Simple Summary
Plutella xylostella is a major pest of Cruciferae vegetables all over the world. Gut bacteria play an important role in the life activities of P. xylostella, but so far, little is known about the source and transmission of gut bacteria of P. xylostella. Therefore, we used the traditional microbial culture method to show that there is a potential correlation between the gut bacteria of P. xylostella and food bacteria, and P. xylostella gut bacteria exhibit vertical and horizontal transmission through eggs. Our research results will contribute to biological pest control based on gut bacteria.
Abstract
Plutella xylostella (L.), commonly known as the diamondback moth, is currently a major worldwide pest. Gut bacteria play an important role in the physiology and insecticide resistance of P. xylostella, but little is known about the sources and transmission routes of its gut bacteria. In this study, traditional microbial culture methods were used to analyze the sources and transmission modes of gut bacteria in P. xylostella, which could help develop pest control strategies based on gut bacteria. The main findings are as follows: gut bacterial diversity was significantly higher in P. xylostella-fed radish sprouts than those fed an artificial diet, indicating a potential association between gut bacteria and food bacteria. In addition, sequence analysis revealed the isolation of Enterobacter sp., Pantoea sp., Cedecea sp., and Pseudomonas sp. from both radish sprouts and P. xylostella. Importantly, Enterobacter sp. was found in all tested samples (radish sprouts, gut of P. xylostella, ovaries, and eggs), suggesting that bacteria acquired from food could be transferred from the gut to the ovaries and eggs. This was confirmed through experiments, which also showed that eggs could carry bacteria and transmit them to the gut, indicating vertical transmission of gut bacteria via eggs. Furthermore, the 3rd instar larvae of P. xylostella with and without gut bacteria were mixed and raised until the 4th instar. Then, we found that all the gut of the 4th instar larvae carried the same bacteria, indicating that the gut bacteria of P. xylostella can be horizontally transmitted through social behavior. This study lays a foundation for further exploration of the sources, transmission, and coevolution of the host of gut bacteria in P. xylostella, and provides new insights into pest control strategies based on the source and transmission of gut bacteria.
1. Introduction
Insects have a complex symbiotic relationship with gut bacteria, which help the hosts digest food [1], resist parasites and pathogens [2,3], facilitate inter-species communication [4], and regulate mating and reproductive systems [5,6]. Therefore, research on insect gut bacteria is particularly important in the field of plant protection. The structure of insect gut bacteria is influenced by the type of food they consume. For example, Diaphorina citri has significant differences in gut bacteria among different hosts, with the highest gut bacteria diversity found in insects that feed on Citrus poonensis cv. Ponkan and the lowest diversity in those that feed on Citrus reticulata cv. Shatangju [7]. Host plants have a significant impact on the structure and composition of gut bacteria in Spodoptera frugiperda [8].
It is worth noting that insects have evolved various mechanisms to vertically transmit beneficial bacteria to their offspring or horizontally spread them within and between populations [9]. Studies have shown that gut bacteria Snodgrassella alvi and Gilliamella apicola in field Bombus terrestris populations can be vertically transmitted from mothers to offspring [10]. Serratia symbiotica, a gut bacterium in Aphidoidea, can also be transmitted vertically from mothers to offspring [11]. Social insects, such as Cryptocercus sp., Reticulitermes speratus, and Apis mellifera, which engage in trophallaxis or coprophagy, can directly or indirectly facilitate the horizontal transmission of gut bacteria, promoting the coevolution of host insects with their gut bacteria [12,13,14]. In an experiment where 20 newly emerged bees and 20 older worker bees from the same hive but marked with different colored paint were mixed and fed with bee bread in a cage, characteristic bacteria were detected in the gut of the newly emerged bees [14], indicating that the gut bacteria of bees can be horizontally transmitted through social activity within the population. Wolbachia of Homalotylus is also capable of horizontal transmission between populations [15].
The diamondback moth Plutella xylostella (L.) (Lepidoptera: Plutellidae), is a major pest of cruciferous vegetables distributed worldwide [16,17]. The life cycle of P. xylostella includes egg, larva, pupa, and adult, with the larvae consisting of four instars. Early studies investigated the abundance and diversity of gut bacteria in P. xylostella at different developmental stages [18]. Subsequently, detailed studies were conducted on gut bacteria in P. xylostella populations collected from different geographic regions in India, revealing that gut bacteria of P. xylostella are influenced by different geographic regions, which may be due to changes in latitude, environmental factors, and the insect’s adaption to its local climate [19]. However, research has shown that both environmental factors and food sources have an impact on the diversity of insect gut bacteria [20]. Our previous research studied the composition of gut bacteria in P. xylostella [21], its functional relationship with host feeding [22], and its relationship with insecticide resistance [23]. However, little is known about the source and transmission mode of gut bacteria in P. xylostella. In this study, we aim to analyze the potential correlation between gut bacteria in P. xylostella and food bacteria, study its vertical and horizontal transmission, lay a foundation for further research on gut bacteria in P. xylostella, and provide ideas for controlling P. xylostella based on the source and transmission of gut bacteria.
2. Materials and Methods
2.1. Feeding P. xylostella
P. xylostella used in this study was from the Institute of Zoology, the Chinese Academy of Sciences, and was domesticated by feeding on an artificial diet. The artificial diet consisted of 6 g of agar mixed with 250 mL of ddH2O, heated in a microwave until fully dissolved, and cooled to about 70 °C. Then, 37.5 g of wheat bran, 20 g of yeast powder, 10 g of sucrose, 3 g of radish seeds, 0.8 g of compound vitamins, 1 g of citric acid, 1 g of nipagin, and 1 g of vitamin C were added, followed by 1 mL of rapeseed oil and 50 μL of linoleic acid. The mixture was stirred well before use. Feeding P. xylostella separately with an artificial diet and radish sprouts resulted in two strains: the artificial diet strain and the radish sprout strain. The larvae possess four instars in both diets. The artificial climate room for rearing larvae was maintained at a temperature of 25 ± 1 °C, a relative humidity (RH) of 40–70%, and a light/dark photoperiod (L:D) of 16:8 h. Radish sprouts, fed upon by the larvae, were grown in an artificial climate room with a temperature of 23 ± 1 °C and an RH of approximately 75%. The variety of radish seeds used was “Spring full ground Nanpanzhou Daiko” (Fuzhou Yongrong Seed Co., Ltd., Fuzhou, China). The artificial climate room for rearing the larvae on radish sprouts was maintained at a temperature of 25 ± 1 °C, an RH of 70–80%, and an L:D photoperiod of 16:8 h. Adult moths were provided with 10% honey water as food.
