Management of Onion Thrips (Thrips tabaci) in Organic Onion Production Using Multiple IPM Tactics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Onion Plants
2.3. Pesticides and Application Technique
2.4. Data Collection
2.5. Data Analysis
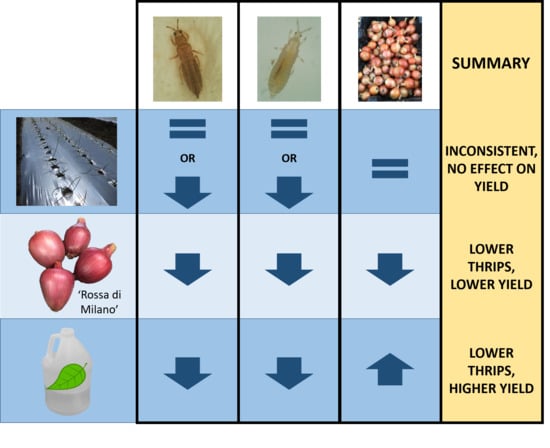
3. Results
3.1. Impact of Treatments on Adult Thrips tabaci Densities
3.2. Impact of Treatments on Larval Thrips tabaci Densities
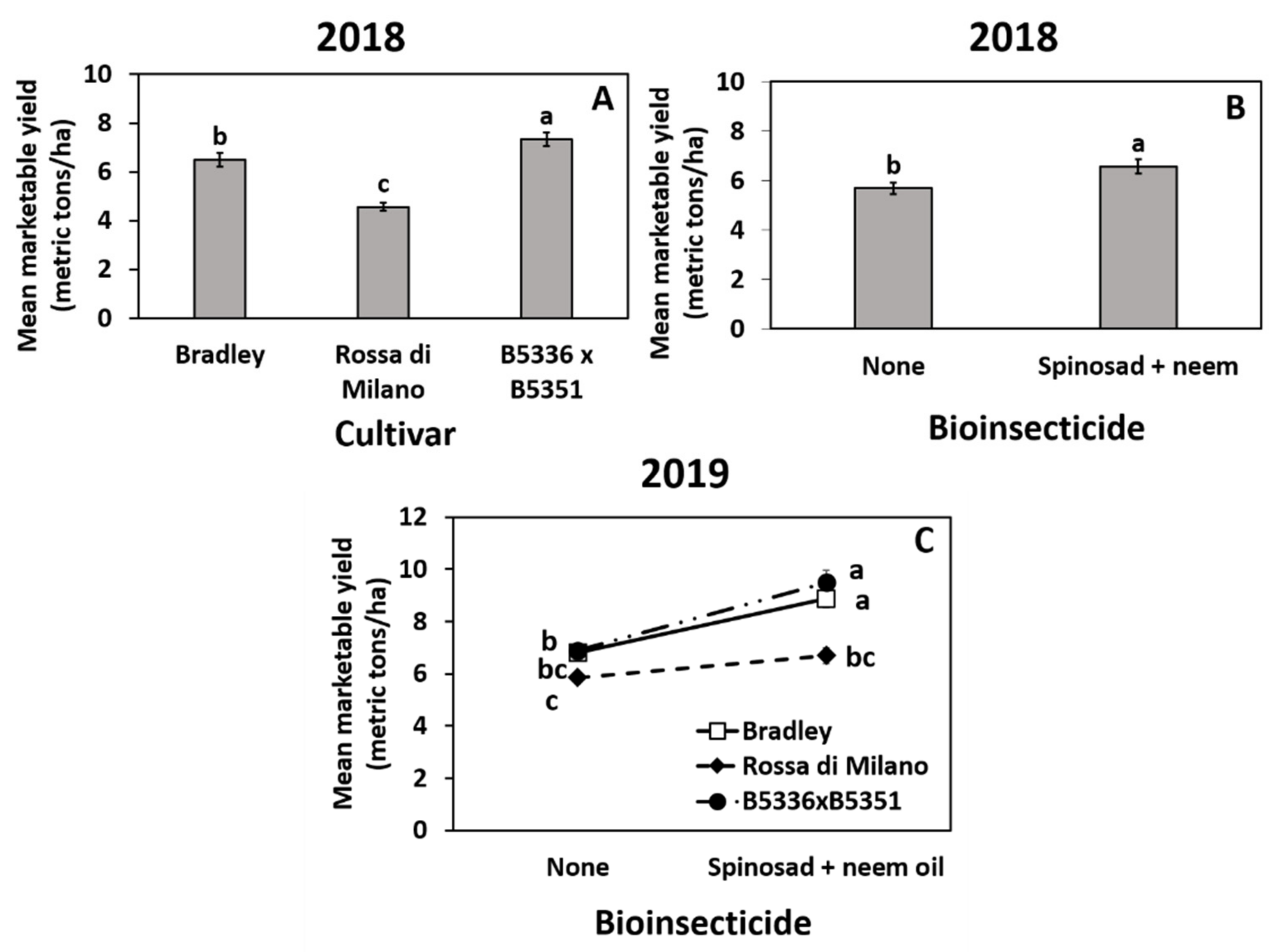
3.3. Impact of Treatments on Marketable Bulb Yields
3.4. Impact of Treatments on Marketable Bulb Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of increasing concern in onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.K.; Garg, H.; Gill, A.K.; Gillett-Kaufman, J.L.; Nault, B.A. Onion thrips (Thysanoptera: Thripidae) biology, ecology, and management in onion production systems. Pest Manag. Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boateng, C.O.; Schwartz, H.F.; Havey, M.J.; Otto, K. Evaluation of onion germplasm for resistance to Iris Yellow Spot (Iris yellow spot virus) and onion thrips, Thrips tabaci. Southwest. Entomol. 2014, 39, 237–260. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Cartwright, B.; Miller, M.E.; Edelson, J. V Injury to onions by Thrips tabaci (Thysanoptera: Thripidae) and its role in the development of purple blotch. Environ. Entomol. 1993, 22, 1266–1277. [Google Scholar] [CrossRef]
- Dutta, B.; Barman, A.K.; Srinivasan, R.; Gitaitis, R. Transmission of Pantoea ananatis and Pantoea agglomerans, causal agents of center rot of onion (Allium cepa L.), by onion thrips (Thrips tabaci Lindeman) through feces. Phytopathology 2014, 104, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Grode, A.; Chen, S.; Walker, E.D.; Szendrei, Z. Onion thrips (Thysanoptera: Thripidae) feeding promotes infection by Pantoea ananatis in onion. J. Econ. Entomol. 2017, 110, 2301–2307. [Google Scholar] [CrossRef]
- Leach, A.; Hay, F.; Harding, R.; Damann, K.C.; Nault, B. Relationship between onion thrips (Thrips tabaci) and Stemphylium vesicarium in the development of Stemphylium leaf blight in onion. Ann. Appl. Biol. 2020, 176, 55–64. [Google Scholar] [CrossRef]
