Thoracic CT Angiographies in Children Using Automated Power Injection with Bolus Tracking Versus Manual Contrast Injection: Analysis of Contrast Enhancement, Image Quality and Radiation Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
- -
- Patients’ characteristics (sex, age, body weight, body mass index at the time of CTA),
- -
- CM volumes and flow rates,
- -
- Venous access routes and sizes of intravenous lines for CM application,
- -
- Method of CM administration (MI or PI),
- -
- Scan parameters (tube voltage, volume computed tomography dose index (CTDIvol), dose length product (DLP), calculation of effective doses (E)),
- -
- CT attenuation values for the calculation of signal-to-noise ratios (SNRs) and contrast-to-noise ratios (CNRs),
- -
- Complications associated with CM administration (extravasation, air embolism and allergic reaction).
2.2. Image Acquisition and Image Processing
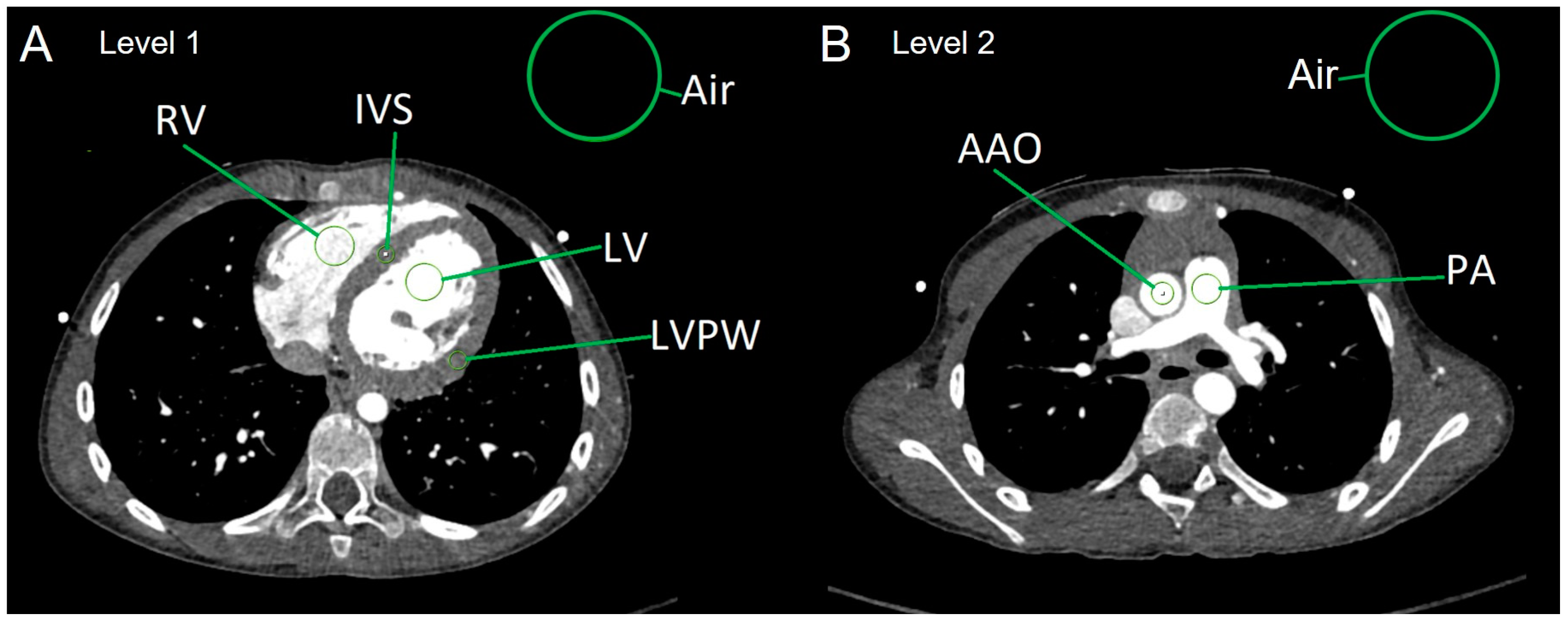
2.3. Analysis of Objective Image Quality
- Average myocardial HUs were calculated in the following way:
- HU[myocardium] = (HU[IVS] + HU[LVPW])/2.
