Mapping Species Distributions of Latoia consocia Walker under Climate Change Using Current Geographical Presence Data and MAXENT (CMIP 6)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
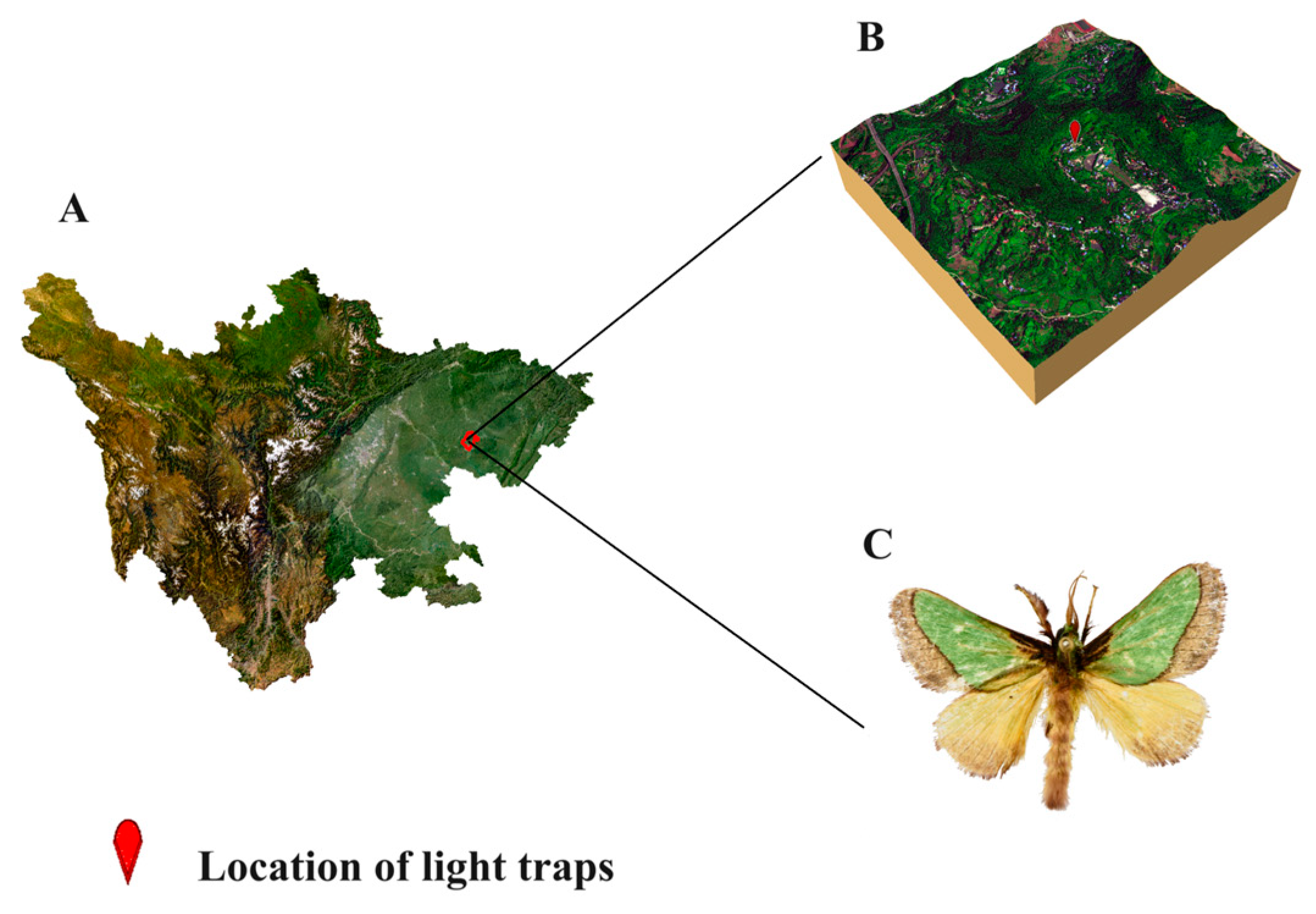
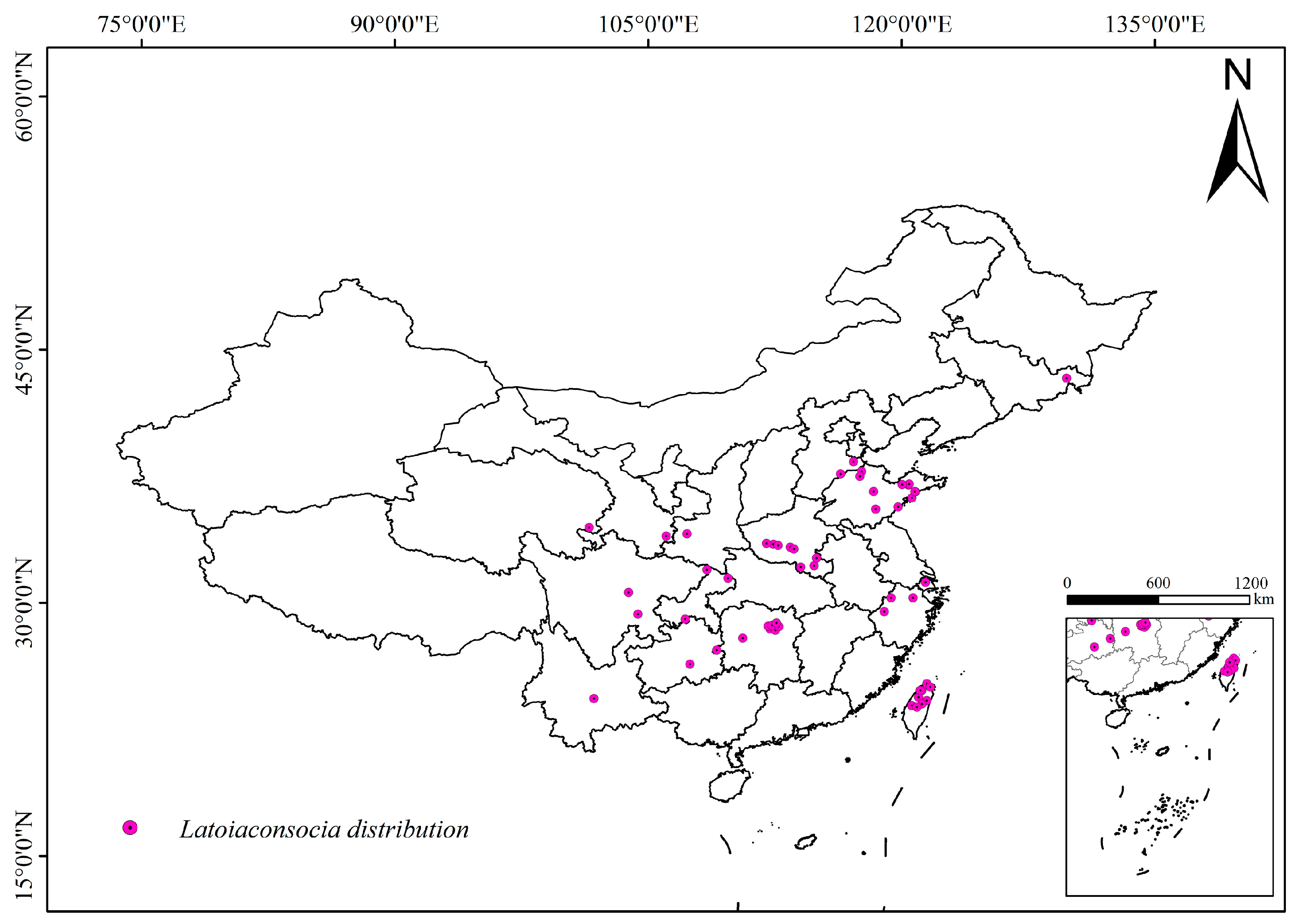
2.1. Species Occurrence Data
2.2. Bioclimatic Variables
2.3. Model Settings and Operation
2.4. Model Evaluation
3. Results
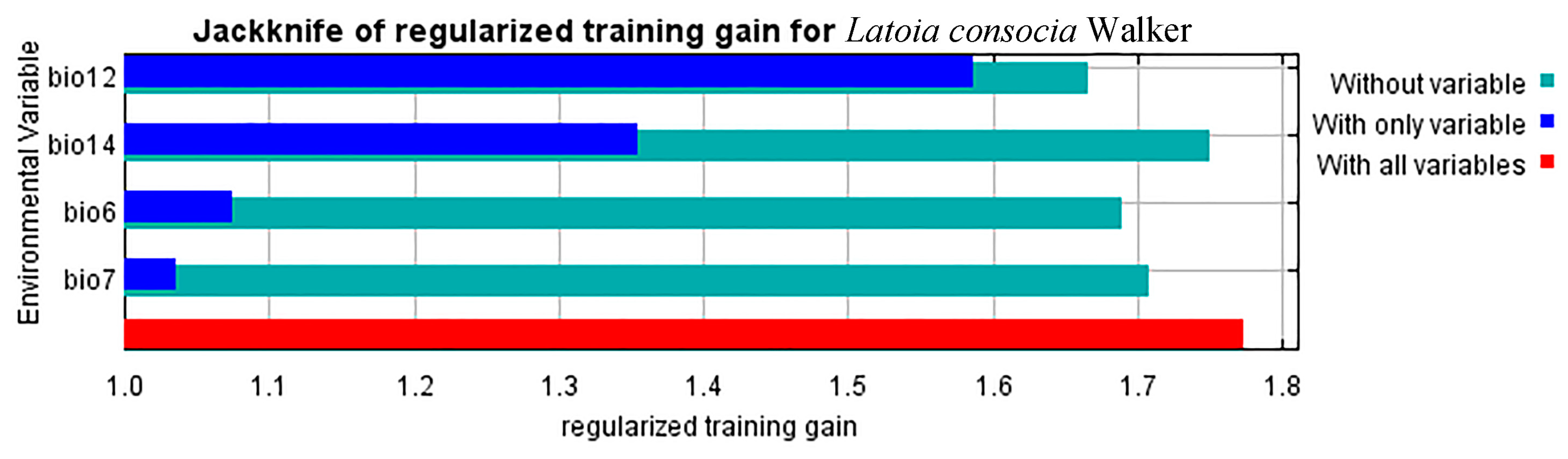
3.1. Key Environment Variable Selection and Model Performance
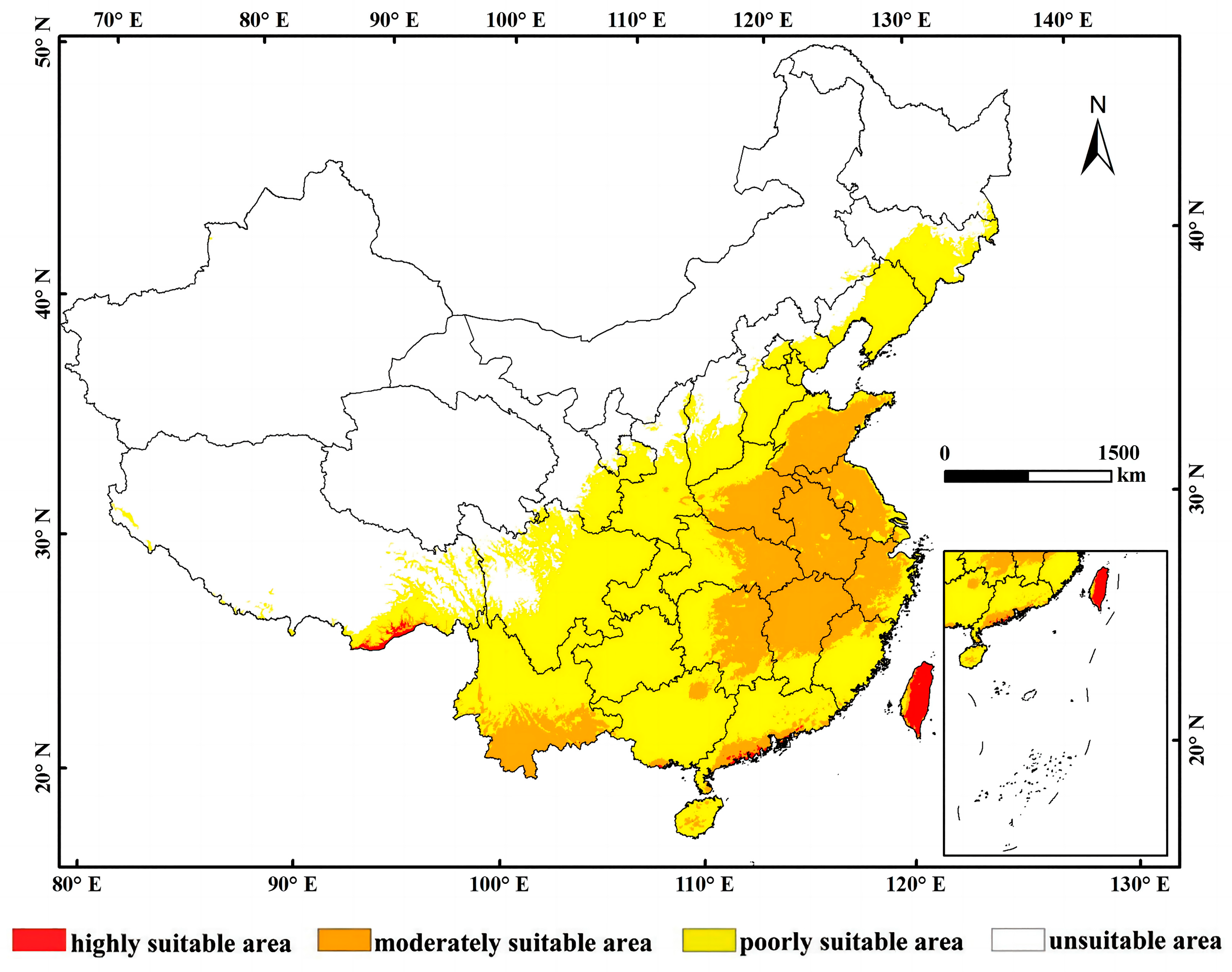
3.2. Current Potential Distribution of L. consocia
3.3. Potential Distribution of L. consocia under Future Climatic Conditions
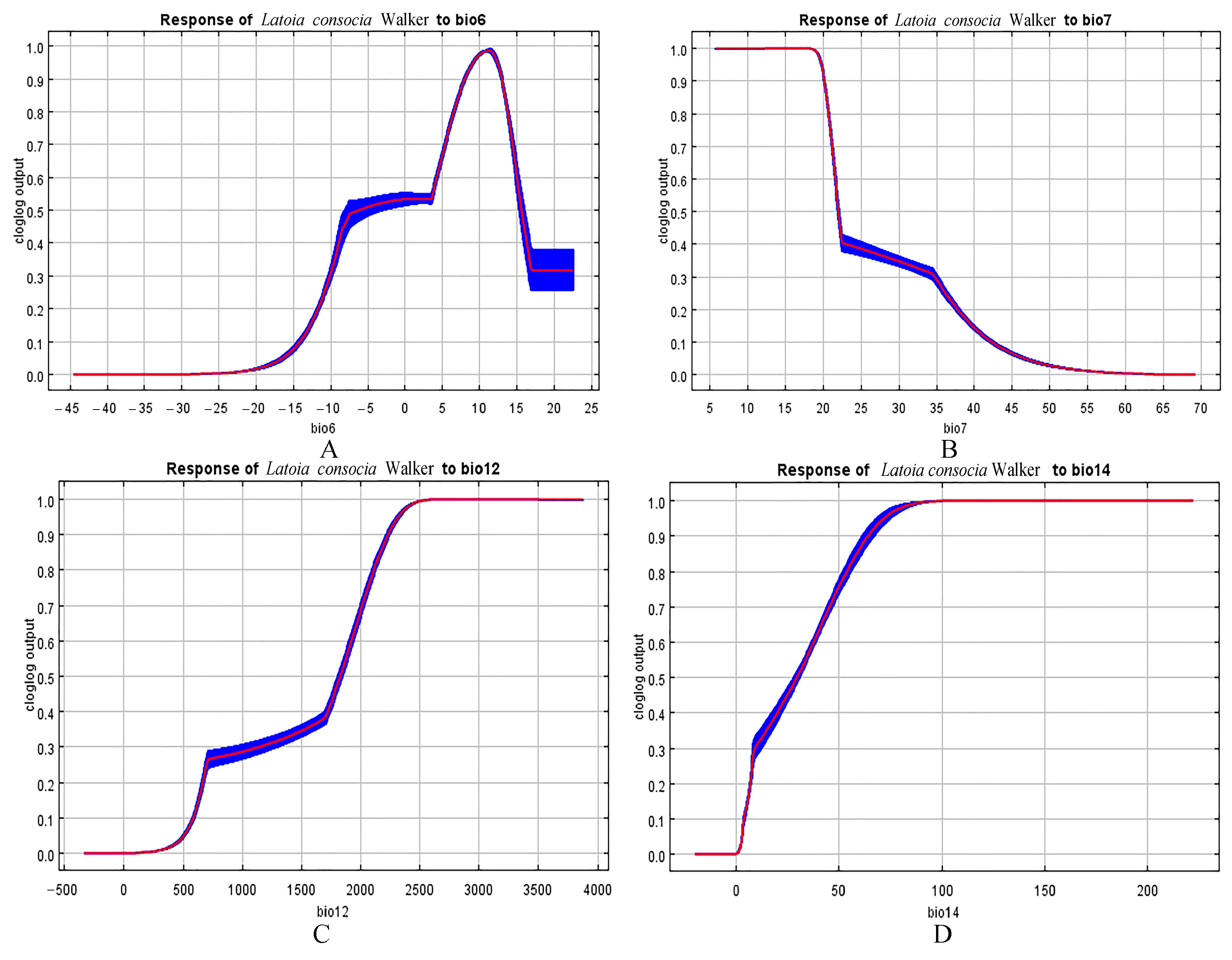
3.4. Environmental Variables That Affect Geographic Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global warming promotes biological invasion of a honey bee pest. Glob. Chang. Biol. 2019, 25, 3571–3994. [Google Scholar] [CrossRef] [PubMed]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yu, W.; Zhao, H.; Xian, X.; Jing, K.; Yang, N.; Lu, X.; Liu, W. Potential Global Distribution of Invasive Alien Species, Anthonomus Grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model. Agriculture 2022, 12, 1759. [Google Scholar] [CrossRef]
- Crimmins, S.M.; Dobrowski, S.Z.; Mynsberge, A.R. Evaluating ensemble forecasts of plant species distributions under climate change. Ecol. Model. 2013, 266, 126–130. [Google Scholar] [CrossRef]
