Durability Requirements for Reinforced Concrete Structures Placed in a Hostile Tropical Coastal Environment
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Coastal Outdoor Exposure Site (COES)
2.2. Characterization of the Atmospheric Corrosivity Categories
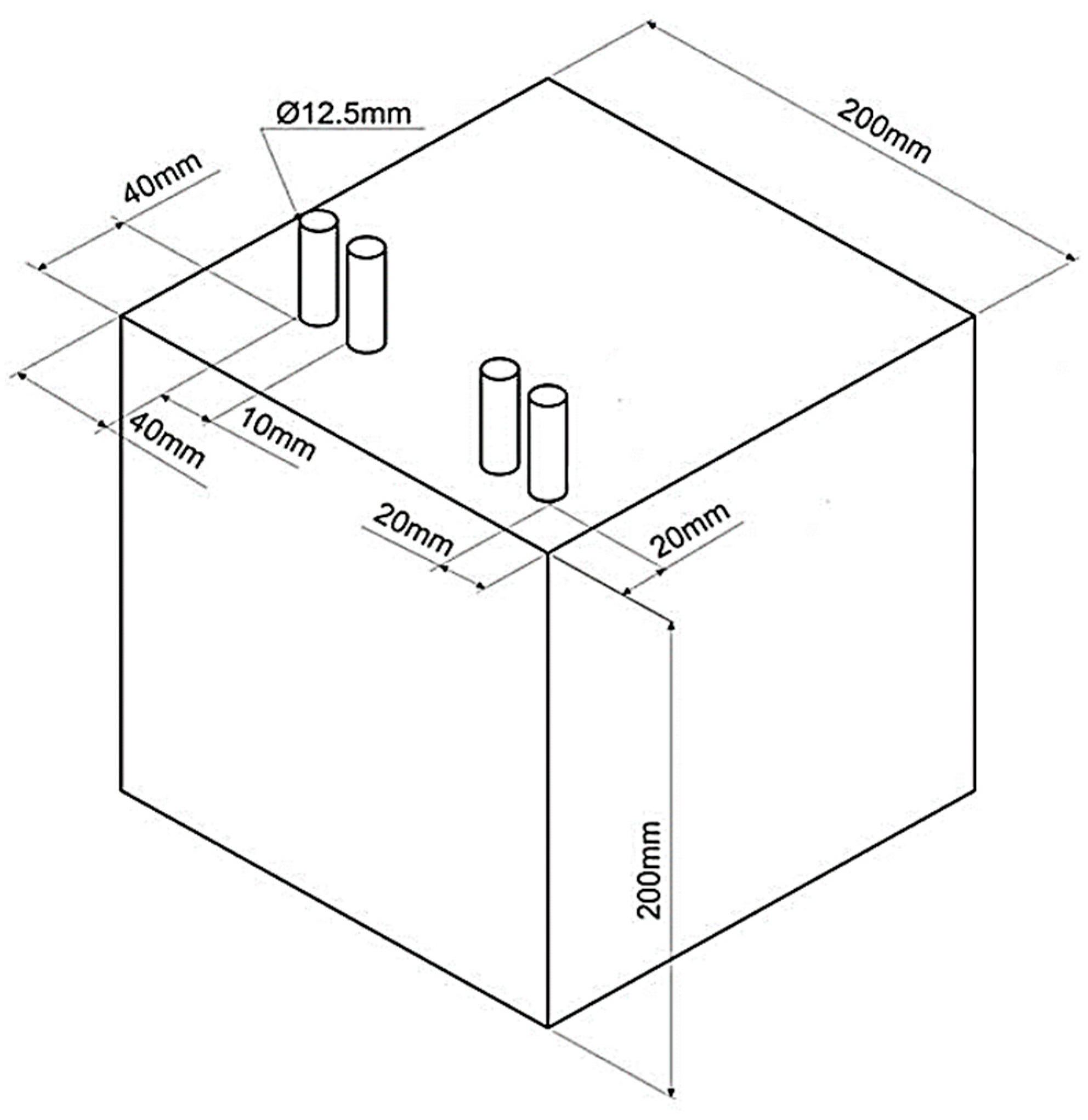
2.3. Preparation of Concrete and Reinforced Concrete (RC) Specimens
2.4. Concrete Quality Analysis
2.4.1. Compressive Strength (fck)
2.4.2. Density (D)
2.4.3. Ultrasonic Pulse Velocity (UPS)
2.4.4. Effective Capillary Porosity (εe-%)