- Calculation of image parameters SNR and CNR:
- SNR of the left ventricle: SNR[LV] = HU[LV]/SD[air]
- SNR of the right ventricle: SNR[RV] = HU[RV]/SD[air]
- SNR of the ascending aorta: SNR[AAO] = HU[AAO]/SD[air]
- SNR of the main PA: SNR[PA] = HU[PA]/SD[air]
- SNR of the myocardium: SNR[myocardium] = HU[myocardium]/SD[air]
- CNR of the left ventricle: CNR[LV] = SNR[LV] − SNR[myocardium]
- CNR of the right ventricle: CNR[RV] = SNR[RV] − SNR[myocardium]
- CNR of the ascending aorta: CNR[AAO] = SNR[AAO] − SNR[myocardium]
- CNR of the main PA: CNR[PA] = SNR[PA] − SNR[myocardium]
2.4. Assessment of Subjective Image Quality
2.5. Analysis of Radiation Exposure
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. CM Administration
3.2.1. Application Route of the CM
3.2.2. CM Volumes, Flow Rates, Modalities of CM Administration and Radiation Exposure
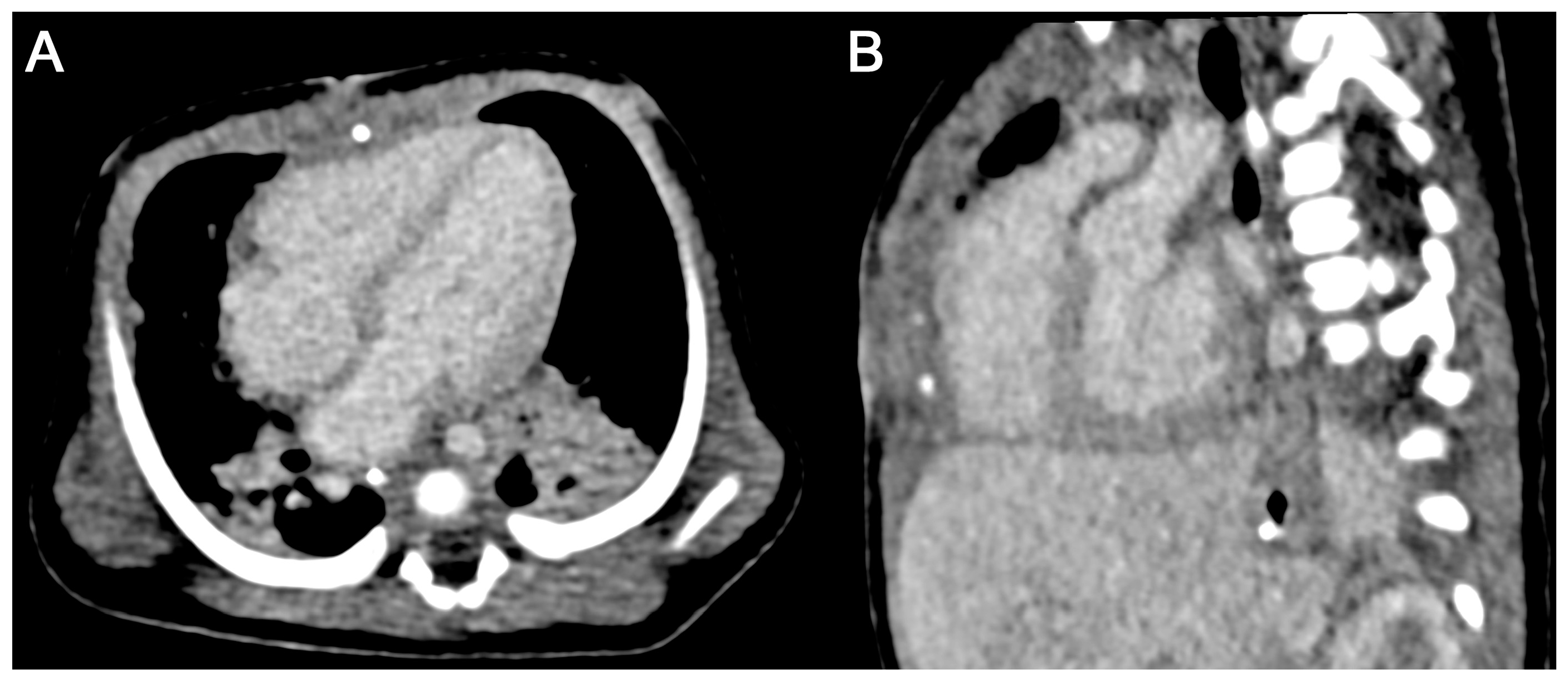
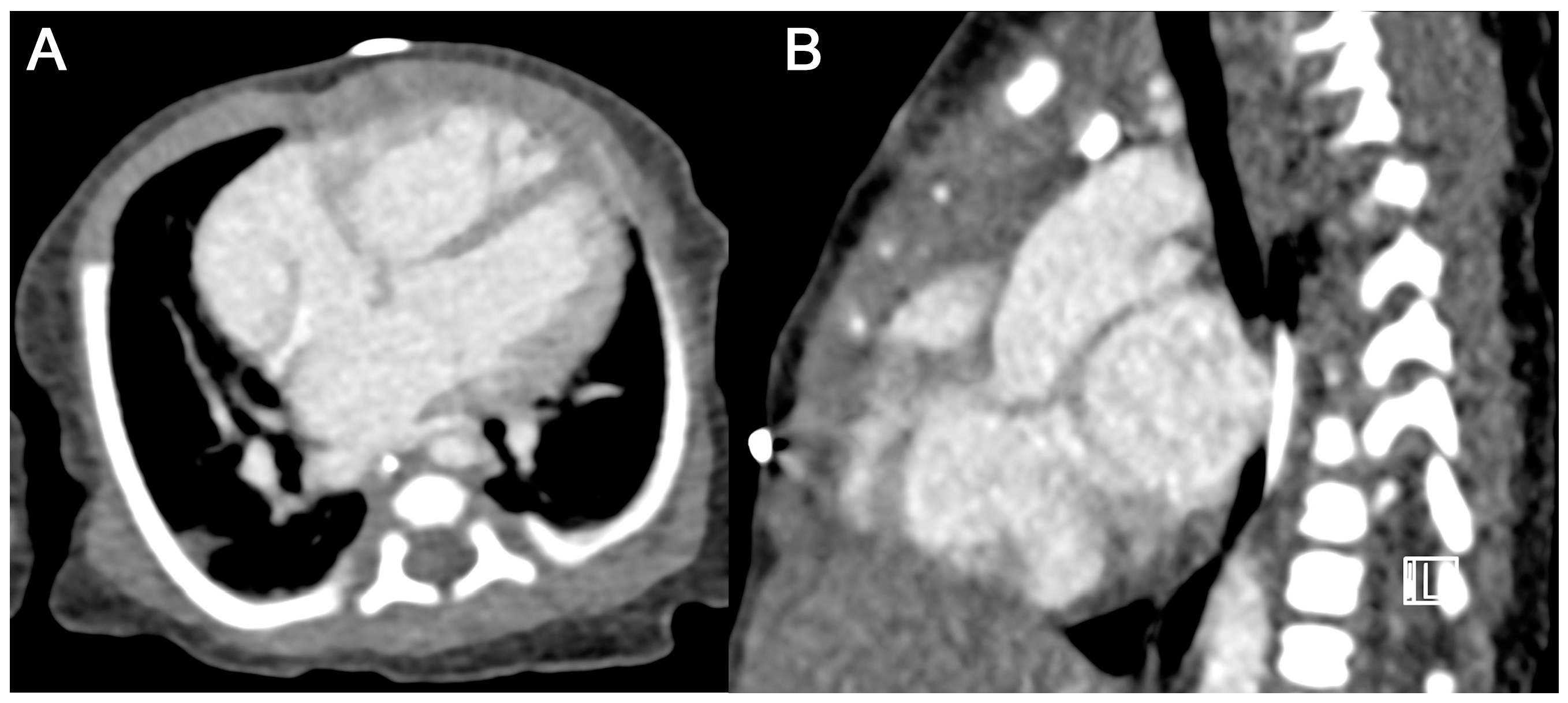
3.2.3. CM-Associated Complications
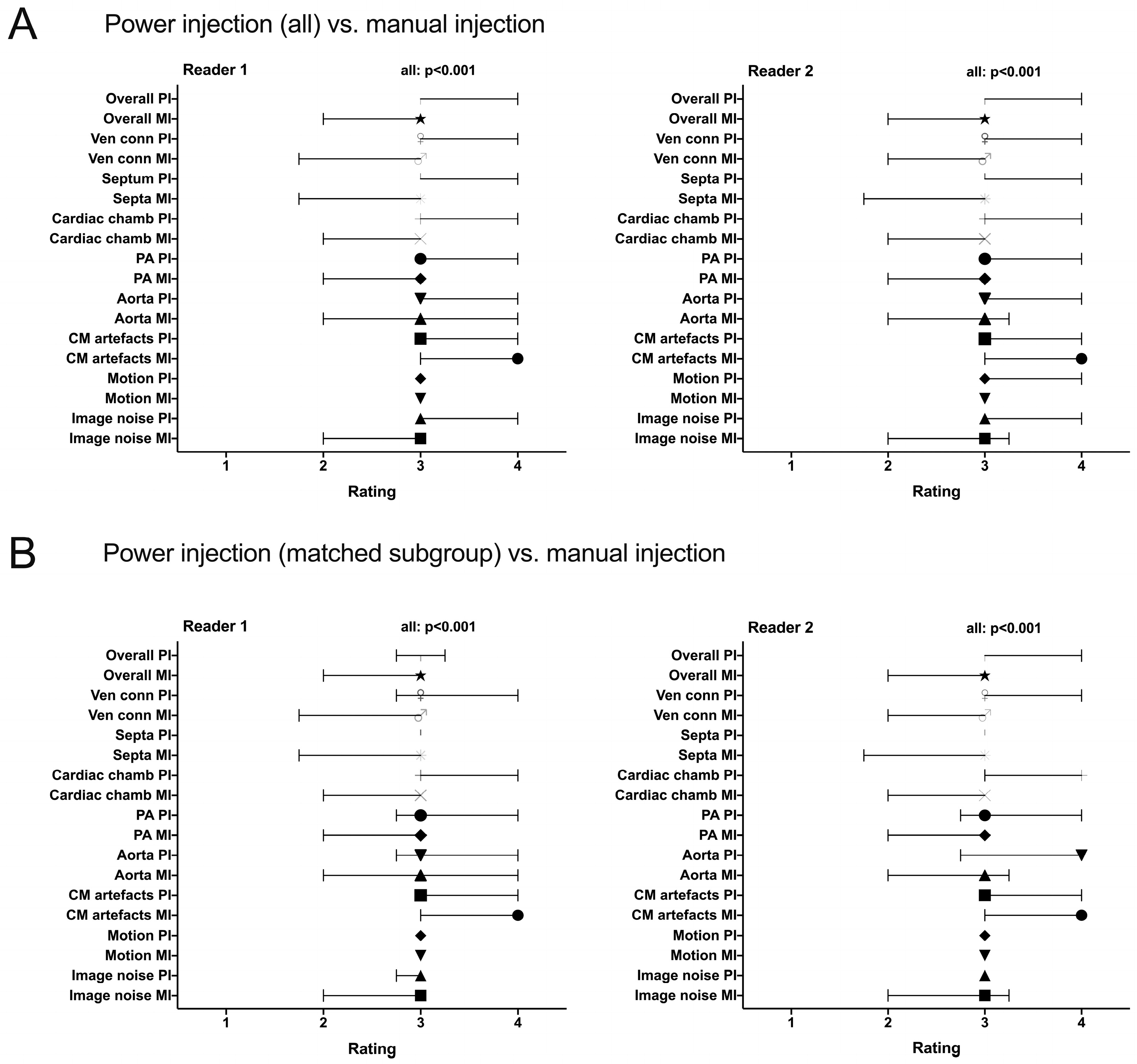
3.3. Image Quality
3.3.1. Objective Image Quality
3.3.2. Subjective Image Quality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO | ascending aorta |
| CM | contrast medium |
| CNR | contrast-to-noise ratio |
| CT | computed tomography |
| CTA | computed tomography angiography |
| CTDIvol | volume computed tomography dose index |
| DLP | dose length product [mGy × cm] |
| DSCT | dual-source computed tomography |
| E | effective dose [mSv] |
| G | Gauge |
| HU | Hounsfield unit(s) |
| IVS | interventricular septum |
| LV | left ventricle |
| LVPW | left ventricular posterior wall |
| MI | manual CM injection |
| PA | main pulmonary artery |
| PI | automated power injection with bolus tracking |
| ROI | region of interest |
| RV | right ventricle |
| SD | standard deviation |
| SNR | signal-to-noise ratio |
References
- Beerbaum, P.B.J. Congenital Heart Disease: Multimodal Imaging Is Every Day’s Routine. Circ. Cardiovasc. Imaging 2017, 10, e006589. [Google Scholar] [CrossRef] [PubMed]
- Caro-Domínguez, P.; Secinaro, A.; Valverde, I.; Fouilloux, V. Imaging and surgical management of congenital heart diseases. Pediatr. Radiol. 2023, 53, 677–694. [Google Scholar] [CrossRef]
- Rajiah, P.S.; Sardá, M.J.; Ashwath, R.; Goerne, H. Palliative Procedures for Congenital Heart Disease: Imaging Findings and Complications. RadioGraphics 2023, 43, e220049. [Google Scholar] [CrossRef]
- Sachdeva, R.; Armstrong, A.K.; Arnaout, R.; Grosse-Wortmann, L.; Han, B.K.; Mertens, L.; Moore, R.A.; Olivieri, L.J.; Parthiban, A.; Powell, A.J. Novel Techniques in Imaging Congenital Heart Disease: JACC Scientific Statement. J. Am. Coll. Cardiol. 2024, 83, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Valente, A.M.; Armstrong, A.K.; Cook, S.C.; Han, B.K.; Lopez, L.; Lui, G.K.; Pickard, S.S.; Powell, A.J.; Bhave, N.M.; et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 Appropriate Use Criteria for Multimodality Imaging During the Follow-Up Care of Patients with Congenital Heart Disease: A Report of the American College of Cardiology Solution Set Oversight Committee and Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, e1–e48. [Google Scholar]
