Evaluation of Lattice Structures for Medical Implants: A Study on the Mechanical Properties of Various Unit Cell Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Specimens
2.2. Specimen Manufacturing
2.3. Specimen Post-Processing
2.4. Compression Tests
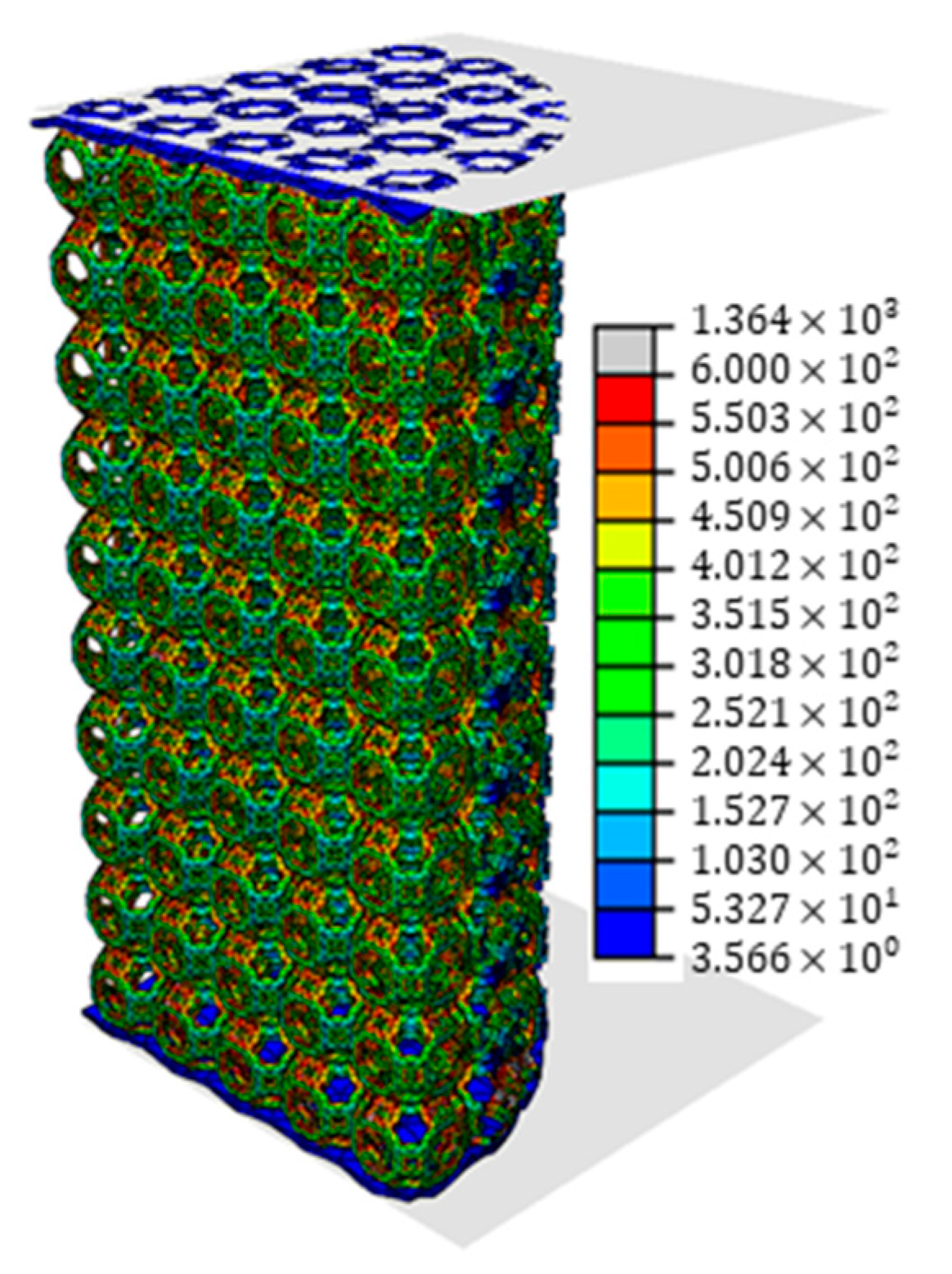
2.5. Finite Element Modelling
3. Results and Discussion
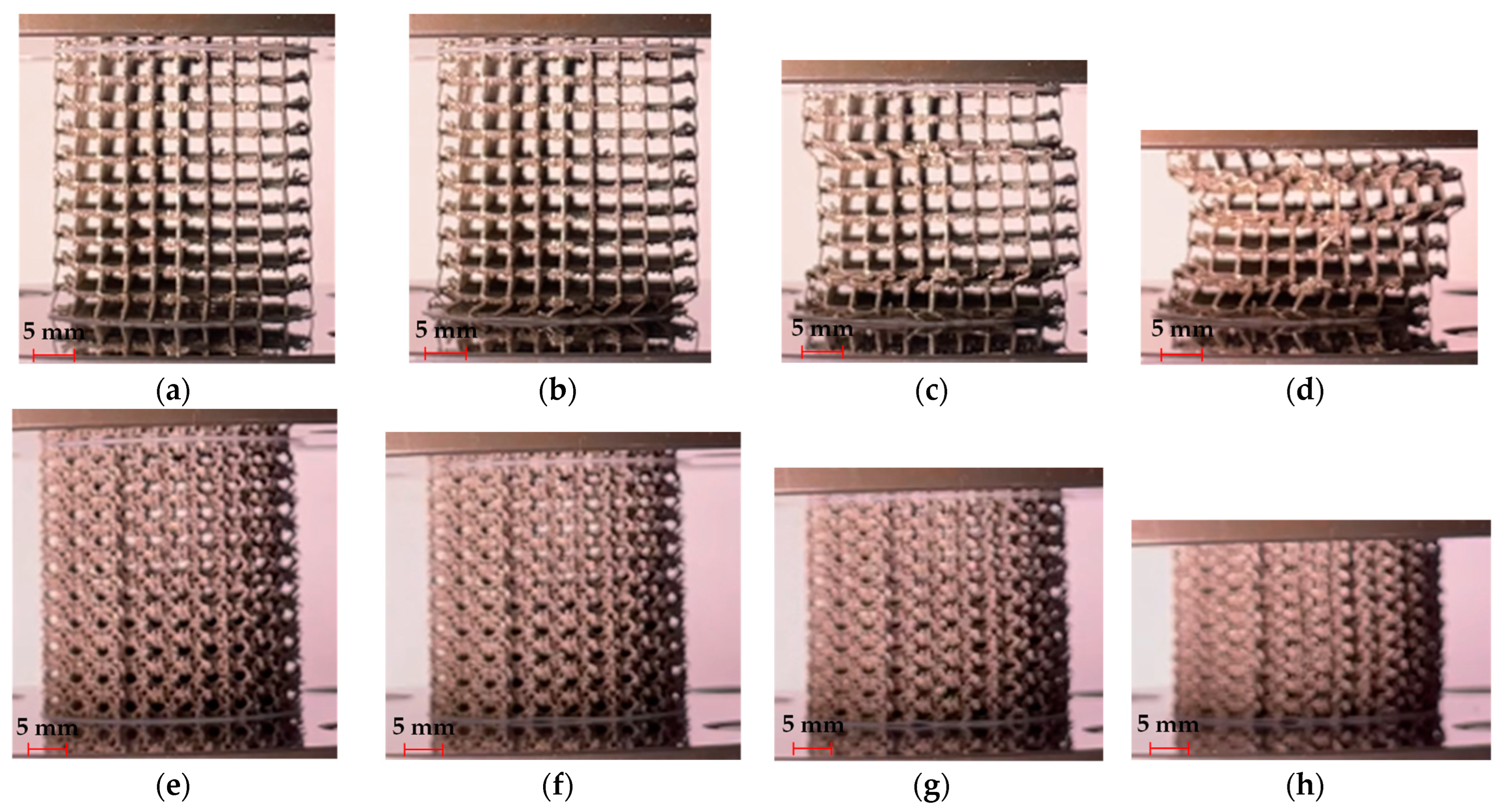
3.1. Specimens and Defects