2.2. Isolation, Culture, and Identification of Symbiotic Bacteria
2.2.1. Isolation and Culture of Bacteria from Radish Sprouts
Radish sprouts were planted using horticultural universal cultivation soil (Rongfeng Horticulture Company, Guangzhou, China). After 3 days, the seeds germinated, and after 7 days, ten radish leaves were randomly selected (each leaf was approximately 87 mm2 in size). The leaves were placed in 2 mL centrifuge tubes with 200 μL of sterile water, and crushed using a pipette tip, two sterilized steel beads were added to each tube, then the tubes were shaken until the leaves were completely dissolved. The culture media include Luria-Bertani medium (LB) [24], nutrient agar (NA) [24], and anaerobic agar (mixing 20.0 g of pancreatic digest of casein, 5.0 g of sodium chloride, 10.0 g of dextrose, 1.0 g of sodium formaldehyde sulfoxylate, 2.0 g of sodium thioglycolate, 0.002 g of aniline blue water soluble, 20 g of agar with 1 L of distilled water, 7.2 of potential hydrogen (pH), then sterilized by high-pressure steam at 121 °C for 30 min after being packaged and sealed). Ten-fold serial (10−1, 10−2, and 10−3) dilutions of dissolved solution of radish leaves were plated on LB and NA mediums. The stock solution and ten-fold serial (10−1 and 10−2) dilutions of dissolved solution of radish leaves were plated on anaerobic agar medium. An amount of 20 μL of each dilution mentioned above was spread onto the culture media, repeated 3 times, and incubated in a 37 °C incubator for 96 h. Isolation and cultivation of bacteria: Individual bacterial colonies with different sizes, colors, and morphologies were isolated and purified five times on LB mediums using an inoculation loop to obtain single clones (Figure S1). After purification, the bacterial strains were cultured in liquid LB mediums and preserved with 25% glycerol at −80 °C.
2.2.2. Isolation and Culture of Gut Bacteria from P. xylostella
Dissection: 30 healthy 4th instar larvae (the P. xylostella start feeding heavily from the beginning of the 4th instar), 30 pupae, and 30 adults of P. xylostella were randomly selected for dissection. The 4th instar larva is a representative larval stage of P. xylostella. Due to a large amount of feeding, there are abundant microorganisms in the gut, and this stage is at the end of the larval stage, making it easier to compare its association with the gut microbiota of pupae and adults. Meanwhile, the insect body is large and easy to dissect; thus, the 4th instar larvae were chosen for study. Before dissection, the adults were frozen at −20 °C for 5 min to immobilize them. The selected insects (the 4th instar larvae, pupae. and adults) were dissected on a UV-sterilized and ultra-clean workbench. Their bodies were washed with sterile water, surface-sterilized with 75% ethanol for 1 min, and then washed again with sterile water. The isolated gut tissues were put into 2 mL centrifuge tubes containing 200 μL sterile water, crushed with a pipette tip, and then two sterilized steel beads were added and shaken until the gut tissues were completely dissolved. The LB, NA, and anaerobic agar mediums were used for bacterial culture. Both ten-fold serial (10−3, 10−4, and 10−5) dilutions of the dissolved solution of larval guts and ten-fold serial (10−2, 10−3, 10−4, and 10−5) dilutions of the dissolved solution of pupal guts were plated on LB, NA, and anaerobic agar mediums. Ten-fold serial (10−2, 10−3, and 10−4) dilutions of the dissolved solution of adult guts were plated on LB and NA mediums. Ten-fold serial (10−1, 10−2, and 10−3) dilutions of the dissolved solution of adult guts were plated on anaerobic agar medium. An amount of 10 μL of each dilution mentioned above was spread onto the culture medium, repeated 3 times, and incubated in a 37 °C incubator for 96 h (Figure S2A–C). Bacterial isolation and cultivation conditions were the same as in Section 2.2.1 (Figure S3A–C).
2.2.3. Isolation and Culture of Bacteria from the Ovary of P. xylostella
Ten healthy female P. xylostella were randomly selected, and the dissection was the same as in Section 2.2.2. The stock solution and ten-fold serial (10−1 and 10−2) dilutions of the dissolved solution of ovaries were plated on LB, NA, and anaerobic agar mediums. An amount of 10 μL of each dilution mentioned above was spread onto the culture medium, repeated 3 times, and incubated in a 37 °C incubator for 96 h (Figure S2D). The isolation and culture conditions of bacteria were the same as in Section 2.2.1 (Figure S3D), and the experiment was repeated three times.
2.2.4. Isolation and Culture of Bacteria from Eggs of P. xylostella
A new oviposition card was placed in the adult rearing cage. After 30 min, 200 eggs of P. xylostella were collected and placed in a 2 mL centrifuge tube with 200 μL sterile water. Two sterilized steel beads were added to the tube, which was then shaken until the eggs were completely dissolved. Ten-fold serial (10−2, 10−3, 10−4, and 10−5) dilutions of the dissolved solution of eggs were plated on LB, NA, and anaerobic agar mediums. An amount of 20 μL of each dilution mentioned above was spread onto the culture medium, repeated 3 times, and incubated in a 37 °C incubator for 96 h (Figure S2E). The isolation and culture conditions of bacteria are the same as in Section 2.2.1 (Figure S3E), and the experiment was repeated three times.
2.2.5. Identification of Bacteria
DNA was extracted from isolated and purified bacteria. DNA Extraction Kit: TaKaRa MiniBEST Bacterial Genomic DNA Extraction Kit Ver.3.0 (TaKaRa Biomedical Technology (Beijing) Co., Ltd., Beijing, China). Amplification was performed as previously described [24]. DNA was amplified using universal primers (27 F, 1492 R). DNA polymerase: Phanta Max Super-fidelity DNA Polymerase (Nanjing Vazyme Biotech Co., Ltd, Nanjing, China). Then, amplified DNA was sent to Boshang Biological Corporation (Shanghai, China) for sequencing, the results were compared by blast, and phylogeny was compared.
