvador, S.; Chan, P. Determining the Number of Clusters/Segments in Hierarchical Clustering/Segmentation Algorithms. In Proceedings of the 16th IEEE International Conference on Tools with Artificial Intelligence, Boca Raton, FL, USA, 15–17 November 2004; pp. 576–584. [Google Scholar]
- Zhang, Y.; Liu, G.; Zheng, L.; Yan, C. A Hierarchical Clustering Strategy of Processing Class Imbalance and Its Application in Fraud Detection. In Proceedings of the 2019 IEEE 21st International Conference on High Performance Computing and Communications; IEEE 17th International Conference on Smart City; IEEE 5th International Conference on Data Science and Systems (HPCC/SmartCity/DSS), Zhangjiajie, China, 10–12 August 2019; pp. 1810–1816. [Google Scholar]
- Yong, Y. The Research of Imbalanced Data Set of Sample Sampling Method Based on K-Means Cluster and Genetic Algorithm. Energy Procedia 2012, 17, 164–170. [Google Scholar] [CrossRef]
- Fang, C.; Jin, W.; Ma, J. Means Algorithms for Clustering Analysis with Frequency Sensitive Discrepancy Metrics. Pattern Recognit. Lett. 2013, 34, 580–586. [Google Scholar] [CrossRef]
- Ismi, D.P.; Panchoo, S.; Murinto, M. K-Means Clustering Based Filter Feature Selection on High Dimensional Data. Int. J. Adv. Intell. Inform. 2016, 2, 38–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, S.; Dai, S.; Luo, L.; Zhu, E.; Xu, H.; Zhu, X.; Yao, C.; Zhou, H. Gaussian Mixture Model Clustering with Incomplete Data. ACM Trans. Multimed. Comput. Commun. Appl. 2021, 17, 1–14. [Google Scholar] [CrossRef]
- Zhuang, X.; Huang, Y.; Palaniappan, K.; Zhao, Y. Gaussian Mixture Density Modeling, Decomposition, and Applications. IEEE Trans. Image Process. 1996, 5, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, S. Measuring the Component Overlapping in the Gaussian Mixture Model. Data Min. Knowl. Disc 2011, 23, 479–502. [Google Scholar] [CrossRef]
- Hu, P.; Gao, W.; Li, Y.; Wu, M.; Hua, F.; Qiao, L. Detection of False Data Injection Attacks in Smart Grids Based on Expectation Maximization. Sensors 2023, 23, 1683. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal Component Analysis. Nat. Rev. Methods Primers 2022, 2, 1–21. [Google Scholar] [CrossRef]
- Duhok Polytechnic University; Salih Hasan, B.M.; Abdulazeez, A.M. A Review of Principal Component Analysis Algorithm for Dimensionality Reduction. JSCDM 2021, 2, 20–30. [Google Scholar] [CrossRef]
- Yan, Z.; Hao, X. Effective Application of Biosensor Analytical Techniques in Drug Testing. Appl. Math. Nonlinear Sci. 2024, 9. [Google Scholar] [CrossRef]
- Qureshi, R.; Irfan, M.; Ali, H.; Khan, A.; Nittala, A.S.; Ali, S.; Shah, A.; Gondal, T.M.; Sadak, F.; Shah, Z.; et al. Artificial Intelligence and Biosensors in Healthcare and Its Clinical Relevance: A Review. IEEE Access 2023, 11, 61600–61620. [Google Scholar] [CrossRef]
- Feng, S.; Chen, R.; Lin, J.; Pan, J.; Chen, G.; Li, Y.; Cheng, M.; Huang, Z.; Chen, J.; Zeng, H. Nasopharyngeal Cancer Detection Based on Blood Plasma Surface-Enhanced Raman Spectroscopy and Multivariate Analysis. Biosens. Bioelectron. 2010, 25, 2414–2419. [Google Scholar] [CrossRef]