- Nagata, T.; Almeida, A.C.L.; Resende, R.d.O.; de Ávila, A.C. The identification of the vector species of Iris Yellow Spot Tospovirus occurring on onion in Brazil. Plant Dis. 1999, 83, 399. [Google Scholar] [CrossRef] [PubMed]
- Kritzman, A.; Lampel, M.; Raccah, B.; Gera, A. Distribution and transmission of Iris yellow spot virus. Plant Dis. 2001, 85, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Gent, D.H.; Du Toit, L.J.; Fichtner, S.F.; Mohan, S.K.; Pappu, H.R.; Schwartz, H.F. Iris yellow spot virus: An emerging threat to onion bulb and seed production. Plant Dis. 2006, 90, 1468–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, E.A.; Harding, R.S.; Scott, J.G.; Nault, B.A. Monitoring Onion Thrips (Thysanoptera: Thripidae) Susceptibility to Spinetoram in New York Onion Fields. J. Econ. Entomol. 2019, 112, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Nault, B.A.; Plate, J.; Zhao, J.-Z. Regional and Temporal Variation in Susceptibility to λ-Cyhalothrin in Onion Thrips, Thrips tabaci (Thysanoptera: Thripidae), in Onion Fields in New York. J. Econ. Entomol. 2003, 96, 1843–1848. [Google Scholar] [CrossRef]
- Shelton, A.M.; Zhao, J.-Z.; Nault, B.A.; Plate, J.; Musser, F.R.; Larentzaki, E. Patterns of insecticide resistance in onion thrips (Thysanoptera: Thripidae) in onion fields in New York. J. Econ. Entomol 2006, 99, 1798–1804. [Google Scholar] [CrossRef]
- MacIntyre Allen, J.K.; Scott-Dupree, C.D.; Tolman, J.H.; Ron Harris, C. Resistance of Thrips tabaci to pyrethroid and organophosphorus insecticides in Ontario, Canada. Pest Manag. Sci. Pest Manag Sci 2005, 61, 809–815. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Waters, T.D.; Lavine, M.D.; Walsh, D.B.; Lavine, L.C. Multiple insecticide resistance in onion thrips populations from Western USA. Pestic. Biochem. Physiol. 2020, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture Organic Regulations. Available online: https://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&sid=3f34f4c22f9aa8e6d9864cc2683cea02&tpl=/ecfrbrowse/Title07/7cfr205_main_02.tpl (accessed on 28 February 2021).