2.3. Vertical Transmission of Gut Bacteria of P. xylostella
2.3.1. Tracing Gut Bacterial Transmission by Resistant Bacteria
1. Preparation of anti-kanamycin Enterobacter sp. RE1-kN: (1) The competent cells of Enterobacter sp. RE1 (GenBank Access Number: MH141495) were prepared, and the anti-kanamycin GFP plasmid (PET28a-EGFP plasmid (Miaoling Biotechnology Corporation, Wuhan, China)) was introduced into them. (2) Some single colonies of anti-kanamycin Enterobacter sp. RE1-KN were selected, and they were shaken at 37 ℃ and 200 rpm overnight, then the shaken bacterial suspension was poured into a sterile 50 mL centrifuge tube in an ultra-clean workbench sterilized by UV for 30 min, and centrifuged for 10 min at 5000 rpm in a high-speed refrigerated centrifuge. (3) The above-mentioned centrifuge tube was shaken on a vortex mixer until the precipitate was dispersed, and then sterile water was added. After shaking evenly, the mixture was centrifuged at 5000 rpm for 10 min, and the supernatant was discarded. This washing step was repeated three times. (4) Then, the above-mentioned centrifuge tube was filled with sterile water, mixed evenly, and diluted the original solution 7 times, then the OD600 value was measured using a UV–Visible spectrophotometer, and the OD600 value of the original solution was calculated.
2. Rearing P. xylostella: (1) 40 mL sterile water and 40 μL of 50 mg/mL kanamycin were added to a sterile glass bottle. (2) An amount of 100 mL artificial diet was poured into a disposable culture dish and cut into 2 × 2 cm square pieces with a blade, put into kanamycin solution, soaked for 30 min, and then they were dried. (3) The 3rd instar larvae were fed with a diet soaked in kanamycin solution for 24 h, and then they were fed with a diet soaked in Enterobacter sp. RE1-KN solution (OD600 = 2.0) for 30 min.
3. Detection: (1) P. xylostella fed on a diet containing Enterobacter sp. RE1-KN, then LB solid mediums containing kanamycin were coated with the gut solution of the P. xylostella (4th instar larvae, pupae, and adults), adult ovaries, and the sterile water, which soaked eggs (The eggs of P. xylostella feeding on the diet containing Enterobacter sp. RE1-KN were brushed onto a sterile weighing paper with a sterile bristle brush. Then, the eggs were placed in a centrifuge tube, and sterile water was added). The plates were sealed and incubated upside down at 37 °C for 12 h. (2) PCR detection: Some single colonies were selected and put into a 1.5 mL centrifuge tube containing 20 μL Elution Buffer (Nanjing Vazyme Biotech Co., Ltd., Nanjing, China) that can elute PET28a-EGFP plasmid from Enterobacter sp. RE1-KN, then it was heated in a water bath at 95 °C for 10 min. After centrifugation, 1 μL of supernatant was obtained, and PCR amplification was performed using T7 primer selection system (Table S1) and procedure (Table S2).
2.3.2. Detection of Egg Surface Bacteria Entering the Gut
1. (1) The sterile artificial diet was sub-packed into a conical flask and dried on an ultra-clean workbench. Sterile artificial diet: 15 g wheat germ powder, 8 g yeast powder, 4 g sucrose, 2.4 g agar, 1.2 g radish seeds, and 100 mL pure water was added into a 250-mL conical flask, then 400 μL rapeseed oil and 25 μL linoleic acid was added. After mixing well, the mouth of the conical flask was wrapped with 8 layers of medical degreased gauze and sealed with sealing film, then the conical flask was sterilized at 115 °C under high-pressure steam for 30 min. Afterward, the mixture (0.032 g multivitamin, 0.04 g sorbic acid, 0.04 g nipagin, 0.04 g Vitamin C, and 5 mL pure water) was filtered and sterilized through a microporous filter film with a pore size of 0.23 μm before being added. (2) The sealing film containing eggs of P. xylostella was washed once with sterile water, sterilized with 1.5% sodium hypochlorite for 15 s, then washed twice with sterile water and dried (Sterile water used to clean the sealing film containing eggs of P. xylostella for the last time was coated on LB solid mediums to test whether the sterilization was complete.), as the control group. The treatment group was left to soak the Enterobacter sp. RE1-KN solution (OD600 = 2.0) for 30 min and dry. (3) The sealing films containing eggs of the control group and the treatment group were put into the glass culture bottles containing sterile artificial diet, each bottle mouth was wrapped with sterile 8-layer medical absorbent gauze, sealed with a rubber band, and then the culture bottles were tilted for cultivation.
2. Detection (1) LB solid mediums containing kanamycin were coated with the gut solution of 4th instar larvae in the treatment group and control group, which were sealed and put into an incubator at 37 °C. After 12 h, the LB solid mediums were observed for colony growth. (2) PCR detection was the same as in Section 2.3.1.
2.4. Horizontal Transmission of Gut Bacteria of P. xylostella
(1) The initial 3rd instar larvae were selected and starved for 12 h. (2) The P. xylostella in the control group were fed with a normal diet after starvation, and the P. xylostella in the treatment group were fed with a diet soaked in Enterobacter sp. RE1-KN solution (OD600 = 2.0) for 30 min. The P. xylostella in both the control and treatment groups was raised for 24 h. (3) 5 larvae in the treatment group and 5 larvae in the control group were placed in the same new insect-rearing box and fed with a normal artificial diet (4 repetitions). Fresh diet was changed once a day and the gut of 4th instar larvae were dissected. (4) PCR detection of gut bacteria was the same as in Section 2.3.1.
3. Results
3.1. Isolation and Identification of Bacteria from Radish Sprouts
In this study, 24 strains of different bacteria were isolated and purified from radish sprouts (Table S3). Phylogenetic analysis showed that the bacteria isolated from radish sprouts were mainly composed of proteobacteria, actinobacteria, and bacteroidetes, of which proteobacteria was the largest phylum (Figure 1).