- D’Alvia, L.; Carraro, S.; Peruzzi, B.; Urciuoli, E.; Apa, L.; Rizzuto, E. A Principal Component Analysis to Detect Cancer Cell Line Aggressiveness. ACTA IMEKO 2023, 12, 1–7. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Brown, A.S. Principal Component Analysis as an Outlier Detection Tool for Polycyclic Aromatic Hydrocarbon Concentrations in Ambient Air. Water Air Soil. Pollut. 2012, 223, 3807–3816. [Google Scholar] [CrossRef]
- Jia, F.; Martin, E.B.; Morris, A.J. Non-Linear Principal Components Analysis with Application to Process Fault Detection. Int. J. Syst. Sci. 2000, 31, 1473–1487. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Chin, W.-L. Blind False Data Injection Attack Using PCA Approximation Method in Smart Grid. IEEE Trans. Smart Grid 2015, 6, 1219–1226. [Google Scholar] [CrossRef]
- Candès, E.J.; Li, X.; Ma, Y.; Wright, J. Robust Principal Component Analysis? J. ACM 2011, 58, 11:1–11:37. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep Learning in Neural Networks: An Overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Aggarwal, C.C. Neural Networks and Deep Learning: A Textbook; Springer International Publishing AG: Cham, Switzerland, 2018; ISBN 9783319944623. [Google Scholar]
- Salman, S.; Liu, X. Overfitting Mechanism and Avoidance in Deep Neural Networks. arXiv 2019, arXiv:1901.06566. [Google Scholar]
- Bekker, A.J.; Goldberger, J. Training Deep Neural-Networks Based on Unreliable Labels. In Proceedings of the 2016 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Shanghai, China, 20–25 March 2016; pp. 2682–2686. [Google Scholar]
- Koutsoukas, A.; Monaghan, K.J.; Li, X.; Huan, J. Deep-Learning: Investigating Deep Neural Networks Hyper-Parameters and Comparison of Performance to Shallow Methods for Modeling Bioactivity Data. J. Cheminform 2017, 9, 42. [Google Scholar] [CrossRef]
- Warr, K. Strengthening Deep Neural Networks: Making AI Less Susceptible to Adversarial Trickery, 1st ed.; O’Reilly Media: Sebastopol, CA, USA, 2019; ISBN 9781492044956. [Google Scholar]
- Nusrat, I.; Jang, S.-B. A Comparison of Regularization Techniques in Deep Neural Networks. Symmetry 2018, 10, 648. [Google Scholar] [CrossRef]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning, Second Edition: An Introduction; MIT Press: Cambridge, MA, USA, 2018; ISBN 9780262039246. [Google Scholar]
- Zhang, K.; Wang, J.; Liu, T.; Luo, Y.; Loh, X.J.; Chen, X. Machine Learning-Reinforced Noninvasive Biosensors for Healthcare. Adv. Healthc. Mater. 2021, 10, 2100734. [Google Scholar] [CrossRef]
- Lyle, C.; Zhang, A.; Jiang, M.; Pineau, J.; Gal, Y. Resolving Causal Confusion in Reinforcement Learning via Robust Exploration; 9 March 2021. Available online: https://openreview.net/pdf?id=DKCXncD4Xtq (accessed on 4 December 2024).
- Aslan, Y. Use of Sample-Splitting and Cross-Fitting Techniques to Mitigate the Risks of Double-Dipping in Behaviour-Agnostic Reinforcement Learning. Bachelor Thesis, Delft University of Technology, Delft, The Netherlands, 2024. [Google Scholar]
- Lavie, O.; Shabtai, A.; Katz, G. A Transferable and Automatic Tuning of Deep Reinforcement Learning for Cost Effective Phishing Detection. arXiv 2022, arXiv:2209.09033. [Google Scholar]
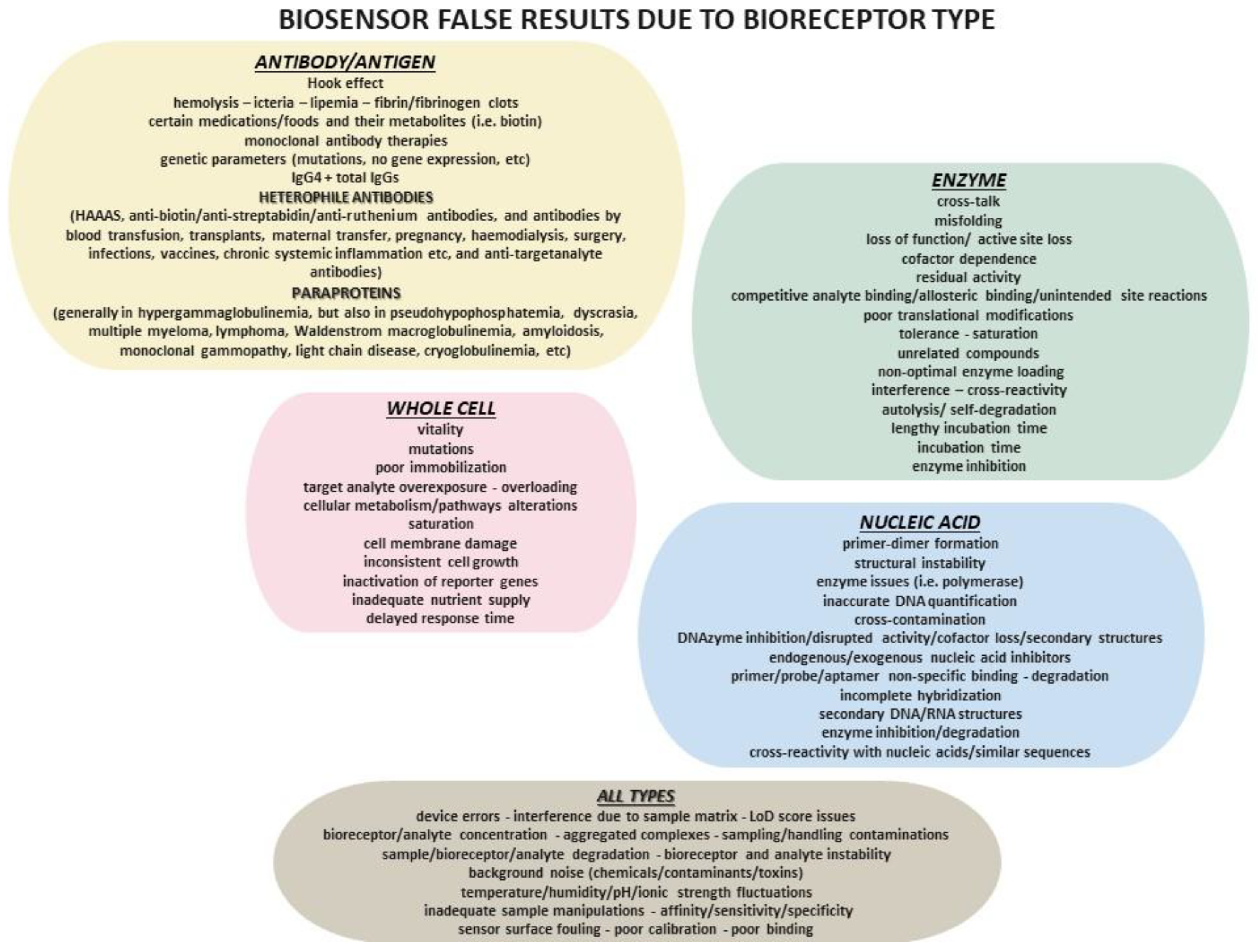