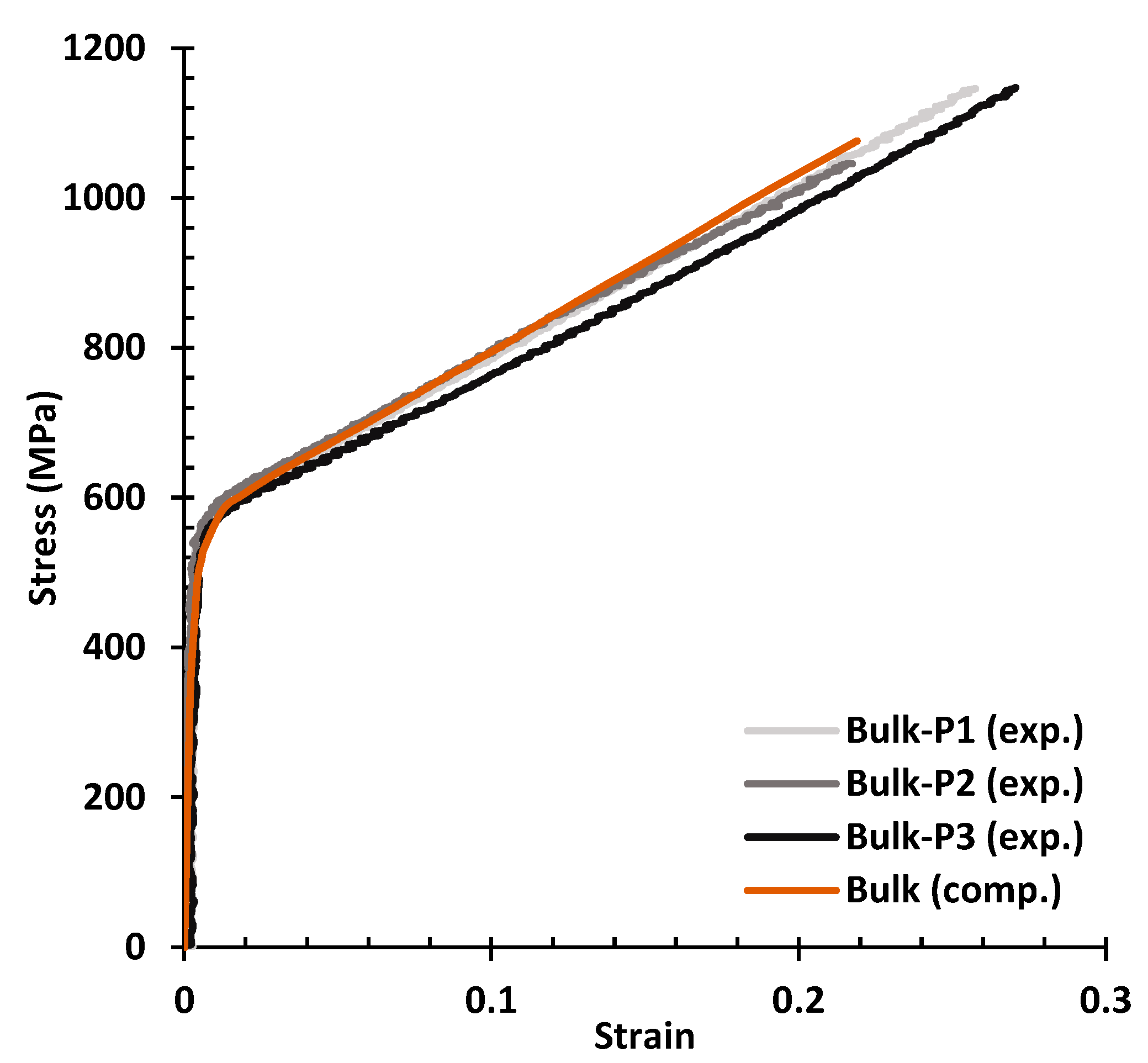
3.2. Finite Element Model Validation
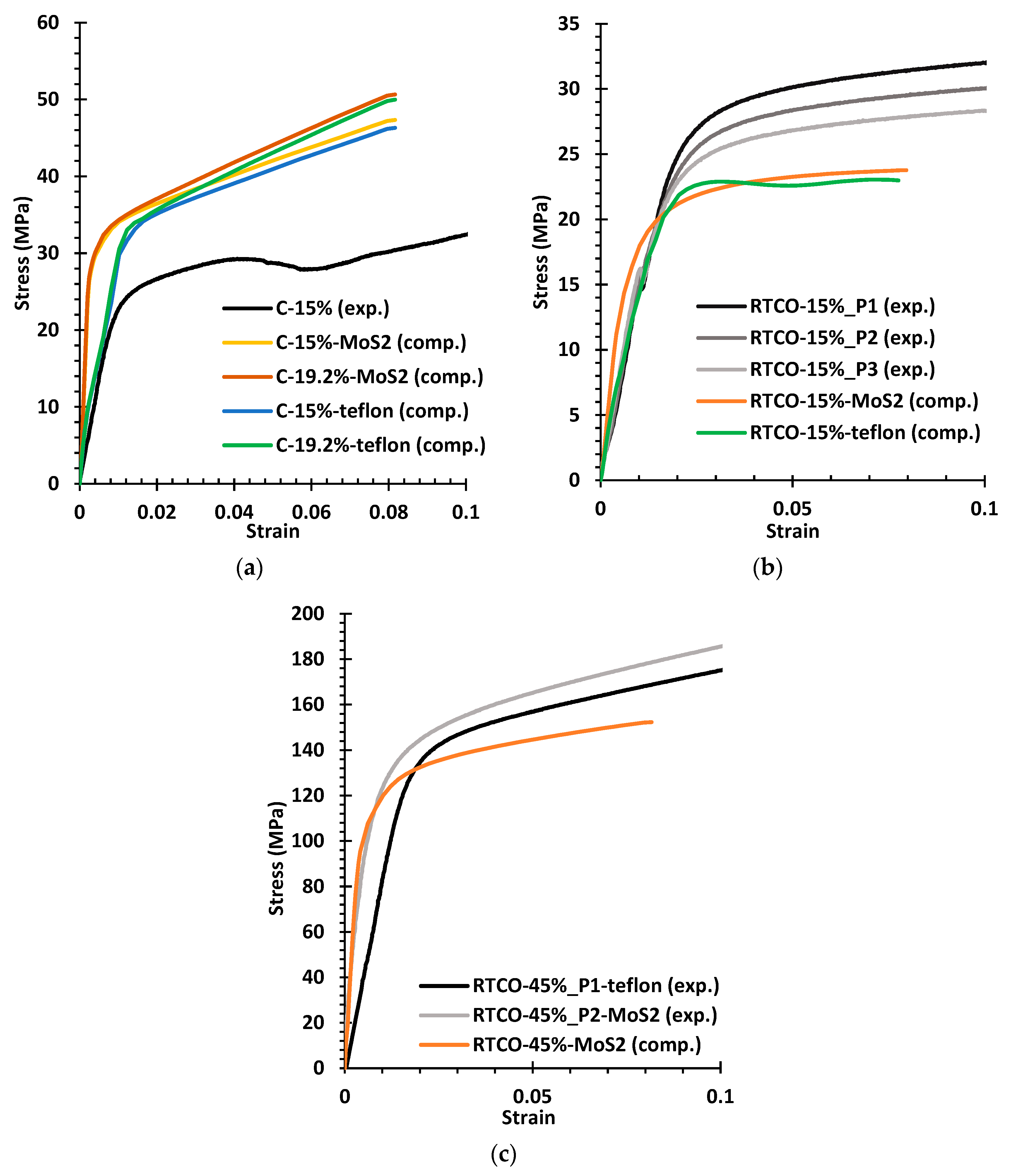
3.3. Experimental Compression Tests
3.4. Discussion of Application in Medical Implants
4. Conclusions
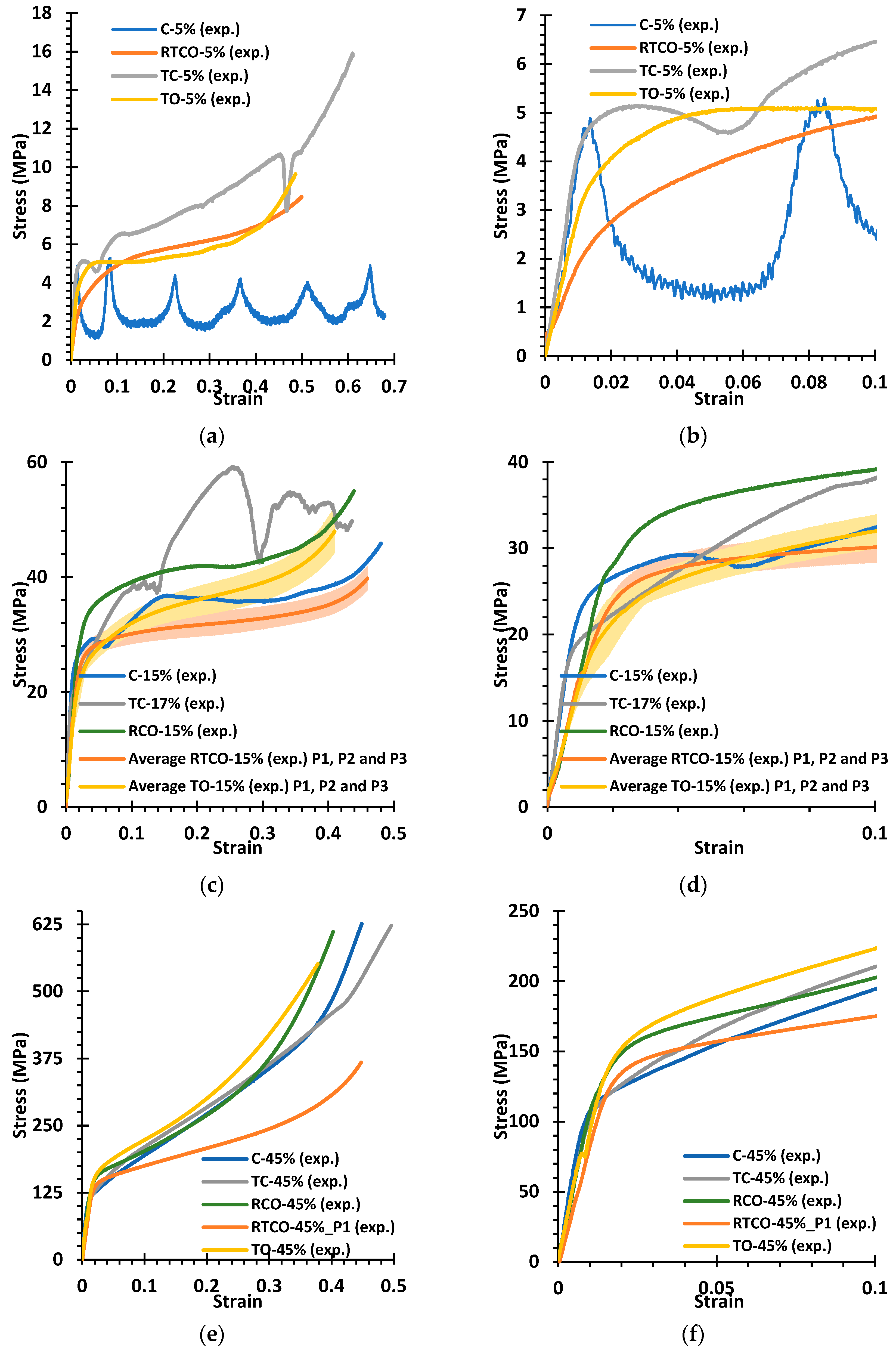
- The increase in mass from the designed to the real specimen, resulting from manufacturing, significantly raises the yield stress and post-yield stresses but has a minimal effect on the Young’s modulus. Other geometrical imperfections also affect mechanical properties such as the tilt of the vertical bars in the C and TC structures, which promotes early buckling, thereby significantly reducing the yield stress.
- Using disulphide molybdenum (MoS2) grease as the lubricant enabled a more direct comparison between the experimental and numerical results. Using a thin layer of Teflon as the lubricant for compressive tests is not the best option when a DIC system is not used to evaluate strains, as it can lead to underestimation of the Young’s modulus.
- The C and TC geometries exhibited instability at design relative densities of 5%, and the RCO geometry could not be fabricated in this case. At a design relative density of 15%, instabilities persisted in the C and TC geometries. Consequently, cell types C, TC, and RCO are unsuitable for medical implant design.