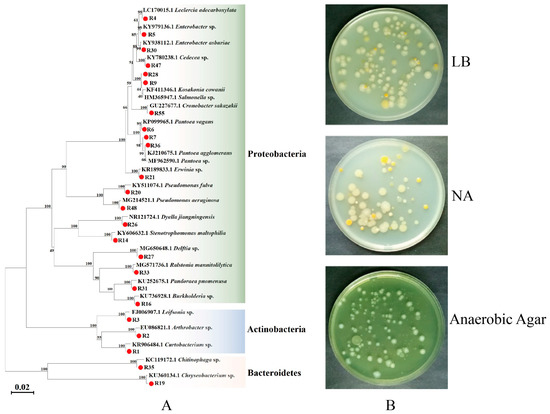
Figure 1.
Phylogenetic analysis of 16S rDNA of bacteria from (A) neighbor-joining tree of bacterial isolates from radish sprouts and their closely related species based on sequencing of the 16S rDNA gene. The numbers corresponding to the red circle in the figure are the bacteria obtained in this study. The nodes’ bootstrap values were based on 1000 replicates. The scaled bar represents 0.02 estimated phylogenetic divergence. (B) Isolation and culture of radish sprout bacteria on Luria-Bertani medium (LB), nutrient agar (NA), and anaerobic agar solid mediums.
3.2. Isolation and Identification of Gut Bacteria of P. xylostella
Seven different strains of bacteria were identified from the gut bacteria of the 4th instar larvae of P. xylostella feeding on radish sprouts. Phylogenetic analysis showed that the bacteria isolated from the gut of the 4th instar larvae of P. xylostella were composed of proteobacteria and actinobacteria, of which proteobacteria was the largest (Figure 2A, Table S4). Six different strains of bacteria were identified in the pupal gut, which was composed of proteobacteria, firmicutes, and actinobacteria (Figure 2B, Table S5). Three different strains of bacteria were identified from the adult gut, composed of proteobacteria and actinobacteria (Figure 2C, Table S6). A total of 12 strains of different bacteria were identified from the ovary, composed of proteobacteria and firmicutes, of which proteobacteria was the largest (Figure 2D, Table S7). A total of 7 strains of different bacteria were identified from the eggs, composed of proteobacteria and firmicutes, of which proteobacteria was the largest (Figure 2E, Table S8). The results showed that the largest phylum of gut symbiotic bacteria of P. xylostella is proteobacteria.

Figure 2.
Neighbor-joining tree of bacterial isolates from P. xylostella and their closely related species based on sequencing of the 16S rDNA gene. The nodes’ bootstrap values were based on 1000 replicates. The scaled bar represents 0.02 estimated phylogenetic divergence. (A–E), respectively, represent phylogenetic analysis of 16S rDNA of bacteria of the 4th instar larval gut, pupal gut, adult gut, ovary, and eggs in P. xylostella. The numbers corresponding to the red circle in the figure are the bacteria obtained in this study: R represents bacteria in radish sprouts, RL represents gut bacteria in the 4th instar larvae of P. xylostella, RP represents gut bacteria in the pupae of P. xylostella, RM represents gut bacteria in adult P. xylostella, OV represents ovarian bacteria in P. xylostella, and SE and RE represent bacteria in eggs of P. xylostella.
3.3. Correlation Analysis between Gut Bacteria of P. xylostella and Food
The common bacteria found through culturing the radish sprouts, the gut of different stages of larvae, ovary, and eggs of P. xylostella were used for phylogenetic analysis. The results showed that the bacteria of the same genus from different sources were clustered in the same branch and closely related, which may be the same species of bacteria (Figure 3). After comparing the bacteria belonging to the same genus in the larval gut, pupal gut, adult gut, ovary, and eggs of P. xylostella that feed on radish sprouts, it was found that these bacteria have a high degree of homology (Table 1). Previous studies suggested that the 16S rDNA sequence identity of the bacteria was more than 97%, which could be considered the same species [25]. Sequence analysis showed that the bacteria of the same genus isolated from radish sprouts and the gut, ovary, and eggs of P. xylostella could be considered the same bacteria (Table 1).
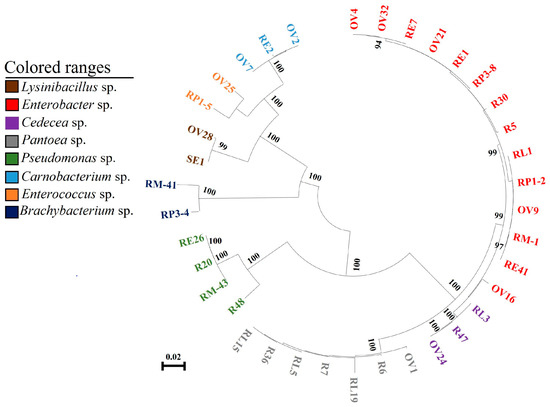
Figure 3.
Neighbor-joining tree of bacterial isolates from P. xylostella. The nodes’ bootstrap values were based on 1000 replicates. The scaled bar represents 0.02 estimated phylogenetic divergence. R represents bacteria in radish sprouts, RL represents gut bacteria in the 4th instar larvae of P. xylostella, RP represents gut bacteria in the pupae of P. xylostella, RM represents gut bacteria in adult P. xylostella, OV represents ovarian bacteria in P. xylostella, and SE and RE represent bacteria in eggs of P. xylostella. As shown in the colored range in the figure, different colors indicate different genera.

Table 1.
Comparison of bacteria of the same genus from different sources.
Proteobacteria and actinobacteria were the main bacteria in the gut of P. xylostella feeding on radish sprouts (Table S9). In proteobacteria, the same Enterobacter sp., Pantoea sp., and Cedecea sp. were found in the 4th instar larval gut of P. xylostella and radish sprouts (Table S10). In addition, the gut bacteria of P. xylostella feeding on radish sprouts and artificial diet were significantly different on the LB medium (Figure S4). These results indicated that the gut bacteria of P. xylostella are potentially related to the food it eats.
The bacteria in the gut, ovaries, and eggs of P. xylostella were mainly composed of bacteria from the phyla proteobacteria and firmicutes (Table S9). P. xylostella had the same Enterobacter sp. in its gut, ovaries, and eggs. The ovaries and the 4th instar larval gut of P. xylostella had the same Enterobacter sp., Pantoea sp., and Cedecea sp., the ovaries and eggs had the same Enterobacter sp., Carnobacterium sp, and Lysinibacillus sp. (Table S10). These indicated that the gut bacteria of P. xylostella may be transferred to the ovary, and the ovary to the egg, to realize the vertical transmission of gut bacteria of P. xylostella.