2.5. Electrochemical Characterization
2.6. Statistical Analysis
2.7. Prediction of Service Life (Sl)
2.8. Microstructural Characterization
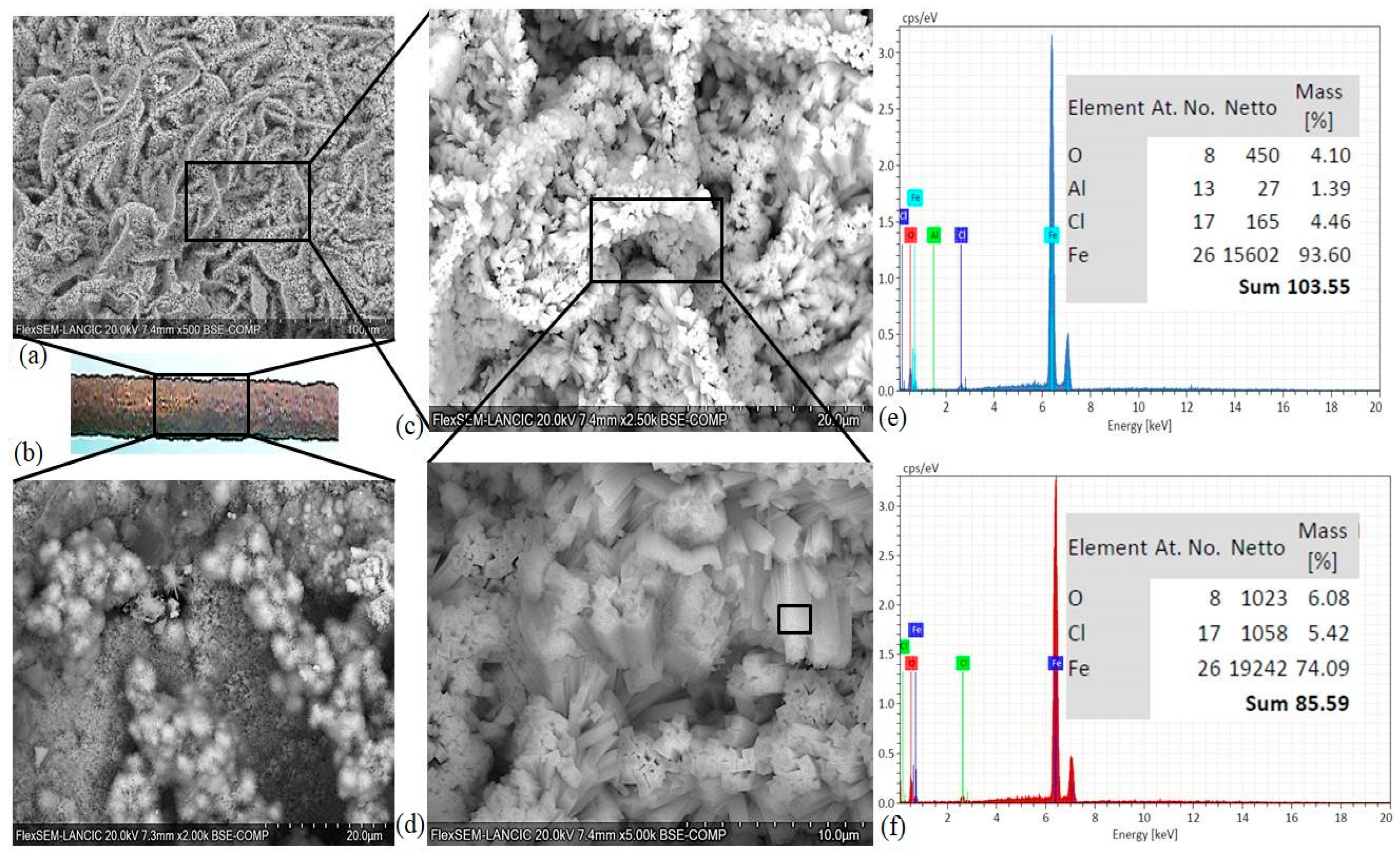
2.8.1. Scanning Electron Microscopy (SEM)
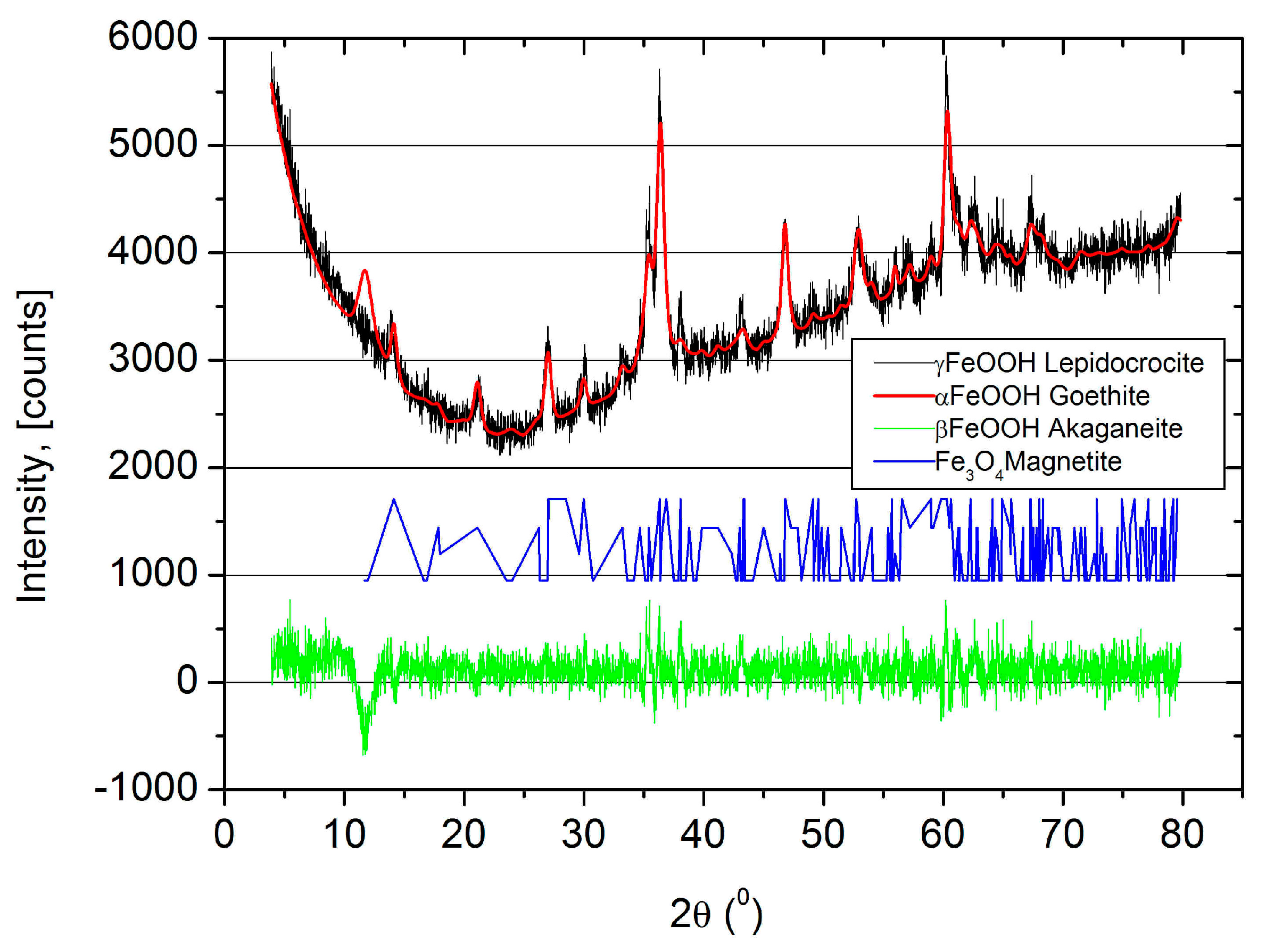
2.8.2. X-ray Diffraction (XRD)
3. Results
3.1. Description of the Typical Atmospheric Environment in the COES
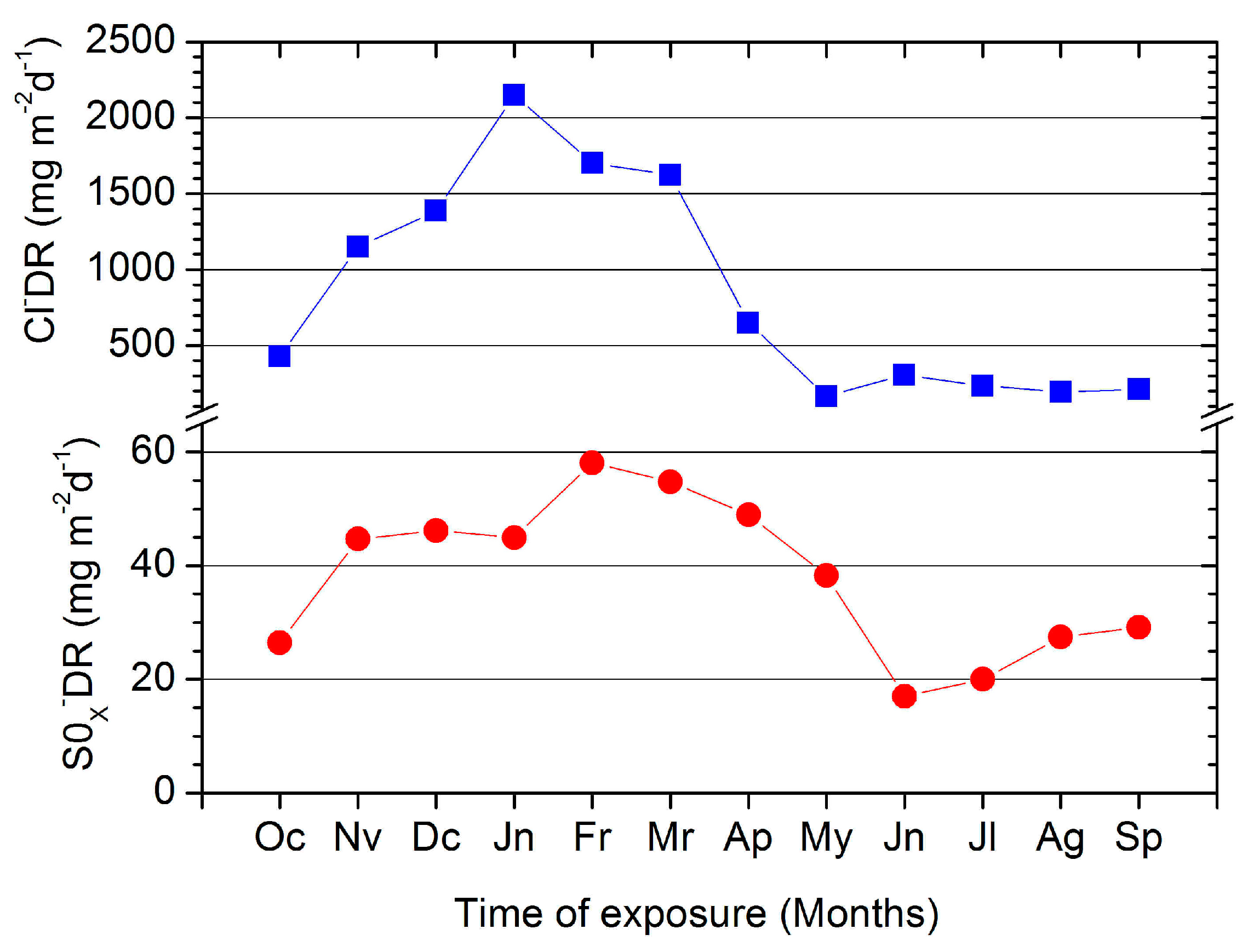
3.1.1. Monthly Mean Values of Cl−DR and SOx−DR
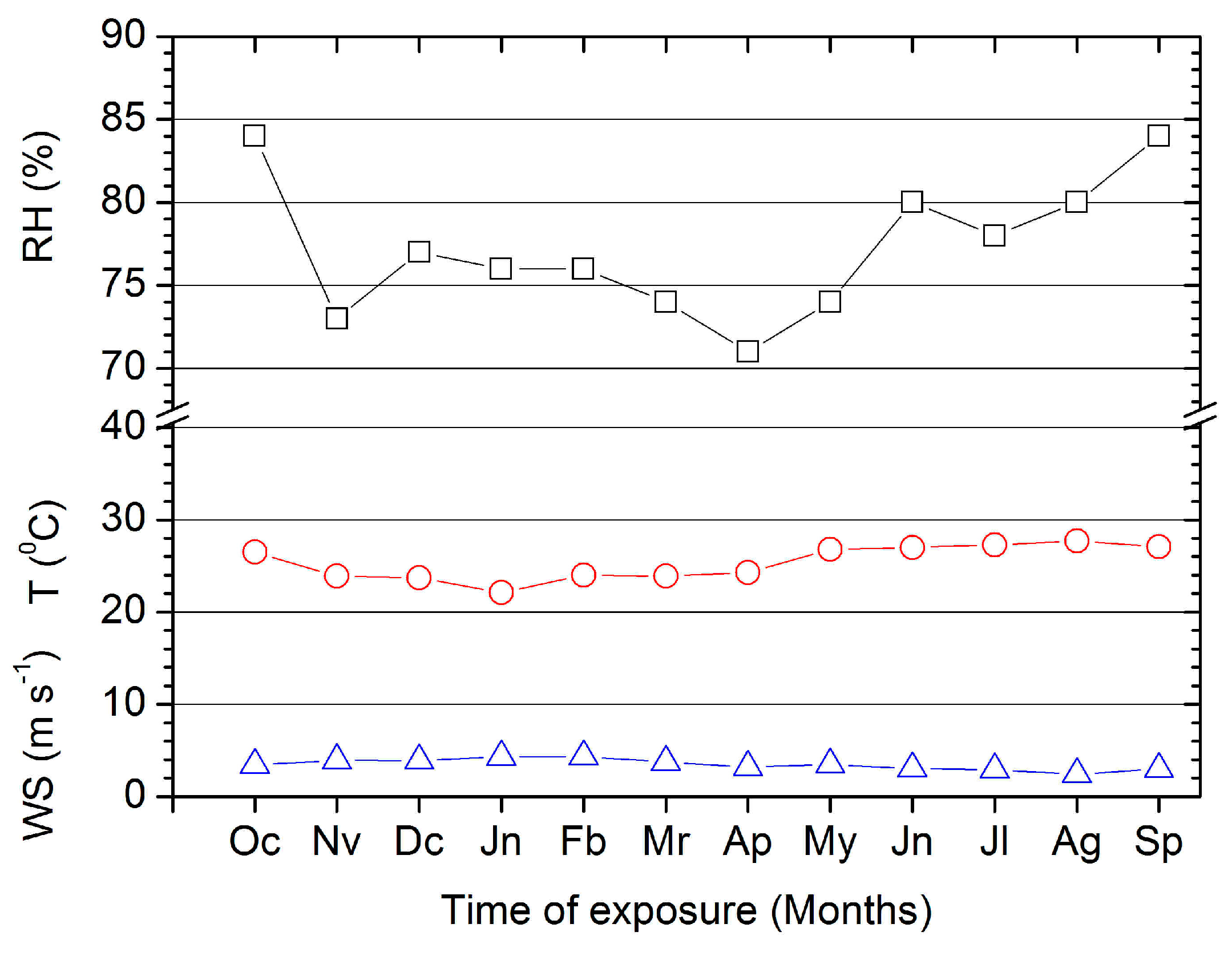
3.1.2. Monthly RH, T, and WS