- The TO and RTCO geometries exhibited consistent and smooth deformation behaviour for all relative densities considered. These unit cells look promising for applications in the implant industry, particularly in functionally graded and/or biodegradable implants, due to their reliable mechanical behaviour.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benedetti, M.; Plessis, A.D.; Ritchie, R.O.; Dallago, M.; Razavi, S.M.J.; Berto, F. Architected cellular materials: A review on their mechanical properties towards fatigue-tolerant design and fabrication. Mater. Sci. Eng. R Rep. 2021, 144, 100606. [Google Scholar] [CrossRef]
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable Iron and Porous Iron: Mechanical Properties, Degradation Behaviour, Manufacturing Routes and Biomedical Applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef]
- Mahmoud, D.; Elbestawi, M. Lattice Structures and Functionally Graded Materials Applications in Additive Manufacturing of Orthopedic Implants: A Review. J. Manuf. Mater. Process. 2017, 1, 13. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yavari, S.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A. Additively Manufactured Open-Cell Porous Biomaterials Made from Six Different Space-Filling Unit Cells: The Mechanical and Morphological Properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef]
- Kadkhodapour, J.; Montazerian, H.; Darabi, A.C.; Anaraki, A.P.; Ahmadi, S.M.; Zadpoor, A.A.; Schmauder, S. Failure mechanisms of additively manufactured porous biomaterials: Effects of porosity and type of unit cell. J. Mech. Behav. Biomed. Mater. 2015, 50, 180–191. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef]
- Shi, X.; Liao, W.; Liu, T.; Zhang, C.; Li, D.; Jiang, W.; Wang, C.; Ren, F. Design optimization of multimorphology surface-based lattice structures with density gradients. Int. J. Adv. Manuf. Technol. 2021, 117, 2013–2028. [Google Scholar] [CrossRef]
- Magarò, P.; Alaimo, G.; Carraturo, M.; Sgambitterra, E.; Maletta, C. A novel methodology for the prediction of the stress–strain response of laser powder bed fusion lattice structure based on a multi-scale approach. Mater. Sci. Eng. A 2023, 863, 144526. [Google Scholar] [CrossRef]
- Płatek, P.; Sienkiewicz, J.; Janiszewski, J.; Jiang, F. Investigations on Mechanical Properties of Lattice Structures with Different Values of Relative Density Made from 316L by Selective Laser Melting (SLM). Materials 2020, 13, 2204. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xiao, D.; Li, Y.; Wen, W.; Zhao, T.; Chen, Z.; Jiang, Y.; Fang, D. Dynamic compressive behavior of a modified additively manufactured rhombic dodecahedron 316L stainless steel lattice structure. Thin-Walled Struct. 2020, 148, 106586. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, D.; Zhang, P.; Zhao, M.; Jafar, S. Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting. Materials 2018, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Yan, Z.; Wang, Y.; Cheng, H.; Zhang, H. Effects of heat treatment on microstructure and mechanical properties of selective laser melted Ti-6Al-4V lattice materials. Int. J. Mech. Sci. 2021, 190, 106042. [Google Scholar] [CrossRef]
- Salama, M.C.; Alves, F.P.D.; Reis, L.; Deus, A.M.; Silva, M.B.; Santos, C.; Carmezim, M.J.; Vaz, M.F. Finite element simulations of mechanical behaviour and degradation of iron lattices. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2023, 237, 1379–1393. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C 2019, 99, 838–852. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.A.; Mol, J.M.C.; Jahr, H.; et al. Additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 101, 609–623. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Dallago, M.; Winiarski, B.; Zanini, F.; Carmignato, S.; Benedetti, M. On the effect of geometrical imperfections and defects on the fatigue strength of cellular lattice structures additively manufactured via Selective Laser Melting. Int. J. Fatigue 2019, 124, 348–360. [Google Scholar] [CrossRef]
- Liu, L.; Kamm, P.; García-Moreno, F.; Banhart, J.; Pasini, D. Elastic and failure response of imperfect three-dimensional metallic lattices: The role of geometric defects induced by Selective Laser Melting. J. Mech. Phys. Solids 2017, 107, 160–184. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Costa, M.M.; Alves, N.; Miranda, G.; Silva, F.S. Selective Laser Melting of Ti6Al4V sub-millimetric cellular structures: Prediction of dimensional deviations and mechanical performance. J. Mech. Behav. Biomed. Mater. 2021, 113, 104123. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Abate, K.M.; Kumar, A.; Jeng, J.Y. A state-of-the-art review on types, design, optimization, and additive manufacturing of cellular structures. Int. J. Adv. Manuf. Technol. 2019, 104, 3489–3510. [Google Scholar] [CrossRef]
- Nogueira, P.; Magrinho, J.P.G.; Silva, M.B.; de Deus, A.M.; Vaz, M.F. Compression properties of cellular iron lattice structures used to mimic bone characteristics. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2024. [Google Scholar] [CrossRef]
- Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M.; Hosseini-Toudeshky, H. Comparison of elastic properties of open-cell metallic biomaterials with different unit cell types. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Xu, Y.; Chen, X.; Xin, L.; Zhang, J.; Li, K.; Sun, Y. Improved mechanical properties and energy absorption of Ti6Al4V laser powder bed fusion lattice structures using curving lattice struts. Mater. Des. 2021, 211, 110140. [Google Scholar] [CrossRef]
- Babamiri, B.B.; Askari, H.; Hazeli, K. Deformation mechanisms and post-yielding behavior of additively manufactured lattice structures. Mater. Des. 2020, 188, 108443. [Google Scholar] [CrossRef]
- Liverani, E.; Fortunato, A. Stiffness prediction and deformation analysis of Cobalt-Chromium lattice structures: From periodic to functionally graded structures produced by additive manufacturing. J. Manuf. Process. 2021, 68, 104–114. [Google Scholar] [CrossRef]
- Ashby, M. The properties of foams and lattices. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 15–30. [Google Scholar] [CrossRef]
- Nogueira, P.; Castresana, K.; Magrinho, J.; Silva, M.B.; de Deus, A.M.; Vaz, M.F. Computational evaluation of the compressive properties of different lattice geometries to be used as temporary implants. Procedia Comput. Sci. 2023, 217, 928–937. [Google Scholar] [CrossRef]
- Nogueira, P.; Magrinho, J.P.G.; Silva, M.B.; de Deus, A.M.; Vaz, M.F. Graded Lattice Structures for Biodegradable Temporary Implants: Computational Evaluation with Two Unit Cell Types; Lecture Notes in Networks and Systems; Springer Nature: Cham, Switzerland, 2023; pp. 534–543. [Google Scholar] [CrossRef]
- ISO 13314; Mechanical Testing of Metals—Ductility Testing—Compression Test for Porous and Cellular Metals (ISO Standard No. 13314:2011(E)). International Organization for Standardization: Geneva, Switzerland, 2011; pp. 1–7. Available online: www.iso.org (accessed on 20 March 2024).