3.4. Analysis of Vertical Transmission of Gut Bacteria from P. xylostella
Enterobacter sp. RE1-KN has kanamycin resistance and can be used as an indicator for screening and identification. The experiment found that there was no Enterobacter sp. RE1-KN in the gut, ovary, and egg surface of P. xylostella feeding with a normal diet, while Enterobacter sp. RE1-KN was detected in the 4th instar larval gut, pupal gut, adult gut, ovary, and egg surface of P. xylostella feeding with a diet containing Enterobacter sp. RE1-KN (Figure 4B,C). The results indicated that the gut bacteria of P. xylostella can be transmitted to the ovaries and eggs.
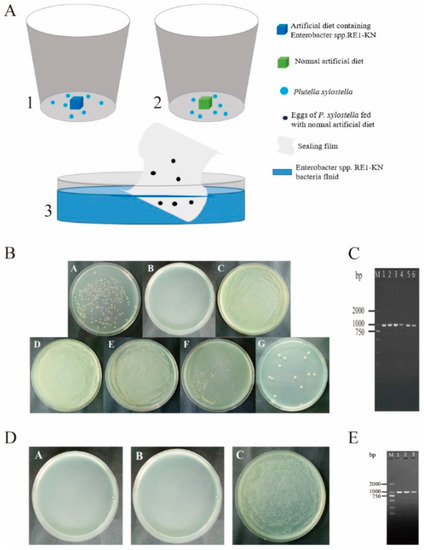
Figure 4.
Validation of vertical transmission of gut bacteria in P. xylostella. (A) Experimental method of vertical transmission of bacteria in the gut of P. xylostella. (A-1) P. xylostella fed with a diet containing Enterobacter sp. RE1-KN, (A-2) P. xylostella fed with a normal diet, (A-3) the sealing film with P. xylostella eggs was soaked in Enterobacter sp. RE1-KN solution; (B) isolation and culture of bacteria from different stages of P. xylostella. (B-A) Enterobacter sp. RE1-KN, (B-B) Control, (B-C) gut of 4th instar larvae, (B-D) pupal gut, (B-E) adult gut, (B-F) adult ovary, (B-G) egg surface. (C) Enterobacter sp. RE1-KN was detected in 4th instar larval gut, pupal gut, adult gut, ovary, and egg surface of P. xylostella feeding with diet containing Enterobacter sp. RE1-KN. (C-M) DL2000 DNA Marker, (C-1) Enterobacter sp. RE1-KN, (C-2) Gut of 4th instar larvae, (C-3) pupal gut, (C-4) adult gut, (C-5) adult ovary, (C-6) egg surface. (D) Isolation and culture of gut bacteria from 4th instar larvae of P. xylostella. (D-A) Sterile water for the last cleaning of eggs: eggs of P. xylostella were sterilized with 1.5% sodium hypochlorite and the sterile water used to clean the eggs for the last time was tested to be free of bacteria with an LB medium, (D-B) gut of the 4th instar larvae were developed from eggs soaked in sterile water, (D-C) gut of the 4th instar larvae which were developed from eggs soaked in Enterobacter sp. RE1-KN solution. (E) The presence of Enterobacter sp. RE1-KN was detected in the gut of the 4th instar larvae which developed from eggs soaked with Enterobacter sp. RE1-KN solution. The PCR amplification (E-1,E-2,E-3) of Enterobacter sp. RE1-KN in the gut of the 4th instar larvae which developed from eggs soaked in Enterobacter sp. RE1-KN solution, (E-M) DL2000 DNA Marker.
It was found that the presence of Enterobacter sp. RE1-KN was not detected in the gut of the 4th instar larvae developed from eggs that were soaked with sterile water, while the presence of Enterobacter sp. RE1-KN was detected in the gut of the 4th instar larvae which developed from eggs soaked with Enterobacter sp. RE1-KN solution (Figure 4D,E). The results showed that the bacteria on the egg surface can spread to the gut of P. xylostella.
3.5. Analysis of Horizontal Transmission of Gut Bacteria from P. xylostella
In mixed feeding of P. xylostella with and without gut bacteria, the survival rates of P. xylostella in four replicates were 70%, 70%, 60%, and 90%, respectively. Importantly, Enterobacter sp. RE1-KN was detected in the gut of all surviving P. xylostella (Figure S5 and Figure 5B). The results showed that gut bacteria of P. xylostella can be horizontally transmitted within populations through social activities.
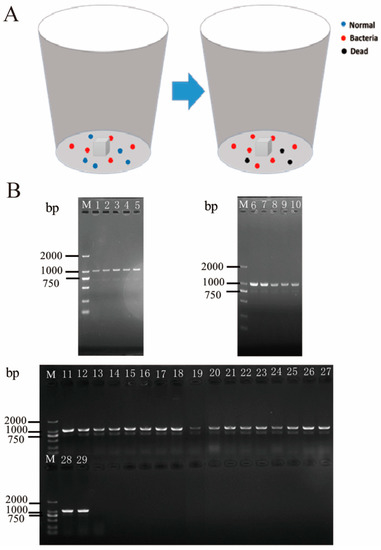
Figure 5.
Validation of horizontal transmission of gut bacteria in P. xylostella. (A) The 1st instar larvae of P. xylostella were fed with a diet containing Enterobacter sp. RE1-KN as the treatment group, while they were fed a normal diet, the same as the control group, and they were raised to the 3rd instar larvae, respectively. Then, the 3rd instar larvae of the control group and treatment group were mixed and reared together as shown in the insect-rearing container on the left, and then reared together until the 4th instar as shown in the container on the right. They were fed on the normal diet when mixed. The experiment was repeated four times. Blue dots represent normal P. xylostella, red dots represent P. xylostella containing Enterobacter sp. RE1-KN, and black dots represent dead P. xylostella. (B) The PCR amplification of Enterobacter sp. RE1-KN in the gut of 4th instar larvae which were developed from the mixed rearing of the 3rd instar larvae. M: DL2000 DNA Marker, 1–29: detection of Enterobacter sp. RE1-KN in each surviving P. xylostella gut.