- Corbett, L.; Forster, J.; Gamlin, W.; Duarte, N.; Burgess, O.; Harkness, A.; Li, W.; Simpson, J.; Bedair, R. A practical guideline for performing a comprehensive transthoracic echocardiogram in the congenital heart disease patient: Consensus recommendations from the British Society of Echocardiography. Echo Res. Pract. 2022, 9, 10. [Google Scholar] [CrossRef]
- Galzerano, D.; Pergola, V.; Eltayeb, A.; Ludovica, F.; Arbili, L.; Tashkandi, L.; Di Michele, S.; Barchitta, A.; Parato, M.V.; Di Salvo, G. Echocardiography in Simple Congenital Heart Diseases: Guiding Adult Patient Management. J. Cardiovasc. Echogr. 2023, 33, 171–182. [Google Scholar] [CrossRef]
- Lopez, L.; Saurers, D.L.; Barker, P.C.; Cohen, M.S.; Colan, S.D.; Dwyer, J.; Forsha, D.; Friedberg, M.K.; Lai, W.W.; Printz, B.F.; et al. Guidelines for Performing a Comprehensive Pediatric Transthoracic Echocardiogram: Recommendations From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2024, 37, 119–170. [Google Scholar] [CrossRef] [PubMed]
- Brotschi, B.; Hug, M.I.; Kretschmar, O.; Rizzi, M.; Albisetti, M. Incidence and predictors of cardiac catheterisation-related arterial thrombosis in children. Heart 2015, 101, 948–953. [Google Scholar] [CrossRef]
- Mori, Y.; Takahashi, K.; Nakanishi, T. Complications of cardiac catheterization in adults and children with congenital heart disease in the current era. Heart Vessel. 2013, 28, 352–359. [Google Scholar] [CrossRef]
- West, R.; Ellis, G.; Brooks, N. Complications of diagnostic cardiac catheterisation: Results from a confidential inquiry into cardiac catheter complications. Heart 2006, 92, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Rompel, O.; Glöckler, M.; Janka, R.; Dittrich, S.; Cesnjevar, R.; Lell, M.M.; Uder, M.; Hammon, M. Third-generation dual-source 70-kVp chest CT angiography with advanced iterative reconstruction in young children: Image quality and radiation dose reduction. Pediatr. Radiol. 2016, 46, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, D.; Shen, Y.; Yang, H.; Chen, C.; Yin, J.; Peng, Y. Improving image quality with model-based iterative reconstruction algorithm for chest CT in children with reduced contrast concentration. La Radiol. Medica 2019, 124, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.A.; Otero, H.J.; White, A.M.; Saul, D.; Biko, D.M. Image Quality of ECG-Triggered High-Pitch, Dual-Source Computed Tomography Angiography for Cardiovascular Assessment in Children. Curr. Probl. Diagn. Radiol. 2020, 49, 23–28. [Google Scholar] [CrossRef]
- Dillman, J.R.; Hernandez, R.J. Role of CT in the Evaluation of Congenital Cardiovascular Disease in Children. Am. J. Roentgenol. 2009, 192, 1219–1231. [Google Scholar] [CrossRef]
- May, L.A.; Atweh, L.A.; Masand, P.; Jadhav, S.P. Technical considerations of cardiac computed tomography in children. Pediatr. Radiol. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Siripornpitak, S.; Pornkul, R.; Khowsathit, P.; Layangool, T.; Promphan, W.; Pongpanich, B. Cardiac CT angiography in children with congenital heart disease. Eur. J. Radiol. 2013, 82, 1067–1082. [Google Scholar] [CrossRef]