3.2. Corrosivity Categories of the Atmosphere
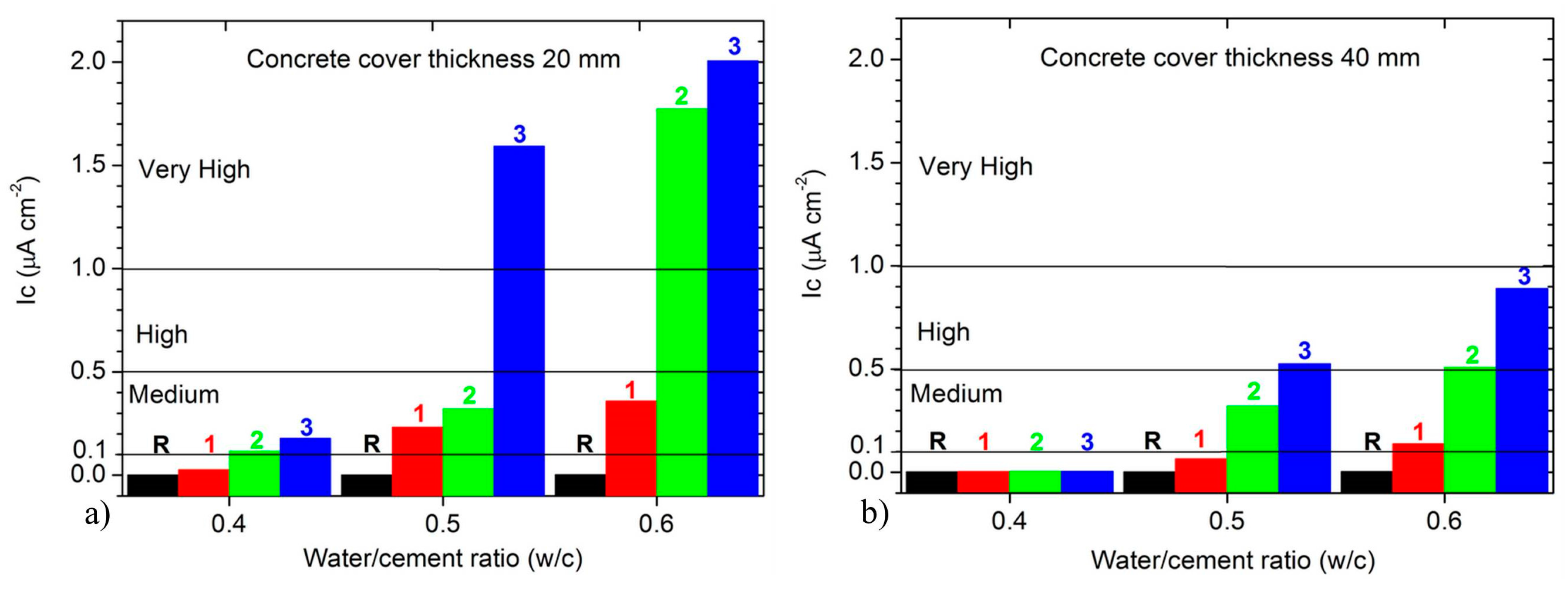
3.3. Electrochemical Corrosion Rate (Ic) and Durability Requirements
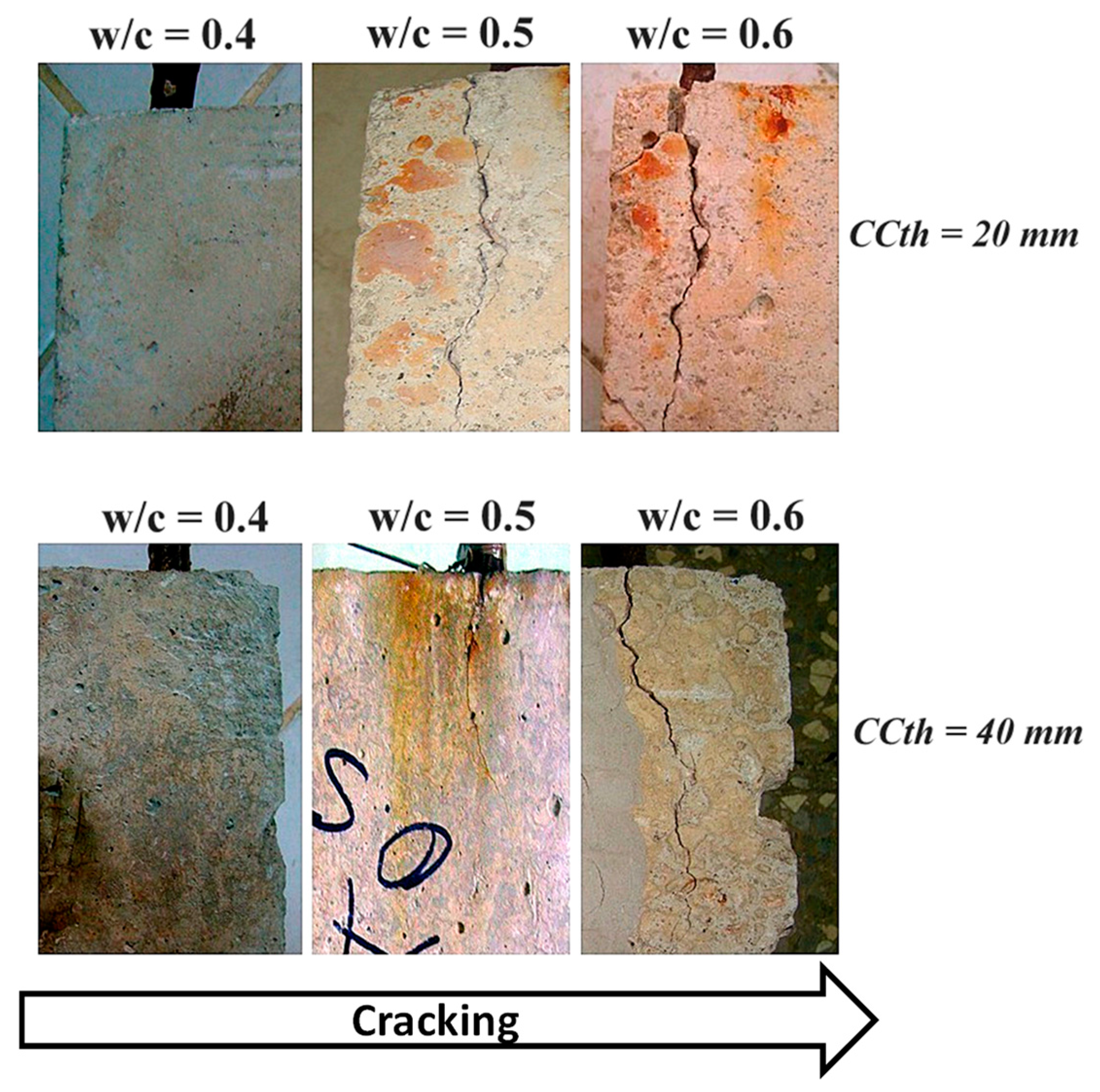
3.4. Macroscopic Analysis
4. Discussion
4.1. Corrosivity of the Atmosphere
4.2. Durability Requirements
4.3. Microstructure of Rust Layers
5. Conclusions
- According to the annual average chloride deposition rate and atmospheric corrosion rate for the metallic materials most commonly used in the construction industry (mainly for carbon