4. Discussion
Whether feeding on rice or maize, proteobacteria was found to be the largest phylum in the gut of Cnaphalocrocis medinalis [26]. Similarly, Bactrocera minax collected from a vegetable field had proteobacteria as the largest phylum in its gut [27]. Furthermore, the largest phylum of gut bacteria in P. xylostella feeding on radish sprouts is also proteobacteria in this study. These show that proteobacteria are widely present in the gut of insects that feed on natural food. Moreover, this study showed that the gut bacteria of P. xylostella are potentially related to the food it eats. These indicate a potential correlation between insect gut bacteria and the food they consume, the phenomenon shared with S. frugiperda, Bactrocera dorsalis, and Nezara viridula [8,28,29,30]. However, studies have shown that both environmental factors and food can affect insect gut microbial diversity [20]. For example, the gut bacteria of P. xylostella can be affected by different geographical regions [19], and there are significant differences in the bacterial community structure of the gut of Musca domestica under field and laboratory conditions [31]. In addition, the gut microflora structure of insects may change during different life stages. For example, the diversity of the gut bacterial community of the larvae of Gastrolina depressa is generally higher than that of adults, and the diversity of the gut bacterial community of 1st and 2nd instar larvae is the highest [32]. Therefore, the diversity of the gut bacterial community of insects is affected by food, environment, and their life history, these factors may work together in the construction of gut bacterial community diversity, affecting the growth and development of insects.
Our previous study also found that different host plants can affect the diversity of gut bacteria of P. xylostella [33]. This study found that many bacteria in food can be transferred to the gut of P. xylostella by the traditional culture method, but this method is based on the similarity of the 16S rDNA of isolated bacteria. Although the similarity of most bacteria is more than 99%, based on the current study, the bacterial taxonomic units have entered the level of strains, and these bacteria with highly similar 16S rDNA belong to the same genus; however, they may belong to different strains [34]. Therefore, it is necessary to study the correlation between gut bacteria of P. xylostella and food more accurately and systematically by bacterial markers and other methods in the future.
In addition, we found that the gut bacteria of P. xylostella can be transmitted to ovaries and eggs, and the bacteria carried by eggs can further spread to the next generation. The gut bacteria of P. xylostella have a route of vertical transmission through the eggs. This phenomenon is similar to Tribolium castaneum, where Knorr et al. fed T. castaneum with fluorescent-labeled Escherichia coli and Pseudomonas entomophila, and traced the labeled bacteria in the female reproductive system and eggs of T. castaneum [35]. The bacterial species Serratia symbiotica was originally characterized as noncultured strains that live as mutualistic symbionts of Aphidoidea and are vertically transmitted through transovarial endocytosis within the mother’s body [11]. Snodgrassella alvi and Gilliamella apicola in Bombus terrestris populations can also be vertically transmitted from the mother to the offspring [10], suggesting that vertical transmission of gut bacteria through eggs is likely a common phenomenon in insects. However, although this study confirmed that eggs carrying Enterobacter sp. RE1-KN can transmit to the offspring, further investigations are needed to examine its stability inside the eggs and in the gut of P. xylostella after multiple generations. Previous studies have shown that some social insects, such as Cryptocercus sp., R. speratus, and A. mellifera, can horizontally transmit gut bacteria through population activities, such as trophallaxis or coprophagy [12,13,14]. This study found that the larvae of P. xylostella can also carry out the horizontal transmission of gut bacteria within the population. Currently, trophallaxis of P. xylostella has not been observed. On the contrary, in the case of food shortage, there was a very serious phenomenon of cannibalism. In this experiment, an adequate amount of food was provided, so cannibalism was not observed. Therefore, the horizontal transmission of gut bacteria in P. xylostella may occur through feces excreted by the larvae, which are transmitted to other P. xylostella living in the same space through food transfer. Another possibility is that during the feeding process of P. xylostella, its oral regurgitation fluid may also contain some gut bacteria, which can also be left on the surface of food and help establish gut microbiota in subsequent feeding by other P. xylostella. However, these specific forms and mechanisms need further determination. This result also suggests that group-living insects, not just social insects, may experience the horizontal transmission of gut bacteria due to the effects of regurgitation fluid and feces when feeding in the same space. In addition, previous studies have found that the horizontal transmission of gut bacteria in some insect species can occur not only within populations but also between populations, such as the gut flora of Xylocopa micans having high homology with those of A. mellifera and Bombus ruderarius [36]. Whether the gut bacteria of P. xylostella can also carry out horizontal transmission among populations with its homologous species or species with the same host needs further study.
In addition, the engineered bacterium Enterobacter sp. RE1-KN, which was constructed to study vertical and horizontal transmission in this study contains a plasmid with the green fluorescent protein (GFP) gene. Our original purpose was to better trace and display the whole process of gut microbial transmission in vivo through fluorescent labeling. However, it is possible that the host bacterium, Enterobacter sp. RE1, lacks the transcription factors necessary for GFP expression from the PET28a-EGFP plasmid but possesses transcription factors containing the kanamycin resistance gene. Therefore, in this study, only kanamycin resistance markers were used to track bacterial migration. Future studies can further construct engineered bacteria that can stably express fluorescent protein for more convenient and visual exploration. The fluorescent labeling and high-throughput sequencing methods can be further combined to study whether the labeled bacteria still exist in the egg and at each growth stage after multiple generations of transmission, to evaluate the stability of this transmission mode, and further study the molecular mechanism of vertical and horizontal transmission. Another point of concern is that the purpose of using an anaerobic agar medium in this paper was to increase the number of bacteria screened, but interestingly, all the bacteria screened on this medium were facultative anaerobes rather than strict anaerobes. This may be due to the relatively straight gut structure of P. xylostella, which is not easy to form a closed anaerobic space in a certain area. It may also be because this anaerobic device can not completely exclude oxygen; therefore, strict anaerobes have not yet been isolated. In the future, more advanced anaerobic culture devices can be used to explore the composition and function of anaerobic bacteria in the gut of P. xylostella. Of course, the extensive presence of facultative anaerobes in the gut of P. xylostella in this study also shows the adaptability of such bacteria to the semi-closed structure of the gut from another perspective. Another disadvantage of this study is that based on the current data, it is not clear whether the main source of gut bacteria of P. xylostella is food or its vertical and horizontal transmission. We only know that P. xylostella can establish gut flora through these three ways, but which is the main way? What is the proportion of the three modes of transmission? These problems cannot be quantified at present, and these problems are related to the establishment and use of biological control methods based on gut bacteria in the future. Therefore, more quantitative experiments need to be designed to determine the main source of gut bacteria in P. xylostella. In addition, this study did not explore which social activities were involved in the horizontal transmission of gut bacteria by P. xylostella. In the future, it is necessary to explore the role of regurgitation fluid and feces from P. xylostella in the horizontal transmission of gut bacteria.