- ASTM F3184-16(2023); Standard Specification for Additive Manufacturing Stainless Steel Alloy (UNS S31603) with Powder Bed Fusion. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- ASTM E9-19; Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature. ASTM: West Conshohocken, PA, USA, 2019. [CrossRef]
- Constantinou, M.C.; Caccese, J.; Harris, H.G. Frictional characteristics of Teflon–steel interfaces under dynamic conditions. Earthq. Eng. Struct. Dyn. 1987, 15, 751–759. [Google Scholar] [CrossRef]
- Rae, P.J.; Dattelbaum, D.M. The properties of poly(tetrafluoroethylene) (PTFE) in compression. Polymer 2004, 45, 7615–7625. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Gupta, R.K. Review on corrosion performance of laser powder-bed fusion printed 316L stainless steel: Effect of processing parameters, manufacturing defects, post-processing, feedstock, and microstructure. Mater. Des. 2022, 221, 110874. [Google Scholar] [CrossRef]
- Choo, H.; Sham, K.-L.; Bohling, J.; Ngo, A.; Xiao, X.; Ren, Y.; Depond, P.J.; Matthews, M.J.; Garlea, E. Effect of laser power on defect, texture, and microstructure of a laser powder bed fusion processed 316L stainless steel. Mater. Des. 2019, 164, 107534. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; University of Michigan: Ann Arbor, MI, USA, 1989. [Google Scholar]
- Zadpoor, A.A. Additively manufactured porous metallic biomaterials. J. Mater. Chem. B 2019, 7, 4088–4117. [Google Scholar] [CrossRef]
- Li, S.J.; Xu, Q.S.; Wang, Z.; Hou, W.T.; Hao, Y.L.; Yang, R.; Murr, L.E. Influence of cell shape on mechanical properties of Ti-6Al-4V meshes fabricated by electron beam melting method. Acta Biomater. 2014, 10, 4537–4547. [Google Scholar] [CrossRef]
- Paiva, J.C.C.; Oliveira, L.; Vaz, M.F.; Costa-de-Oliveira, S. Biodegradable Bone Implants as a New Hope to Reduce Device-Associated Infections—A Systematic Review. Bioengineering 2022, 9, 409. [Google Scholar] [CrossRef]
- Sharma, P.; Jain, K.G.; Pandey, P.M.; Mohanty, S. In vitro degradation behaviour, cytocompatibility and hemocompatibility of topologically ordered porous iron scaffold prepared using 3D printing and pressureless microwave sintering. Mater. Sci. Eng. C 2020, 106, 110247. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. A novel manufacturing route for the fabrication of topologically-ordered open-cell porous iron scaffold. Mater. Lett. 2018, 222, 160–163. [Google Scholar] [CrossRef]