- Kumar, P. Assessment of impact of climate change on Rhododendrons in Sikkim Himalayas using Maxent modelling: Limitations and challenges. Biodivers. Conserv. 2012, 21, 1251–1266. [Google Scholar] [CrossRef]
- Korell, L.; Auge, H.; Chase, J.M.; Harpole, S.; Knight, T.M. We need more realistic climate change experiments for understanding ecosystems of the future. Glob. Chang. Biol. 2020, 26, 325–327. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, D.; Zhuo, Z. Predicting the Impact of Climate Change on the Geographical Distribution of Leafhopper, Cicadella viridis in China through the MaxEnt Model. Insects 2023, 14, 586. [Google Scholar] [CrossRef]
- Moore, F. On the lepidopterous insects of bengal. Proc. Zool. Soc. Lond. 1865, 33, 755–823. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, L.; Liu, Z. Preliminary Study on the Feeding Preference of Parasa Consocia to Eight Fruit Trees. J. Fruit Resour. Guoshu 2023, 4, 28–29. [Google Scholar]
- Zhang, Z.; Hooper, J.; van Soest, R.; Pisera, A.; Crowther, A.; Tyler, S.; Schilling, S.; Eschmeyer, W.; Fong, J.; Blackburn, D. Animal biodiversity: An outline of higher-level classification and taxonomic richness. Zootaxa 2011, 3148, 7–237. [Google Scholar]
- Wu, C.S. Analysis on the host plant diversity of slug caterpillar moths in China. For. Pest Dis. 2010, 29, 1–4. [Google Scholar]
- Meng, J.; Jing, L.; Huifeng, Z.; Chunsheng, W.; Aibing, Z. Species diversity and global distribution of Limacodidae (Lepidoptera) using online databases. Biodivers. Sci. 2019, 27, 778–786. [Google Scholar] [CrossRef]