Finally, in microbial research, insects are subjected to aseptic treatment before being dissected, but the aseptic-treated insect body is not usually sampled for the detection of bacteria, as we generally believe that bacteria on the surface of insects soaked in alcohol and pure water will be killed. However, in order to ensure the high reliability of the research, subsequent microbiological studies require the sterile validation of sterilized insect bodies. Additionally, in this study, all PCR experiments were conducted using a culture medium containing kanamycin, the recombinant bacterium Enterobacter sp. RE1-KN with kanamycin resistance was first isolated from P. xylostella by the selective medium, and then PCR amplification was performed using the specific primer T7 of the recombinant plasmid. The length of the primer also met theoretical expectations, and the entire experiment strictly followed aseptic procedures, so the contamination can be ruled out in theory. However, from a rigorous experimental perspective, negative controls should be added to make the experiment more rigorous. In future studies, we will add both negative and positive controls to improve the reliability and rigor of the experiment.
5. Conclusions
The gut bacteria of P. xylostella is related to its food. P. xylostella can obtain these bacteria from its diet to establish its gut flora and transmit the bacteria to the next generation via the ovary and egg. In addition, the gut bacteria of P. xylostella can be vertically transmitted through eggs and horizontally transmitted within the population. This study laid a foundation for further research on the gut bacteria of P. xylostella in the future and provided a new idea for the control of P. xylostella from the perspective of the source and transmission modes of gut bacteria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14060504/s1, Figure S1: Bacteria strains isolated and purified from radish sprouts;; Figure S2: Isolation of bacteria from P. xylostella at different stages; Figure S3: Bacteria strains isolated and purified from P. xylostella at different stages; Figure S4: Isolation of gut bacteria from 4th instar larvae of P. xylostella; Figure S5: Statistics on the number of P. xylostella with Enterobacter spp. RE1-KN in mixed feeding; Table S1: The amplification system for PCR; Table S2: The response procedure for PCR; Table S3: Blast-based alignment of 16S rDNA of bacteria from radish sprouts; Table S4: Blast-based alignment of 16S rDNA from the 4th larval gut bacteria of P. xylostella; Table S5: Blast-based alignment of 16S rDNA from the pupal gut bacteria of P. xylostella; Table S6 Blast-based alignment of 16S rDNA from the adult gut bacteria of P. xylostella; Table S7: Blast-based alignment of 16S rDNA from the adult ovary bacteria of P. xylostella; Table S8: Blast-based alignment of 16S rDNA from bacteria in the egg of P. xylostella; Table S9: Different sources of bacteria in Phylum level; Table S10: Different sources of bacteria in Genus’s level.
Author Contributions
X.X. conceived and designed the experiments; Q.A. and S.H. performed the experiments; X.X., Q.A. and S.H. analyzed the data; X.X., S.H. and Q.A. interpreted the results and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project of the Natural Science Foundation of Fujian Province, China (2022J01126), the National Natural Science Foundation of China (Nos. 31871968), the project of the National Key Research and Development Program of China (2017YFE0122000), and the open project of Fujian Key Laboratory of crop pest monitoring and control (MIMCP-201902).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions, e.g., privacy or ethics.
Acknowledgments
We appreciate the language modifications made to this article by Muhammad Rehan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Watanabe, H.; Tokuda, G. Cellulolytic systems in insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef]
- Gao, H.; Bai, L.; Jiang, Y.M.; Huang, W.; Wang, L.L.; Li, S.G.; Zhu, G.D.; Wang, D.Q.; Huang, Z.H.; Li, X.H.; et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat. Microbiol. 2021, 6, 806–817. [Google Scholar] [CrossRef]
- Koch, H.; Schmid-Hempel, P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl. Acad. Sci. USA 2011, 108, 19288–19292. [Google Scholar] [CrossRef]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. A note: Gut bacteria produce components of a locust cohesion pheromone. J. Appl. Microbiol. 2002, 92, 759. [Google Scholar] [CrossRef]
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, I.; Rosenberg, E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Segal, D.; Zilberrosenberg, I. Symbiotic bacteria are responsible for diet-induced mating preference in Drosophila melanogaster, providing support for the hologenome concept of evolution. Gut Microbes. 2011, 2, 190–192. [Google Scholar] [CrossRef]
- Meng, L.X.; Xia, C.X.; Jin, Z.X.; Zhang, H.Y. Investigation of gut bacterial communities of asian citrus psyllid (Diaphorina citri) reared on different host plants. Insects 2022, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Li, Y.H.; Sun, Z.X.; Du, E.W.; Lu, Z.H.; Li, H.; Gui, F.R. Effects of host plants on bacterial community structure in larvae midgut of Spodoptera frugiperda. Insects 2022, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Onchuru, T.O.; Martinez, A.J.; Ingham, C.S.; Kaltenpoth, M. Transmission of mutualistic bacteria in social and gregarious insects. Curr. Opin. Insect Sci. 2018, 28, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Abrol, D.P.; Li, J.; Schmid-Hempel, P. Diversity and evolutionary patterns of bacterial gut associates of corbiculate bees. Mol. Ecol. 2013, 22, 2028–2044. [Google Scholar] [CrossRef] [PubMed]