steel), the coastal city of Havana can be considered among the coastal cities with the highest atmospheric corrosivity categories worldwide. An annual average atmospheric corrosion rate over the maximum limit established in the ISO 9223:2012 for the extreme (CX) atmospheric corrosivity category for carbon steel was determined. An increase in the range established for the extreme (CX) atmospheric corrosivity category in a coastal environment at a short distance from the shoreline could be considered. This result was confirmed from the visual observation of the carbon steel specimens.
- According to the behavior and prediction of the annual average Ic, considered as an indicator of the atmospheric corrosion rate of reinforcement steel in RC versus the time of exposure, the Sl from the sum of the time-to-corrosion-initiation (ti) and the time-to-corrosion-induced concrete cracking (tcc) was determined and predicted as a function of the durability requirements. The model established in the ISO 9224-2012 to predict the corrosivity category of the atmosphere for the metallic materials that are most often used in the construction industry (carbon steel, galvanized steel) for times of exposure over one year can be used to predict the Sl in RC structures in the coastal city of Havana and in all Cuban coastal environments.
- The two statistical methods used allowed for the demonstration of the most important durability requirements to ensure a long Sl of more than 70 years in RC structures in the Cuban coastal environment exposed to extreme (CX) and above-extreme (>CX) corrosivity categories. This requirement is as follows: w/c ratio of 0.4, fck, and εe, with a CCTh of 40 mm.
- It was confirmed that the occurrence of cracking in the RC specimens with w/c ratios of 0.5 and 0.6 for both CCTh values tested is an indicator of the end of the Sl, in less than five years, for RC structures exposed to very high (C5) and extreme (CX) atmospheric corrosivity categories in the coastal city of Havana. The results were confirmed by the visual observation of the RC specimens placed at the COES during the three years of this study.