- Cohen, J.; Asrani, P.; Lee, S.; Frush, D.; Han, B.K.; Chelliah, A.; Farooqi, K.M. Cardiovascular computed tomography in pediatric congenital heart disease: A state of the art review. J. Cardiovasc. Comput. Tomogr. 2022, 16, 467–482. [Google Scholar] [CrossRef]
- Zucker, E.J. Cardiac Computed Tomography in Congenital Heart Disease. Radiol. Clin. N. Am. 2024, 62, 435–452. [Google Scholar] [CrossRef]
- Pop, M. Cardiothoracic CTA in Infants Referred for Aortic Arch Evaluation-Retrospective Comparison of Iomeprol 350, Ioversol 350, Iopromide 370 and Iodixanol 320. Children 2021, 8, 949. [Google Scholar] [CrossRef]
- Zapala, M.A.; Zurakowski, D.; Lee, E.Y. Comparison of Mechanical Versus Hand Administration of IV Contrast Agents for Pediatric Pulmonary CT Angiography. Am. J. Roentgenol. 2017, 208, 632–636. [Google Scholar] [CrossRef]
- Amaral, J.G.; Traubici, J.; BenDavid, G.; Reintamm, G.; Daneman, A. Safety of Power Injector Use in Children as Measured by Incidence of Extravasation. Am. J. Roentgenol. 2006, 187, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.A.; White, A.M.; Shepherd, A.M.; Mecca, P.; Biko, D.M.; Saul, D.; Otero, H.J. Contrast Extravasation using Power Injectors for Contrast-Enhanced Computed Tomography in Children: Frequency and Injury Severity. Acad. Radiol. 2019, 26, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Indrajit, I.K.; Sivasankar, R.; D′Souza, J.; Pant, R.; Negi, R.S.; Sahu, S.; Pi, H. Pressure injectors for radiologists: A review and what is new. Indian J. Radiol. Imaging 2015, 25, 2–10. [Google Scholar] [CrossRef]
- Han, B.K.; Rigsby, C.K.; Leipsic, J.; Bardo, D.; Abbara, S.; Ghoshhajra, B.; Lesser, J.R.; Raman, S.V.; Crean, A.M.; Nicol, E.D.; et al. Computed Tomography Imaging in Patients with Congenital Heart Disease, Part 2: Technical Recommendations. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT): Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2015, 9, 493–513. [Google Scholar]
- Hong, S.H.; Goo, H.W.; Maeda, E.; Choo, K.S.; Tsai, I.C. User-Friendly Vendor-Specific Guideline for Pediatric Cardiothoracic Computed Tomography Provided by the Asian Society of Cardiovascular Imaging Congenital Heart Disease Study Group: Part 1. Imaging Techniques. Korean J. Radiol. 2019, 20, 190–204. [Google Scholar] [CrossRef]
- Goo, H.W.; Park, I.-S.; Ko, J.K.; Kim, Y.H.; Seo, D.-M.; Park, J.-J. Computed tomography for the diagnosis of congenital heart disease in pediatric and adult patients. Int. J. Cardiovasc. Imaging 2005, 21, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W.; Park, I.-S.; Ko, J.K.; Kim, Y.H.; Seo, D.-M.; Yun, T.-J.; Park, J.-J.; Yoon, C.H. CT of Congenital Heart Disease: Normal Anatomy and Typical Pathologic Conditions. RadioGraphics 2003, 23, S147–S165. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Weinstein, O.; Sade, M.Y.; Shelef, I.; Novack, V.; Abu Tailakh, M.; Levy, J. The association between exposure to radiation and the incidence of cataract. Int. Ophthalmol. 2021, 41, 237–242. [Google Scholar] [CrossRef]