| Generation | Key Features | Examples | Technological Advancements |
|---|---|---|---|
| 1st | basic integration of biological and physicochemical components for basically qualitative/semi-quantitative measurements | enzyme-based biosensors | simple early-stage biosensors on direct biological interactions |
| 2nd | better sensitivity and selectivity with a focus on quantitative measurements | immunosensors, DNA sensors | usage of more refined biological recognition elements like antibodies |
| 3rd | incorporation of advanced materials and nanotechnology, with miniaturization and higher specificity | nanobiosensors, lab-on-a-chip | boosted sensor surface chemistry or microfluidics for faster results |
| 4th | real-time detection with wireless communication and incorporation with mobile devices and cloud technologies | wearable biosensors, implantable sensors | design of real-time continuous monitoring systems which provide real-time transmission of data |
| 5th | fully incorporated, smart biosensors with AI and ML for predictive analytics | AI-powered diagnostic sensors | AI-driven data analysis for personalized medicine and diagnostics |
| Biosensor Method | Biosensor Transducer Type | Reasons for Possible False Results |
|---|---|---|
| electrochemical | voltametric |
|
| amperometric |
| |
| impedimetric |
| |
| potentiometric |
| |
| conductometric |
| |
| optical | colorimetric |
|
| fluorescent |
| |
| optical fiber |
| |
| surface-enhanced Raman scattering |
| |
| photonic crystal |
| |
| thermal |
| |
| acoustic |
| |
| mechanical |
| |
| magnetic |
| |
| Biosensor Technology Type | Reasons for Possible False Results | |
|---|---|---|
| SPR |
| |
| microfluidic | paper based |
|
| polymer based |
| |
| nanotechnology | metal nanoparticles |
|
| nanostructured surface based |
| |
| quantum dots based |
| |
| implantable |
| |
| Biosensor Algorithm Used | Reasons for Possible False Results | |
|---|---|---|
| Supervised learning | classification |
|
| regression |
| |
| unsupervised learning | clustering |
|
| dimensionality reduction (PCA) |
| |
| deep learning neutral networks |
| |
| reinforced learning |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goumas, G.; Vlachothanasi, E.N.; Fradelos, E.C.; Mouliou, D.S. Biosensors, Artificial Intelligence Biosensors, False Results and Novel Future Perspectives. Diagnostics 2025, 15, 1037. https://doi.org/10.3390/diagnostics15081037
Goumas G, Vlachothanasi EN, Fradelos EC, Mouliou DS. Biosensors, Artificial Intelligence Biosensors, False Results and Novel Future Perspectives. Diagnostics. 2025; 15(8):1037. https://doi.org/10.3390/diagnostics15081037
Chicago/Turabian StyleGoumas, Georgios, Efthymia N. Vlachothanasi, Evangelos C. Fradelos, and Dimitra S. Mouliou. 2025. "Biosensors, Artificial Intelligence Biosensors, False Results and Novel Future Perspectives" Diagnostics 15, no. 8: 1037. https://doi.org/10.3390/diagnostics15081037
APA StyleGoumas, G., Vlachothanasi, E. N., Fradelos, E. C., & Mouliou, D. S. (2025). Biosensors, Artificial Intelligence Biosensors, False Results and Novel Future Perspectives. Diagnostics, 15(8), 1037. https://doi.org/10.3390/diagnostics15081037