- Wang, F.; Ju, R.; Du, Y. Comparison of the bionomics of five species of Limacodidae in city virescence. For. Pest Dis. 2006, 25, 11–15. [Google Scholar]
- Wang, J.; Wu, D.; Lu, A.; Xu, L.; Chen, Y.; Zhu, W. Experiment on Control of Biological Pesticides on Latoia consocia. J. Zhejiang For. Sci. Technol. 2008, 3, 66–68. [Google Scholar]
- Greeney, H.F.; Dyer, L.A.; Smilanich, A.M. Feeding by lepidopteran larvae is dangerous: A review of caterpillars’ chemical, physiological, morphological, and behavioral defenses against natural enemies. ISJ-Invertebr. Surviv. J. 2012, 9, 7–34. [Google Scholar]
- Hossler, E.W. Caterpillars and moths. Dermatol. Ther. 2009, 22, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Dixuan, W.; Youming, Z.; Sai, G.; Lini, C. Hazards of Latoia consocia on fruit trees and their integrated control. Fruit Grow. Friend 2019, 12, 19–42. [Google Scholar]
- Chungang, G. Infestation characteristics and management of balsam camphor Latoia consocia in Nantong area. Agric. Jilin 2016, 24, 90. [Google Scholar]
- Rongzh, L. A new host castor for Latoia consocia. China Public Health 2005, 2, 39–40. [Google Scholar]
- Jiyu, Q.; Xiuqing, Y.; Zhi, L. Study on dermatitis of pathogenicity lepidoptera euileidae caterpillar and its clinic experiment. Plant Prot. 1996, 3, 50. [Google Scholar]
- Ju, R.; Wang, F.; Li, Y. Effects of tem perature on the developm ent and survival ofexperm en talpopu lation ofParasa consocia. J. Plant Prot. 2007, 5, 466–470. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Cao, B.; Bai, C.; Wu, K.; Xue, Y.; Yang, J.; Gao, P.; Liang, H.; Li, G. Concentrated conservation and utilization: Four medicinal crops for diabetes treatment showed similar habitat distribution patterns in China. Ind. Crops Prod. 2020, 152, 112478. [Google Scholar] [CrossRef]
- Lompo, O.; Dimobe, K.; Mbayngone, E.; Savadogo, S.; Sambaré, O.; Thiombiano, A.; Ouédraogo, A. Climate influence on the distribution of the yellow plum (Ximenia Americana L.) in Burkina Faso. Trees For. People 2021, 4, 100072. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R. Potential geographical distribution of the edangred plant Isoetes under human activities using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e2186. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Rinnan, D.S.; Lawler, J. Climate-niche factor analysis: A spatial approach to quantifying species vulnerability to climate change. Ecography 2019, 42, 1494–1503. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar]
- Khan, A.M.; Li, Q.; Saqib, Z.; Khan, N.; Habib, T.; Khalid, N.; Majeed, M.; Tariq, A. MaxEnt Modelling and Impact of Climate Change on Habitat Suitability Variations of Economically Important Chilgoza Pine (Pinus gerardiana Wall.) in South Asia. Forests 2022, 13, 715. [Google Scholar] [CrossRef]
- Rong, Z.; Zhao, C.; Liu, J.; Gao, Y.; Zang, F.; Guo, Z.; Mao, Y.; Wang, L. Modeling the Effect of Climate Change on the Potential Distribution of Qinghai Spruce (Picea crassifolia Kom.) in Qilian Mountains. Forests 2019, 10, 62. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic Niche Shifts Are Rare among Terrestrial Plant Invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Tong, G.; Zhang, Q.; Xu, L.; Sang, Z.; Li, Y. A Study on the Suitable Areas for Growing Apricot Kernels in China Based on the MaxEnt Model. Sustainability 2023, 15, 9635. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Wang, R.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with MaxEnt modeling. Glob. Ecol. Conserv. 2019, 19, e691. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PLoS ONE 2018, 13, e192153. [Google Scholar] [CrossRef] [PubMed]
- Worthington, T.A.; Zhang, T.; Logue, D.R.; Mittelstet, A.R.; Brewer, S.K. Landscape and flow metrics affecting the distribution of a federally-threatened fish: Improving management, model fit, and model transferability. Ecol. Model. 2016, 342, 1–18. [Google Scholar] [CrossRef]
- Čengić, M.; Rost, J.; Remenska, D.; Janse, J.H.; Huijbregts, M.A.; Schipper, A.M. On the importance of predictor choice, modelling technique, and number of pseudo-absences for bioclimatic envelope model performance. Ecol. Evol. 2020, 10, 12307–12317. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Wu, D.; Zhu, C. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e856. [Google Scholar] [CrossRef]
- Alfonso-Corrado, C.; Naranjo-Luna, F.; Clark-Tapia, R.; Campos, J.; Rojas-Soto, O.; Luna-Krauletz, M. Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest. Forests 2017, 8, 261. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: Synthesis Report; Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Kogo, B.K.; Kumar, L.; Koech, R.; Kariyawasam, C.S. Modelling Climate Suitability for Rainfed Maize Cultivation in Kenya Using a Maximum Entropy (MaxENT) Approach. Agronomy 2019, 9, 727. [Google Scholar] [CrossRef]
- Elith, J.; HGraham, C.; PAnderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; JHijmans, R.; Huettmann, F.; RLeathwick, J.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Vanagas, G. Receiver operating characteristic curves and comparison of cardiac surgery risk stratification systems. Interact. CardioVascular Thorac. Surg. 2004, 3, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wei, Y.; Wang, X. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecol. 2015, 17, 140–145. [Google Scholar] [CrossRef]
- Li, R.; Xu, M.; Wong, M.H.; Qiu, S.; Sheng, Q.; Li, X.; Song, Z. Climate change-induced decline in bamboo habitats and species diversity: Implications for giant panda conservation. Divers. Distrib. 2015, 21, 379–391. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Lin, F.; Yang, W.; Gaisberger, H.; Christopher, K.; Zheng, Y. MaxEnt modelling for predicting the potential distribution of a near threatened rosewood species (Dalbergia cultrata Graham ex Benth). Ecol. Eng. 2019, 141, 105612. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Q.; Gao, Y. Improving ecological niche model transferability to predict the potential distribution of invasive exotic species. Biodivers. Sci. 2014, 22, 223–230. [Google Scholar]