- Goodman, T.R.; Mustafa, A.; Rowe, E. Pediatric CT radiation exposure: Where we were, and where we are now. Pediatr. Radiol. 2019, 49, 469–478. [Google Scholar] [CrossRef]
- Shah, N.B.; Platt, S.L. ALARA: Is there a cause for alarm? Reducing radiation risks from computed tomography scanning in children. Curr. Opin. Pediatr. 2008, 20, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, S.; Liu, J.; Yang, L.; Huang, Z.; Luo, C.; Li, X. Feasibility of high-pitch spiral dual-source CT angiography in children with complex congenital heart disease compared to retrospective-gated spiral acquisition. Clin. Radiol. 2017, 72, 864–870. [Google Scholar] [CrossRef]
- Nau, D.; Wuest, W.; Rompel, O.; Hammon, M.; Gloeckler, M.; Toka, O.; Dittrich, S.; Rueffer, A.; Cesnjevar, R.; Lell, M.M.; et al. Evaluation of ventricular septal defects using high pitch computed tomography angiography of the chest in children with complex congenital heart defects below one year of age. J. Cardiovasc. Comput. Tomogr. 2019, 13, 226–233. [Google Scholar] [CrossRef]
- Bae, K.; Jeon, K.N.; Cho, S.B.; Park, S.E.; Moon, J.I.; Baek, H.J.; Choi, B.H. Improved Opacification of a Suboptimally Enhanced Pulmonary Artery in Chest CT: Experience Using a Dual-Layer Detector Spectral CT. Am. J. Roentgenol. 2018, 210, 734–741. [Google Scholar] [CrossRef]
- Chatzaraki, V.; Kubik-Huch, R.A.; Thali, M.; Niemann, T. Quantifying image quality in chest computed tomography angiography: Evaluation of different contrast-to-noise ratio measurement methods. Acta Radiol. 2022, 63, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Padole, A.M.; Sagar, P.; Westra, S.J.; Lim, R.; Nimkin, K.; Kalra, M.K.; Gee, M.S.; Rehani, M.M. Development and validation of image quality scoring criteria (IQSC) for pediatric CT: A preliminary study. Insights Imaging 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT Protocols: Sex- and Age-specific Conversion Factors Used to Determine Effective Dose from Dose-Length Product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef]
- Abadi, M.; Moore, D.R. Selection of Circular Proposals in Building Projects: An MCDM Model for Lifecycle Circularity Assessments Using AHP. Buildings 2022, 12, 1110. [Google Scholar] [CrossRef]
- Hell, M.M.; Bittner, D.; Schuhbaeck, A.; Muschiol, G.; Brand, M.; Lell, M.; Uder, M.; Achenbach, S.; Marwan, M. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: Feasibility, image quality, radiation dose, and effect of iterative reconstruction. J. Cardiovasc. Comput. Tomogr. 2014, 8, 418–425. [Google Scholar] [CrossRef]
- Achenbach, S.; on behalf of the X-ACT Study Group; Paul, J.-F.; Laurent, F.; Becker, H.-C.; Rengo, M.; Caudron, J.; Leschka, S.; Vignaux, O.; Knobloch, G.; et al. Comparative assessment of image quality for coronary CT angiography with iobitridol and two contrast agents with higher iodine concentrations: Iopromide and iomeprol. A multicentre randomized double-blind trial. Eur. Radiol. 2017, 27, 821–830. [Google Scholar] [CrossRef]
- Stocker, T.J.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Deseive, S.; Heckner, M.; Bax, J.J.; Kitagawa, K.; Marques, H.; et al. Application of Low Tube Potentials in CCTA: Results From the PROTECTION VI Study. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 425–434. [Google Scholar] [CrossRef]