- Li, Y.; Lietaert, K.; Li, W.; Zhang, X.Y.; Leeflang, M.A.; Zhou, J.; Zadpoor, A.A. Corrosion fatigue behavior of additively manufactured biodegradable porous iron. Corros. Sci. 2019, 156, 106–116. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef]
- Carluccio, D.; Xu, C.; Venezuela, J.; Cao, Y.; Kent, D.; Bermingham, M.; Demir, A.G.; Previtali, B.; Ye, Q.; Dargusch, M. Additively manufactured iron-manganese for biodegradable porous load-bearing bone scaffold applications. Acta Biomater. 2020, 103, 346–360. [Google Scholar] [CrossRef]
- Fatima, M.; Canhao, H.; Eurico, J. Bone: A Composite Natural Material. In Advances in Composite Materials—Analysis of Natural and Man-Made Materials; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Linde, F.; Gothgen, C.B.; Hvid, I.; Pongsoipetch, B. Mechanical properties of trabecular bone by a non-destructive compression testing approach. Eng. Med. 1988, 17, 23–29. [Google Scholar] [CrossRef]
- McCalden, R.W.; McGlough, J.A.; Barker, M.B.; Court-Brown, C.M. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization and microstructure. J. Bone Jt. Surg. Ser. A 1993, 75, 1193–1205. [Google Scholar] [CrossRef]
- Öhman, C.; Baleani, M.; Pani, C.; Taddei, F.; Alberghini, M.; Viceconti, M.; Manfrini, M. Compressive behaviour of child and adult cortical bone. Bone 2011, 49, 769–776. [Google Scholar] [CrossRef]
- Røhl, L.; Larsen, E.; Linde, F.; Odgaard, A.; Jørgensen, J. Tensile and compressive properties of cancellous bone. J. Biomech. 1991, 24, 1143–1149. [Google Scholar] [CrossRef]
- Shi, W.; Liu, B.; Li, J.; Han, Y.; Zhang, Y.; Guo, B.; Lin, Y. Mechanical Properties Evaluation Based on the Variable Gradient of Body-Centered-Cubic-Based Lattice Structure Prepared by Laser Powder Bed Fusion. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Saleh, B.; Jiang, J.; Fathi, R.; Al-hababi, T.; Xu, Q.; Wang, L.; Song, D.; Ma, A. 30 Years of functionally graded materials: An overview of manufacturing methods, Applications and Future Challenges. Compos. Part B Eng. 2020, 201, 108376. [Google Scholar] [CrossRef]
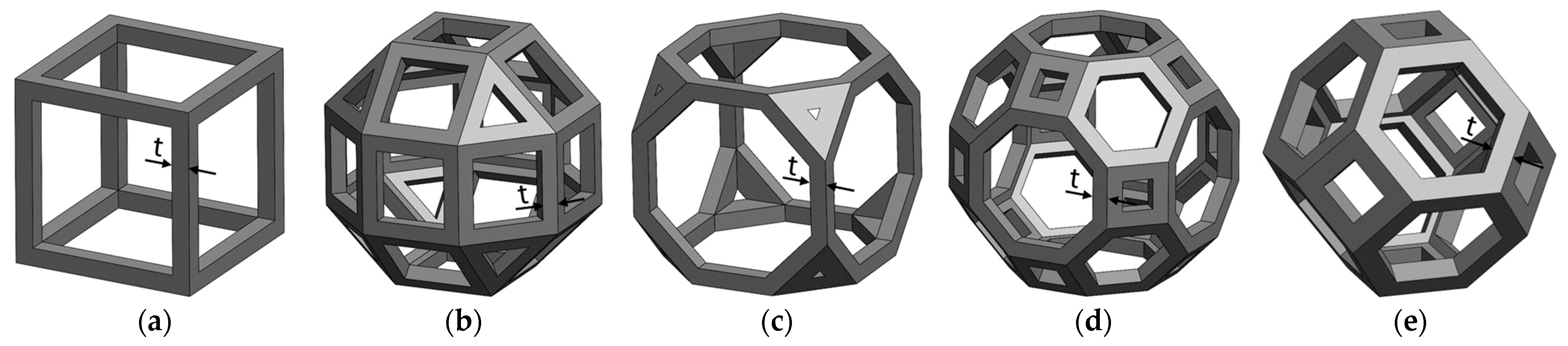


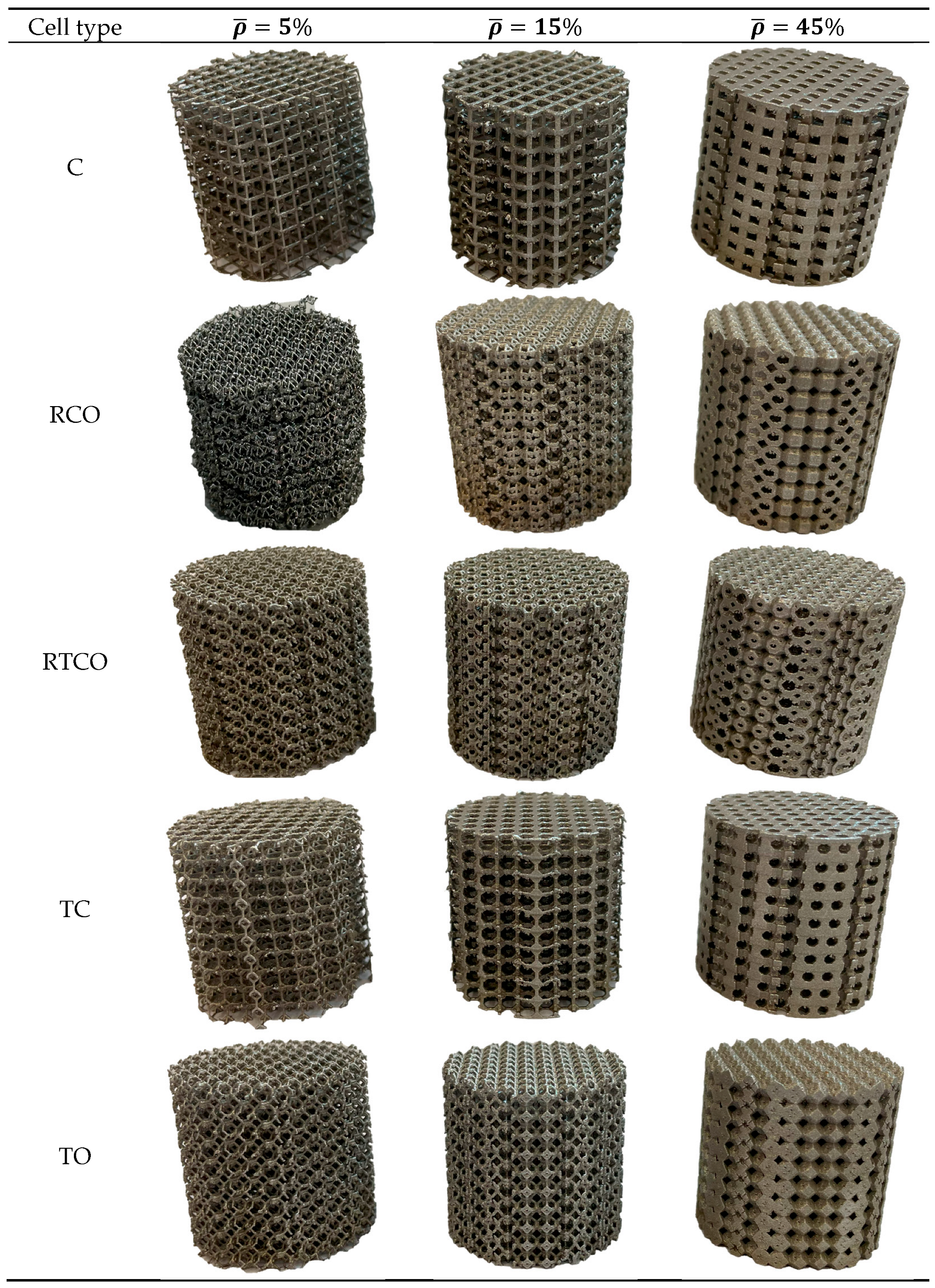

| Specimen | Theoretical Strut Thickness (mm) | Theoretical Relative Density (%) | Real Relative Density (%) | Mass Increment (%) |
|---|---|---|---|---|
| C-5% | 0.24 | 5 | 7.9 | 57.8 |
| C-15% | 0.43 | 15 | 19.2 | 28.0 |
| C-45% | 0.82 | 45 | 49.4 | 9.8 |
| RCO-15% | 0.28 | 15 | 24.3 | 62.0 |
| RCO-45% | 0.64 | 45 | 59.5 | 32.1 |
| RTCO-5% | 0.16 | 5 | 11.6 | 132.6 |
| RTCO-15%_P1 | 0.29 | 15 | 23.8 | 58.7 |
| RTCO-15%_P2 | 0.29 | 15 | 23.2 | 54.7 |
| RTCO-15%_P3 | 0.29 | 15 | 22.5 | 49.8 |