- He, Q.; Zhou, G. Climate-associated distribution of summer maize in China from 1961 to 2010. Agric. Ecosyst. Environ. 2016, 232, 326–335. [Google Scholar] [CrossRef]
- Wan, J.Z.; Yu, J.H.; Yin, G.J.; Song, Z.M.; Wei, D.X.; Wang, C.J. Effects of soil properties on the spatial distribution of forest vegetation across China. Glob. Ecol. Conserv. 2019, 18, e635. [Google Scholar] [CrossRef]
- Aguilar-Soto, V.; Melgoza-Castillo, A.; Villarreal-Guerrero, F.; Wehenkel, C.; Pinedo-Alvarez, C. Modeling the Potential Distribution of Picea chihuahuana Martínez, an Endangered Species at the Sierra Madre Occidental, Mexico. Forests 2015, 6, 692–707. [Google Scholar] [CrossRef]


| Code | bio6 | bio7 | bio12 |
|---|---|---|---|
| bio7 | −0.069 | ||
| bio12 | 0.695 ** | 0.531 ** | |
| bio14 | 0.648 ** | 0.333 ** | 0.845 ** |
| Variable | Environment Variables | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| Bio 12 | Annual precipitation | 74.8 | 27.75 |
| Bio 14 | Precipitation of driest month | 13.41 | 8.56 |
| Bio 6 | Min. temperature of coldest month | 9.22 | 53.12 |
| Bio 7 | Temperature annual range | 2.57 | 10.58 |
| Province | Highly Suitable Area (km2) | Total (104 km2) * | Percentage of Highly Suitable Areas in Province (%) |
|---|---|---|---|
| Taiwan | 1450.00 | 3.60 | 4.028 |
| Tibet | 472.00 | 120.28 | 0.039 |
| Guangdong | 261.00 | 17.98 | 0.145 |
| Hong Kong | 33.00 | 0.11 | 3.000 |
| Guangxi | 23.00 | 23.76 | 0.010 |
| Yunnan | 3.00 | 39.41 | 0.001 |
| Hainan | 1.00 | 3.54 | 0.003 |