- Nagy, E.; Tschauner, S.; Marterer, R.; Riedl, R.; Sorantin, E. Chest CTA in children younger than two years—A retrospective comparison of three contrast injection protocols. Sci. Rep. 2019, 9, 18109. [Google Scholar] [CrossRef] [PubMed]
- Saake, M.; Lell, M.M.; Rompel, O.; Gloeckler, M.; May, M.; Eller, A.; Achenbach, S.; Uder, M.; Wuest, W. Contrast medium application in pediatric high-pitch cardiovascular CT angiography: Manual or power injection? J. Cardiovasc. Comput. Tomogr. 2014, 8, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yoshiura, T.; Masuda, T.; Tahara, M.; Kobayashi, Y.; Kikuhara, Y.; Ishibashi, T.; Nonaka, H.; Oku, T.; Sato, T.; Funama, Y. Cardiac computed tomography angiography with and without bolus tracking methods in infants with congenital heart disease. Radiat. Prot. Dosim. 2024, 200, 251–258. [Google Scholar] [CrossRef]
- Forte, E.; Monti, S.; Parente, C.A.; Beyer, L.; De Rosa, R.; Infante, T.; Cavaliere, C.; Cademartiri, F.; Salvatore, M.; Stroszczynski, C.; et al. Image Quality and Dose Reduction by Dual Source Computed Tomography Coronary Angiography: Protocol Comparison. Dose-Response 2018, 16, 1559325818805838. [Google Scholar] [CrossRef]
- Esquivel, M.F.D.; Miller, E.; Bijelić, V.; Barrowman, N.; Putnins, R. CT contrast extravasation in children: A single-center experience and systematic review. Pediatr. Radiol. 2024, 54, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, H.; Tang, S.; Chen, M.; Li, T.; He, L. Application of dual-energy CT with prospective ECG-gating in cardiac CT angiography for children: Radiation and contrast agent dose. Eur. J. Radiol. 2024, 170, 111229. [Google Scholar] [CrossRef]
- Stroeder, J.; Fries, P.; Raczeck, P.; Buecker, A.; Jagoda, P. Prospective safety evaluation of automated iomeprol 400 injections for CT through peripheral venous cannulas. Clin. Radiol. 2020, 75, 396.e1–396.e6. [Google Scholar] [CrossRef]
- Valentin, J. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Lu, R.; Naisula, D.; Bi, F.; Liu, X.; Wang, S.; Sun, J. Image quality assessment of the CARE kV for pediatric CT angiography: A feasibility study of over 50% effective dose reduction. J. Radiat. Res. Appl. Sci. 2025, 18, 101386. [Google Scholar] [CrossRef]
- Doss, M. Linear No-Threshold Model VS. Radiation Hormesis. Dose Response 2013, 11, 480–497. [Google Scholar] [CrossRef]
- Siegel, J.A.; McCollough, C.H.; Orton, C.G. Advocating for use of the ALARA principle in the context of medical imaging fails to recognize that the risk is hypothetical and so serves to reinforce patients’ fears of radiation. Med. Phys. 2017, 44, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, M.; Ishimaru, T.; Belsky, J.L. Incidence of Leukemia in Atomic Bomb Survivors Belonging to a Fixed Cohort in Hiroshima and Nagasaki, 1950–1971 Radiation dose, years after exposure, age at exposure, and type of leukemia. J. Radiat. Res. 1978, 19, 262–282. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ Br. Med. J. 