| RTCO-45%_P1 | 0.62 | 45 | 53.5 | 18.8 |
| RTCO-45%_P2 | 0.62 | 45 | 52.8 | 17.3 |
| TC-5% | 0.20 | 5 | 9.1 | 82.0 |
| TC-17% | 0.35 | 17 | 20.2 | 18.7 |
| TC-45% | 0.73 | 45 | 48.3 | 7.3 |
| TO-5% | 0.20 | 5 | 9.7 | 94.6 |
| TO-15%_P1 | 0.38 | 15 | 22.6 | 50.7 |
| TO-15%_P2 | 0.38 | 15 | 21.5 | 43.5 |
| TO-15%_P3 | 0.38 | 15 | 22.1 | 47.6 |
| TO-45% | 0.91 | 45 | 53.4 | 18.6 |
| Experimental Specimen | Young’s Modulus | Yield Stress | Computational Model | Young’s Modulus | Yield Stress |
|---|---|---|---|---|---|
| C-5% | 0.403 | 4.7 | C-15%-MoS2 | 11.375 | 29.1 |
| C-15% | 2.645 | 22.9 | C-19.2%-MoS2 | 12.030 | 29.6 |
| C-45% | 13.958 | 109.1 | C-15%-Teflon | 2.921 | 29.8 |
| RCO-15% | 1.591 | 28.6 | C-19.2%-Teflon | 3.113 | 30.5 |
| RCO-45% | 10.426 | 125.9 | |||
| RTCO-5% | 0.166 | 2.5 | |||
| RTCO-15%_P1 | 1.434 | 19.8 | RTCO-15%-MoS2 | 2.777 | 16.4 |
| RTCO-15%_P2 | 1.501 | 19.4 | RTCO-15%-Teflon | 1.507 | 16.9 |
| RTCO-15%_P3 | 1.634 | 21.3 | |||
| RTCO-45%_P1 | 8.548 | 128.5 | |||
| RTCO-45%_P2 | 19.552 | 119.5 | RTCO-45%-MoS2 | 24.634 | 107.8 |
| TC-5% | 0.430 | 4.7 | |||
| TC-17% | 3.024 | 19.0 | |||
| TC-45% | 12.015 | 111.3 | |||
| TO-5% | 0.298 | 3.6 | |||
| TO-15%_P1 | 1.442 | 23.3 | |||
| TO-15%_P2 | 1.380 | 18.7 | |||
| TO-15%_P3 | 1.166 | 15.2 | |||
| TO-45% | 10.986 | 130.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, P.; Lopes, P.; Oliveira, L.; Alves, J.L.; Magrinho, J.P.G.; Deus, A.M.d.; Vaz, M.F.; Silva, M.B. Evaluation of Lattice Structures for Medical Implants: A Study on the Mechanical Properties of Various Unit Cell Types. Metals 2024, 14, 780. https://doi.org/10.3390/met14070780
Nogueira P, Lopes P, Oliveira L, Alves JL, Magrinho JPG, Deus AMd, Vaz MF, Silva MB. Evaluation of Lattice Structures for Medical Implants: A Study on the Mechanical Properties of Various Unit Cell Types. Metals. 2024; 14(7):780. https://doi.org/10.3390/met14070780
Chicago/Turabian StyleNogueira, Pedro, Pedro Lopes, Luís Oliveira, Jorge L. Alves, João P. G. Magrinho, Augusto Moita de Deus, M. Fátima Vaz, and M. Beatriz Silva. 2024. "Evaluation of Lattice Structures for Medical Implants: A Study on the Mechanical Properties of Various Unit Cell Types" Metals 14, no. 7: 780. https://doi.org/10.3390/met14070780
APA StyleNogueira, P., Lopes, P., Oliveira, L., Alves, J. L., Magrinho, J. P. G., Deus, A. M. d., Vaz, M. F., & Silva, M. B. (2024). Evaluation of Lattice Structures for Medical Implants: A Study on the Mechanical Properties of Various Unit Cell Types. Metals, 14(7), 780. https://doi.org/10.3390/met14070780