- Waterer, D. Effect of Soil Mulches and Herbicides on Production Economics of Warm Season Vegetable Crops in a Cool Climate. HortTechnology 2000, 10, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.; Gallandt, E.R. A systems comparison of contrasting organic weed management strategies. Weed Sci. 2018, 66, 109–120. [Google Scholar] [CrossRef]
- Chakraborty, D.; Nagarajan, S.; Aggarwal, P.; Gupta, V.K.; Tomar, R.K.; Garg, R.N.; Sahoo, R.N.; Sarkar, A.; Chopra, U.K.; Sundara Sarma, K.S.; et al. Effect of mulching on soil and plant water status, and the growth and yield of wheat (Triticum aestivum L.) in a semi-arid environment. Agric. Water Manag. 2008, 95, 1323–1334. [Google Scholar] [CrossRef]
- Zribi W; Aragüés R; Medina E; Faci JM Efficiency of inorganic and organic mulching materials for soil evaporation control. Soil Tillage Res. 2015, 148, 40–45. [CrossRef] [Green Version]
- Qin, W.; Hu, C.; Oenema, O. Soil mulching significantly enhances yields and water and nitrogen use efficiencies of maize and wheat: A meta-analysis. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Heißner, A.; Schmidt, S.; von Elsner, B. Comparison of plastic films with different optical properties for soil covering in horticulture: Test under simulated environmental conditions. J. Sci. Food Agric. 2005, 85, 539–548. [Google Scholar] [CrossRef]
- Lamont, W.J. Plastic mulches for the production of vegetable rops. Horttechnology 1993, 3, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Lamont, W.J. Plastics: Modifying the microclimate for the production of vegetable crops. Horttechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E. Effect of living (buckwheat) and UV reflective mulches with and without imidacloprid on whiteflies, aphids and marketable yields of zucchini squash. Int. J. Pest Manag. 2010, 56, 31–39. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E.; Webb, S.E. Suppression of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae) and incidence of Cucurbit leaf crumple virus, a whitefly-transmitted virus of zucchini squash new to Florida, with mulches and imidacloprid. Florida Entomol. 2008, 91, 460–465. [Google Scholar] [CrossRef]
- Frank, D.L.; Liburd, O.E. Effects of living and synthetic mulch on the population dynamics of whiteflies and aphids, their associated natural enemies, and insect-transmitted plant diseases in zucchini. J. Environ. Entomol. 2005, 34, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Reitz, S.R.; Yearby, E.L.; Funderburk, J.E.; Stavisky, J.; Timur Momol, M.; Olson, S.M. Integrated management tactics for Frankliniella thrips (Thysanoptera: Thripidae) in field-grown pepper. J. Econ. Entomol. 2003, 96, 1201–1214. [Google Scholar] [CrossRef]
- Terry, L.I. Host selection, communication and reproductive behaviour. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 175–196. [Google Scholar]
- Stavisky, J.; Funderburk, J.; Brodbeck, B.V.; Olson, S.M.; Andersen, P.C. Population dynamics of Frankliniella spp. and tomato spotted wilt incidence as influenced by cultural management tactics in tomato. J. Econ. Entomol. 2002, 95, 1216–1221. [Google Scholar] [CrossRef]
- Vos, J.G.M.; Uhan, T.S.; Sutatya, R. Integrated crop management of hot pepper (Capsicum spp.) under tropical lowland conditions: Effects of rice straw and plastic mulches on crop health. Crop Prot. 1995, 14, 445–452. [Google Scholar] [CrossRef]
- Razzak, M.A.; Seal, D.R. Effect of plastic mulch on the abundance of Thrips palmi Karny (Thysanoptera: Thripidae) and yield of jalapeno pepper in south Florida. Proc. Florida State Hortic. Soc. 2017, 130, 124–128. [Google Scholar]
- Lu, F.M. Color preference and using silver mulches to control the onion thrips, Thrips tabaci Lindeman. Chin. J. Entomol. 1990, 10, 337–342. [Google Scholar]
- Van Toor, R.F.; Till, C.M.; James, D.E.; Teulon, D.A.J. Evaluation of UF reflective mulches for protection against thrips (Thrips tabaci) in onion (Allium cepa) crops. N. Z. Plant Prot. 2004, 57, 209–213. [Google Scholar]