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Sato, S.; Kanie, Y.; Tanaka, T.; Inai, R.; Akagi, N.; Morimitsu, Y.; Kanazawa, S. Image Quality of Coronary Computed Tomography Angiography with 320-Row Area Detector Computed Tomography in Children with Congenital Heart Disease. Pediatr. Cardiol. 2016, 37, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousily, F.; Shifrin, R.Y.; Fricker, F.J.; Feranec, N.; Quinn, N.S.; Chandran, A. Use of 320-Detector Computed Tomographic Angiography for Infants and Young Children with Congenital Heart Disease. Pediatr. Cardiol. 2011, 32, 426–432. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Luo, Z.; Wang, D.; Bai, J.; Han, D.; Shen, B. Initial experience on the application of 320-row CT angiography with low-dose prospective ECG-triggered in children with congenital heart disease. Int. J. Cardiovasc. Imaging 2012, 28, 1787–1797. [Google Scholar] [CrossRef]
- Thorne, M.C. Background radiation: Natural and man-made. J. Radiol. Prot. 2003, 23, 29–42. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhao, H.; Jia, Y.; Ren, J.; Xu, J.; Hao, Y.; Zheng, M. Image quality and radiation dose of dual-source CT cardiac angiography using prospective ECG-triggering technique in pediatric patients with congenital heart disease. J. Cardiothorac. Surg. 2016, 11, 47. [Google Scholar] [CrossRef]
- Hanneman, K.; Newman, B.; Chan, F. Congenital Variants and Anomalies of the Aortic Arch. RadioGraphics 2017, 37, 32–51. [Google Scholar] [CrossRef]
- Trinavarat, P. Computed tomographic angiography (CTA) of major thoracic vessels in children—A pictorial assay on common findings also discussing CTA technique. Eur. J. Radiol. 2013, 82, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Roik, D.; Kucińska, B.; Roik, M.; Werner, B. Image quality and radiation doses of volumetric 320-row computed tomography angiography with prospective electrocardiogram-gating in the assessment of coronary arteries in neonates and infants. Kardiol. Pol. 2022, 80, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Spanaki, A.; Kabir, S.; Stephenson, N.; van Poppel, M.P.M.; Benetti, V.; Simpson, J. 3D Approaches in Complex CHD: Where Are We? Funny Printing and Beautiful Images, or a Useful Tool? J. Cardiovasc. Dev. Dis. 2022, 9, 269. [Google Scholar] [CrossRef] [PubMed]


| Parameter | PI (All) n = 119 | MI (All) n = 18 | p |
|---|---|---|---|
| Male/female, n | 58/61 | 13/5 | |
| Age (years) | 4.4 ± 5.3 | 0.59 ± 0.95 | 0.04 |
| Body weight (kg) | 17.1 ± 16.2 | 6.1 ± 2.7 | 0.05 |
| Height (m) | 0.95 ± 0.41 | 0.63 ± 0.12 | 0.03 |
| BMI (kg/m2) | 15.1 ± 3.2 | 14.8 ± 2.0 | 0.74 |
| CTDIvol (mGy) | 1.13 ± 1.51 | 0.45 ± 0.22 | 0.01 |
| DLP (mGy × cm) | 23.4 ± 26.2 | 7.8 ± 4.6 | 0.003 |
| E (mSv) | 0.83 ± 0.7 | 0.53 ± 0.3 | 0.04 |
| Parameter | PI (Matched Subgroup) n = 18 | MI (All) n = 18 | p |
|---|---|---|---|
| Male/female, n | 11/7 | 13/5 | |
| Age (years) | 0.71 ± 1.03 | 0.59 ± 0.95 | 0.57 |
| Body weight (kg) | 6.6 ± 3.2 | 6.1 ± 2.7 | 0.74 |
| Height (m) | 0.65 ± 1.3 | 0.63 ± 0.12 | 0.64 |
| BMI (kg/m2) | 14.6 ± 1.7 | 14.8 ± 2.0 | 0.90 |
| CTDIvol (mGy) | 0.44 ± 0.2 | 0.45 ± 0.22 | 0.76 |
| DLP (mGy × cm) | 6.8 ± 3.5 | 7.8 ± 4.6 | 0.62 |