- Perreau, J.; Patel, D.J.; Anderson, H.; Maeda, G.P.; Elston, K.M.; Barrick, J.E.; Moran, N.A. Vertical Transmission at the Path-ogen-Symbiont Interface: Serratia symbiotica and Aphids. mBio 2021, 12, e00359-21. [Google Scholar] [CrossRef]
- Hongoh, Y.; Deevong, P.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Vongkaluang, C.; Noparatnaraporn, N.; Kudo, T. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 2005, 71, 6590–6599. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, Y.; Ekpornprasit, L.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Noparatnaraporn, N.; Kudo, T. Intra-colony variation of bacterial gut microbiota among castes and ages in the fungus-growing termite macrotermes gilvus. Mol. Ecol. 2006, 15, 505–516. [Google Scholar] [CrossRef]
- Martinson, V.G.; Moy, J.; Moran, N.A. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl. Environ. Micr. 2012, 78, 2830–2840. [Google Scholar] [CrossRef]
- Shaikevich, E.; Romanov, D. Symbiotic Wolbachia bacteria in coccinellid parasitoids: Genetic diversity, horizontal transfer, and recombination. Int. Microbiol. 2022, 26, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Saeed, R.; Ali, H.S.; Sarfraz, A.S.; Syed, M.Z. Effect of different host plants on the fitness of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2010, 29, 178–182. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress and prospects. Ann. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Pan, Q.J.; Tian, H.G.; Douglas, A.E.; Li, T.X. Bacteria abundance and diversity of different life stages of Plutella xylostella (Lepidoptera: Plutellidae), revealed by bacteria culture-dependent and PCR-DGGE methods. Insect Sci. 2015, 22, 375–385. [Google Scholar] [CrossRef]
- Kaur, M.; Thakur, M.; Sagar, V.; Sharma, R. Diversity of culturable gut bacteria of diamondback moth, Plutella xylostella (Lin-naeus) (Lepidoptera: Yponomeutidae) collected from different geographical regions of India. Mol. Biol. Rep. 2022, 49, 7475–7481. [Google Scholar] [CrossRef]
- Li, G.N.; Sun, J.J.; Meng, Y.J.; Yang, C.F.; Chen, Z.; Wu, Y.F.; Tian, L.; Song, F.; Cai, W.Z.; Zhang, X.; et al. The impact of en-vironmental habitats and diets on the gut microbiota diversity of True Bugs (Hemiptera: Heteroptera). Biology 2022, 11, 1039. [Google Scholar] [CrossRef]
- Xia, X.F.; Zheng, D.D.; Zhong, H.Z.; Qin, B.C.; Gurr, G.M.; Vasseur, L.; Lin, H.L.; Bai, J.L.; He, W.Y.; You, M.S. DNA Se-quencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852. [Google Scholar]
- Xia, X.F.; Gurr, G.M.; Vasseur, L.; Zheng, D.D.; Zhong, H.Z.; Qin, B.C.; Lin, J.H.; Wang, Y.; Song, F.Q.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Sun, B.T.; Gurr, G.M.; Vasseur, L.; Xue, M.Q.; You, M.S. Gut microbiota mediate insecticide resistance in the dia-mondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Xia, X.F.; Zheng, D.D.; Lin, H.L.; You, M.S. Isolation and identification of bacteria from the larval midgut of the diamondback moth, Plutella xylostella. Chin. J. Appl. Entomol. 2013, 50, 770–776. [Google Scholar]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, X.G.; Xu, H.X.; Liu, Y.H.; Lu, Z.X. Effects of host plant and insect generation on shaping of the gut microbiota in the Rice Leaffolder, Cnaphalocrocis medinalis. Front. Microbiol. 2022, 13, 824224. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Yao, Z.C.; Zheng, W.W.; Zhang, H.Y. Bacterial communities in the gut and reproductive organs of Bactrocera minax (Diptera: Tephritidae) based on 454 pyrosequencing. PLoS ONE 2014, 9, e106988. [Google Scholar] [CrossRef]
- Mason, C.J.; Hoover, K.; Felton, G.W. Effects of maize (Zea mays) genotypes and microbial sources in shaping fall armyworm (Spodoptera frugiperda) gut bacterial communities. Sci. Rep. 2021, 11, 4429. [Google Scholar] [CrossRef] [PubMed]
- Akami, M.; Ren, X.M.; Wang, Y.H.; Mansour, A.; Cao, S.; Qi, X.W.; Ngakou, A.; Ngane, R.; Annie, N.; Niu, C.Y. Host fruits shape the changes in the gut microbiota and development of Bactrocera dorsalis (Diptera: Tephritidae) larvae. Int. J. Trop. Insect. Sci. 2022, 42, 2127–2141. [Google Scholar] [CrossRef]
- Medina, V.; Rosso, B.E.; Soria, M.; Gutkind, G.O.; Pagano, E.A.; Zavala, J.A. Feeding on soybean crops changed gut bacteria diversity of the southern green stinkbug (Nezara viridula) and reduced negative effects of some associated bacteria. Pest Manag. Sci. 2022, 78, 4608–4617. [Google Scholar] [CrossRef]
- Voulgari-Kokota, A.; Beukeboom, L.W.; Wertheim, B.; Salles, J.F. Houseflies harbor less diverse microbiota under laboratory conditions but maintain a consistent set of host-associated bacteria. Sci. Rep. 2022, 12, 11132. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Q.; Chen, X.T.; Li, S.Q.; Luo, J.; Han, R.H.; Xu, L. Composition and diversity of gut bacterial community in different life stages of a leaf beetle Gastrolina Depressa. Microb. Ecol. 2022. [Google Scholar] [CrossRef]
- Wu, X.L.; Xia, X.F.; Chen, J.H.; Geoff, M.G.; You, M.S. Effects of different foods on intestinal bacterial diversity of diamondback moth larvae. Acta Entomol. Sin. 2019, 62, 1172–1185. [Google Scholar]
- Meng, Y.J.; Li, S.; Zhang, C.; Zheng, H. Strain-level profiling with picodroplet microfluidic cultivation reveals host-specific adaption of honeybee gut symbionts. Microbiome 2022, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Knorr, E.; Schmidtberg, H.; Arslan, D.; Bingsohn, L.; Vilcinskas, A. Translocation of bacteria from the gut to the eggs triggers maternal transgenerational immune priming in Tribolium castaneum. Biol. Lett. 2015, 11, 20150885. [Google Scholar] [CrossRef]
- Holley, J.C.; Jackson, M.N.; Pham, A.T.; Hatcher, S.C.; Moran, N.A. Carpenter bees (Xylocopa) harbor a distinctive gut microbiome related to that of honey bees and bumble bees. Appl. Environ. Microb. 2022, 88, e0020322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).