- Cramer, C.S.; Singh, N.; Kamal, N.; Pappu, H.R. Screening onion plant introduction accessions for tolerance to onion thrips and iris yellow spot. HortScience 2014, 49, 1253–1261. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fail, J.; Deutschlander, M.; Nault, B.A.; Shelton, A.M. Characterization of Resistance, Evaluation of the Attractiveness of Plant Odors, and Effect of Leaf Color on Different Onion Cultivars to Onion Thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2012, 105, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Munaiz, E.D.; Groves, R.L.; Havey, M.J. Amounts and types of epicuticular leaf waxes among onion accessions selected for reduced damage by onion thrips. J. Am. Soc. Hortic. Sci. 2020, 145, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Shelton, A.M. Evaluation of onion cultivars for resistance to onion thrips (Thysanoptera: Thripidae) and iris yellow spot virus. J. Econ. Entomol. 2010, 103, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Damon, S.J.; Groves, R.L.; Havey, M.J. Variation for epicutic- ular waxes and numbers of Thrips tabaci on onion foliage. J. Am. Soc. Hortic. Sci. 2014, 139, 495–501. [Google Scholar]
- Damon, S.J.; Havey, M.J. Quantitative trait loci controlling amounts and types of epicuticular waxes in onion. J. Am. Soc. Hortic. Sci. 2014, 139, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.K.; Molenaar, N.D. Powdery mildew caused by Leveillula taurica on glossy leaf genotypes of onion in Idaho. Plant Dis. 2005, 89, 431. [Google Scholar] [CrossRef]
- Leach, A.; Reiners, S.; Nault, B. Challenges in integrated pest management: A case study of onion thrips and bacterial bulb rot in onion. Crop Prot. 2020, 105123. [Google Scholar] [CrossRef]
- Leach, A.; Reiners, S.; Fuchs, M.; Nault, B. Evaluating integrated pest management tactics for onion thrips and pathogens they transmit to onion. Agric. Ecosyst. Environ. 2017, 250, 89–101. [Google Scholar] [CrossRef]
- Nault, B.A.; Huseth, A.S. Evaluating an action threshold-based insecticide program on onion cultivars varying in resistance to onion thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2016, 109, 1772–1778. [Google Scholar] [CrossRef]
- Thompson, G.D.; Dutton, R.; Sparks, T.C. Spinosad—A case study: An example from a natural products discovery programme. Pest Manag. Sci. 2000, 56, 696–702. [Google Scholar] [CrossRef]
- Nault, B.A.; Hessney, M. Lou Onion thrips control in onion, 2005. Arthropod Manag. Tests 2005, 31, 1–2. [Google Scholar]
- Waters, T.D.; Skoczylas, J.C. Thrips control on dry bulb onions in Washington state, 2012. Arthropod Manag. Tests 2015, 40, 1. [Google Scholar] [CrossRef] [Green Version]
- Nault, B.A.; Hessney, M. Lou Onion thrips control in onion, 2009. Arthropod Manag. Tests 2010, 35, 1–2. [Google Scholar] [CrossRef]
- Leach, A.B.; Hoepting, C.A.; Nault, B.A. Grower adoption of insecticide resistance management practices increase with extension-based program. Pest Manag. Sci. 2019, 75, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.Z.; Chapman, S.A.; Crubaugh, L.K.; Groves, R.L. Evaluation of foliar insecticides for the control of onion thrips in dry-bulb onion, 2018. Arthropod Manag. Tests 2019, 44, 1–2. [Google Scholar] [CrossRef]
- Bradford, B.Z.; Chapman, S.A.; Groves, R.L. Evaluation of Foliar Insecticides for the Control of Onion Thrips in Dry-Bulb Onion in Wisconsin, 2019. Arthropod Manag. Tests 2020, 45, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Nault, B.A.; Huseth, A.S.; Smith, E.A. Onion thrips control in onion, 2013. Arthropod Manag. Tests 2014, 39, 2013–2014. [Google Scholar] [CrossRef] [Green Version]
- Reiners, S.; Wallace, J.; Curtis, P.D.; Helms, M.; Landers, A.J.; McGrath, M.T.; Nault, B.A.; Seaman, A. 2019 Cornell integrated crop and pest management guidelines for commercial vegetable production. Cornell Coop. Ext. 2019. [Google Scholar]
- Brewster, J.L. Onions and Other Vegetable Alliums, 2nd ed.; CABI International: Wallingford, UK, 2008. [Google Scholar]
- Iglesias, L.; Groves, R.L.; Bradford, B.; Harding, R.S.; Nault, B.A. Evaluating combinations of bioinsecticides and adjuvants for managing Thrips tabaci (thysanoptera: Thripidae) in onion production systems. Crop Prot. 2021, 142, 105527. [Google Scholar] [CrossRef]
- Agricultural Marketing Service United States Standards for Grades of Bermuda-Granex-Grano Type Onions; United States Department of Agriculture: Washington, WA, USA, 2014.