| E (mSv) | 0.52 ± 0.3 | 0.53 ± 0.3 | 0.76 |
| Structure | Attenuation (HU) | SNR | CNR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PI (All) n = 119 | MI (All) n = 18 | p | PI (All) n = 119 | MI (All) n = 18 | p | PI (All) n = 119 | MI (All) n = 18 | p | |
| LV | 547 ± 218 | 331 ± 184 | <0.001 | 29.6 ± 13.1 | 25.9 ± 20.5 | 0.11 | 22.2 ± 11.3 | 16.0 ± 17.2 | 0.007 |
| RV | 569 ± 279 | 264 ± 132 | <0.001 | 30.5 ± 15.6 | 19.9 ± 11.2 | <0.001 | 22.9 ± 14.9 | 10.6 ± 8.7 | <0.001 |
| AAO | 577 ± 228 | 325 ± 171 | <0.001 | 34.6 ± 14.2 | 25.6 ± 23.7 | <0.001 | 27.1 ± 13.2 | 15.7 ± 20.4 | <0.001 |
| PA | 568 ± 298 | 294 ± 141 | <0.001 | 34.4 ± 19.4 | 21.5 ± 12.6 | <0.001 | 27.4 ± 18.5 | 11.6 ± 10.1 | <0.001 |
| Structure | Attenuation (HU) | SNR | CNR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PI (Subgroup) n = 18 | MI (All) n = 18 | p | PI (Subgroup) n = 18 | MI (All) n = 18 | p | PI (Subgroup) n = 18 | MI (All) n = 18 | p | |
| LV | 621 ± 228 | 331 ± 184 | <0.001 | 35.3 ± 17.1 | 25.9 ± 20.5 | 0.07 | 27.2 ± 14.6 | 16.0 ± 17.2 | 0.009 |
| RV | 528 ± 262 | 264 ± 132 | <0.001 | 30.8 ± 16.3 | 19.9 ± 11.2 | 0.008 | 22.7 ± 15.0 | 10.6 ± 8.7 | 0.007 |
| AAO | 660 ± 285 | 325 ± 171 | <0.001 | 39.6 ± 19.0 | 25.6 ± 23.7 | 0.005 | 31.5 ± 16.7 | 15.7 ± 20.4 | 0.001 |
| PA | 521 ± 259 | 294 ± 141 | 0.002 | 32.7 ± 15.1 | 21.5 ± 12.6 | 0.02 | 24.6 ± 13.9 | 11.6 ± 10.1 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, J.; Driulini, D.; Altmeyer, K.; Wagenpfeil, G.; Poryo, M.; Giebels, C.; Bücker, A.; Massmann, A.; Abdul-Khaliq, H.; Fries, P. Thoracic CT Angiographies in Children Using Automated Power Injection with Bolus Tracking Versus Manual Contrast Injection: Analysis of Contrast Enhancement, Image Quality and Radiation Exposure. Diagnostics 2025, 15, 1103. https://doi.org/10.3390/diagnostics15091103
Pfeifer J, Driulini D, Altmeyer K, Wagenpfeil G, Poryo M, Giebels C, Bücker A, Massmann A, Abdul-Khaliq H, Fries P. Thoracic CT Angiographies in Children Using Automated Power Injection with Bolus Tracking Versus Manual Contrast Injection: Analysis of Contrast Enhancement, Image Quality and Radiation Exposure. Diagnostics. 2025; 15(9):1103. https://doi.org/10.3390/diagnostics15091103
Chicago/Turabian StylePfeifer, Jochen, Deborah Driulini, Katrin Altmeyer, Gudrun Wagenpfeil, Martin Poryo, Christian Giebels, Arno Bücker, Alexander Massmann, Hashim Abdul-Khaliq, and Peter Fries. 2025. "Thoracic CT Angiographies in Children Using Automated Power Injection with Bolus Tracking Versus Manual Contrast Injection: Analysis of Contrast Enhancement, Image Quality and Radiation Exposure" Diagnostics 15, no. 9: 1103. https://doi.org/10.3390/diagnostics15091103
APA StylePfeifer, J., Driulini, D., Altmeyer, K., Wagenpfeil, G., Poryo, M., Giebels, C., Bücker, A., Massmann, A., Abdul-Khaliq, H., & Fries, P. (2025). Thoracic CT Angiographies in Children Using Automated Power Injection with Bolus Tracking Versus Manual Contrast Injection: Analysis of Contrast Enhancement, Image Quality and Radiation Exposure. Diagnostics, 15(9), 1103. https://doi.org/10.3390/diagnostics15091103