| China | 2243.00 | 960.00 | / |
| Decade Scenarios | Predicted Area (104 km2) | Comparison with Current Distribution (%) | |||||
|---|---|---|---|---|---|---|---|
| Poorly Suitable Aera | Moderately Suitable Aera | Highly Suitable Aera | Poorly Suitable Aera | Moderately Suitable Aera | Highly Suitable Aera | ||
| Current | 35.78 | 19.39 | 0.22 | ||||
| 2050s | SSP1-2.6 | 36.33 | 18.75 | 0.31 | 1.55 | −3.31 | 39.32 |
| SSP2-4.5 | 36.51 | 18.63 | 0.16 | 2.03 | −3.89 | −26.48 | |
| SSP5-8.5 | 35.72 | 19.20 | 0.36 | −0.18 | −0.95 | 60.41 | |
| 2090s | SSP1-2.6 | 36.81 | 18.39 | 0.20 | 2.88 | −5.16 | −13.02 |
| SSP2-4.5 | 35.89 | 19.29 | 0.22 | 0.31 | −0.53 | −3.52 | |
| SSP5-8.5 | 36.78 | 18.31 | 0.30 | 2.79 | −5.55 | 34.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Xu, D.; Peng, Y.; Zhuo, Z. Mapping Species Distributions of Latoia consocia Walker under Climate Change Using Current Geographical Presence Data and MAXENT (CMIP 6). Insects 2024, 15, 756. https://doi.org/10.3390/insects15100756
Wu Y, Xu D, Peng Y, Zhuo Z. Mapping Species Distributions of Latoia consocia Walker under Climate Change Using Current Geographical Presence Data and MAXENT (CMIP 6). Insects. 2024; 15(10):756. https://doi.org/10.3390/insects15100756
Chicago/Turabian StyleWu, Yuhan, Danping Xu, Yaqin Peng, and Zhihang Zhuo. 2024. "Mapping Species Distributions of Latoia consocia Walker under Climate Change Using Current Geographical Presence Data and MAXENT (CMIP 6)" Insects 15, no. 10: 756. https://doi.org/10.3390/insects15100756