- Kramer-Wilson, K.; Cranshaw, W. Onion thrips control, foliar trial 2, 1997. Arthropod Manag. Tests 1998, 23, 111–112. [Google Scholar]
- Khaliq, A.; Khan, A.; Afzal, M.; Tahir, H.M.; Raza, A.M.; Khan, A.M. Field evaluation of selected botanicals and commercial synthetic insecticides against Thrips tabaci Lindeman (Thysanoptera: Thripidae) populations and predators in onion field plots. Crop Prot. 2014, 62, 10–15. [Google Scholar] [CrossRef]
- Malik, M.F.; Nawaz, M.; Haffez, Z. Efficacy of Synthetic Insecticide and Botanical Infusions Against Onion Thrips in Balochistan, Pakistan-I. Asian J. Plant Sci. 2003, 2, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Fitiwy, I.; Gebretsadkan, A.; Ayimut, K. Evaluation of botanicals for onion thrips, Thrips tabaci Lindeman, (Thysanoptera: Thripidae) control at Gum Selassa, South Tigray, Ethiopia. Momona Ethiop. J. Sci. 2015, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Damon, S.J.; Groves, R.L.; Havey, M.J. Variation for epicuticular waxes on onion foliage and impacts on numbers of onion thrips. J. Am. Soc. Horticult. Sci. 2014, 139, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Loges, V.; Lemos, M.A.; Resende, L.V.; Menezes, D.; Candeia, J.A.; Dos Santos, F.V. Resistência de cultivares e híbridos de cebola a tripes. Hortic. Bras. 2004, 22, 222–225. [Google Scholar] [CrossRef]
- Nault, B.A.; Shelton, A.M. Impact of insecticide efficacy on developing action thresholds for pest management: A case study of onion thrips (Thysanoptera: Thripidae) on onion. J. Econ. Entomol. 2010, 103, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Fournier, F.; Boivin, G.; Stewart, R.K. Effect of Thrips tabaci (Thysanoptera: Thripidae) on yellow onion yields and economic thresholds for its management. J. Econ. Entomol. 1995, 88, 1401–1407. [Google Scholar] [CrossRef]
- Hoepting, C.; Klotzbach, K.; Reid, J.; Gugino, B. Stop the Rot! Using Cultural Practices to Reduce Bacterial Bulb Decay in Onions; Cornell Cooperative Extension: Ithaca, NY, USA, 2011. [Google Scholar]
- Bag, S.; Schwartz, H.F.; Cramer, C.S.; Havey, M.J.; Pappu, H.R. Iris yellow spot virus (Tospovirus: Bunyaviridae): From obscurity to research priority. Mol. Plant Pathol. 2015, 16, 224–237. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Level | Manufacturer | Rate |
|---|---|---|---|
| Mulch | White | FilmTech Corp., Allentown, PA | - |
| Silver | Rain-Flo Irrigation, East Earl, PA | - | |
| Cultivar | “Bradley” | Waxy hybrid from Bejo Seeds, Geneva, NY | - |
| “Rossa di Milano” | Open pollinated population with semi-glossy foliage from Johnny’s Selected Seeds, Winslow, ME | - | |
| B5336AxB5351C | Experimental hybrid with semi-glossy foliage developed by MJH | - | |
| Pesticide | Spinosad + neem oil (tank mix) | Corteva Agriscience, Indianapolis, IN + Certis USA, LLC, Columbia, MD | 0.6 L/ha + 1% v:v |
| None | - | - |
| Reflective Mulch (Mean ± SE) | White Mulch (Mean ± SE) | ||||
|---|---|---|---|---|---|
| Date | Biopesticide | None | Spinosad + Neem Oil | None | Spinosad + Neem Oil |
| 07/09/19 | 0.19 ± 0.01a | 0.17 ± 0.02b | 0.43 ± 0.02a | 0.33 ± 0.02b | |
| 07/16/19 | 0.62 ± 0.06a | 0.45 ± 0.04b | 1.07 ± 0.09a | 0.75 ± 0.06c | |
| 07/24/19 | 1.12 ± 0.09a | 0.69 ± 0.06b | 1.80 ± 0.11b | 1.08 ± 0.08c | |
| 07/31/19 | 1.79 ± 0.16a | 0.68 ± 0.05b | 2.93 ± 0.18c | 0.95 ± 0.08d | |
| 08/08/19 | 7.83 ± 0.85a | 0.80 ± 0.07b | 9.30 ± 0.65c | 1.24 ± 0.09d | |
| 08/14/19 | 5.91 ± 0.39b | 0.59 ± 0.06a | 4.70 ± 0.35c | 0.82 ± 0.07c | |
| 08/22/19 | 0.61 ± 0.06a | 0.65 ± 0.08ab | 0.47 ± 0.03b | 0.70 ± 0.07ab | |
| Reflective (Mean ±SE) | White (Mean ±SE) | ||||||
|---|---|---|---|---|---|---|---|
| Date | Cultivar | Bradley | Rossa | B5336xB5351 | Bradley | Rossa | B5336xB5351 |
| 07/09/19 | 0.22 ± 0.02c | 0.15 ± 0.01c | 0.18 ± 0.02bc | 0.40 ± 0.03a | 0.37 ± 0.02ab | 0.37 ± 0.03ab | |
| 07/16/19 | 0.68 ± 0.06b | 0.40 ± 0.05c | 0.53 ± 0.08bc | 1.10 ± 0.10a | 0.72 ± 0.06ab | 0.91 ± 0.11ab | |
| 07/24/19 | 1.00 ± 0.08a | 0.71 ± 0.06b | 0.99 ± 0.15ab | 1.49 ± 0.13a | 1.27 ± 0.16ab | 1.56 ± 0.18a | |
| 07/31/19 | 1.49 ± 0.25bc | 0.89 ± 0.13d | 1.33 ± 0.22bc | 2.10 ± 0.39a | 1.68 ± 0.33cd | 2.04 ± 0.29ab | |
| 08/08/19 | 4.87 ± 1.48ab | 3.17 ± 0.79c | 4.89 ± 1.44ab | 4.98 ± 1.33a | 4.57 ± 1.05bc | 6.26 ± 1.55ab | |
| 08/14/19 | 3.58 ± 0.94a | 2.68 ± 0.72b | 3.49 ± 0.92a | 2.49 ± 0.53ab | 2.85 ± 0.70a | 2.95 ± 0.74ab | |
| 08/22/19 | 0.64 ± 0.05 | 0.70 ± 0.11 | 0.53 ± 0.10 | 0.54 ± 0.07 | 0.62 ± 0.08 | 0.59 ± 0.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, L.; Havey, M.J.; Nault, B.A. Management of Onion Thrips (Thrips tabaci) in Organic Onion Production Using Multiple IPM Tactics. Insects 2021, 12, 207. https://doi.org/10.3390/insects12030207
Iglesias L, Havey MJ, Nault BA. Management of Onion Thrips (Thrips tabaci) in Organic Onion Production Using Multiple IPM Tactics. Insects. 2021; 12(3):207. https://doi.org/10.3390/insects12030207
Chicago/Turabian StyleIglesias, Lindsy, Michael J. Havey, and Brian A. Nault. 2021. "Management of Onion Thrips (Thrips tabaci) in Organic Onion Production Using Multiple IPM Tactics" Insects 12, no. 3: 207. https://doi.org/10.3390/insects12030207
APA StyleIglesias, L., Havey, M. J., & Nault, B. A. (2021). Management of Onion Thrips (Thrips tabaci) in Organic Onion Production Using Multiple IPM Tactics. Insects, 12(3), 207. https://doi.org/10.3390/insects12030207