- A significant difference between the chemical composition of the oxide layers developed in the freely exposed carbon steel specimens and the reinforcement steel embedded in the RC was not observed, but the presence of akaganeite with different morphologies was reported for the reinforced steel. It seems that crystalline-phase akaganeite could be one of the reasons for the corrosion product expansion of the reinforcement steel, which subsequently led to cracking in the specimens with a CCTh of 20 and 40 mm in the RC prepared with w/c ratios of 0.5 and 0.6.
- Since only one type of cement was considered in this work, the proposed protocol could be applied in future research to study the durability of RC containing supplementary cementitious materials or made using green formulations, recycled concrete aggregates, and/or sand to provide a large variety of solutions to ensure the sustainability and durability of constructions in these tropical environments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abosrra, L.; Ashour, A.F.; Youseffi, M. Corrosion of steel reinforcement in concrete of different compressive strengths. Constr. Build. Mater. 2011, 25, 3915–3925. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Wu, Y.; Jin, W. Critical thickness of rust layer at inner and out surface cracking of concrete cover in reinforced concrete structures. Corr. Sci. 2012, 59, 316–323. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 37, 125–138. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhattacharjee, B. Rebar corrosion in chloride environment. Constr. Build. Mater. 2011, 25, 2565–2575. [Google Scholar] [CrossRef]
- Ann, K.Y.; Ahn, J.H.; Ryou, J.S. The importance of chloride content at the concrete surface in assessing the time to corrosion of steel in concrete structures. Constr. Build. Mater. 2009, 23, 239–245. [Google Scholar] [CrossRef]
- Narasimhan, H.; Chew, M.Y. Integration of durability with structural design: An optimal life cycle cost based design procedure for reinforced concrete structures. Constr. Build. Mater. 2009, 23, 918–929. [Google Scholar] [CrossRef]
- Liu, P.; Yu, Z.; Lu, Z.; Chen, Y.; Liu, X. Predictive convection environment depth of chloride in concrete under chloride environment. Cem. Concr. Compos. 2016, 72, 257–267. [Google Scholar] [CrossRef]
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Initial corrosion presented by chloride threshold penetration of concrete up to 10 year-results under marine site. Constr. Build. Mater. 2012, 37, 693–698. [Google Scholar] [CrossRef]
- Castañeda, A.; Rodríguez, M. Las pérdidas económicas causadas por el fenómeno de la corrosión atmosférica del acero de refuerzo embebido en el hormigón armado. Resultadospreliminares. Rev. CENIC. Chem. Sci. 2014, 45, 52–59. [Google Scholar]
- Rincon, L.F.; Moscoso, Y.M.; Hamami, A.E.A.; Matos, J.C.; Bastidas-Arteaga, E. Degradation Models and Maintenance Strategies for Reinforced Concrete Structures in Coastal Environments under Climate Change: A Review. Buildings 2024, 14, 562. [Google Scholar] [CrossRef]
- Muralidharan, S.; Vedalakshmi, R.; Saraswathi, V.; Joseph, J.; Palaniswamy, N. Studies on the aspects of chloride ion determination in different types of concrete under macro-cell corrosion conditions. Build. Environ. 2005, 40, 1275–1281. [Google Scholar] [CrossRef]
- Güneyisi, E.; Özturan, T.; Gesoglu, M. Effect of initial curing on chloride ingress and corrosion resistance characteristics of concretes made with plain and blended cements. Build. Environ. 2007, 42, 2676–2685. [Google Scholar] [CrossRef]
- Yiğiter, H.; Yazıcı, H.; Aydın, S. Effects of cement type, water/cement ratio and cement content on sea water resistance of concrete. Build. Environ. 2007, 42, 1770–1776. [Google Scholar] [CrossRef]
- Mohamed, N.; Boulfiza, M.; Evitts, R. Corrosion of Carbon Steel and Corrosion-Resistant Rebars in Concrete Structures Under Chloride Ion Attack. J. Mater. Eng. Perform. 2012, 22, 787–795. [Google Scholar] [CrossRef]
- Castañeda, A.; Corvo, F.; O’Reilly, V. Comparación entre el pronóstico de corrosiónbasado en la medición de potenciales y la determinación de la velocidad de corrosión de la barra de refuerzomediantetécnicaselectroquímicas. Mater. Construcción 2003, 53, 155–164. [Google Scholar]
- Castañeda, A.; Corvo, F.; González, J. Estudiocomparativo de la corrosióndelacero de refuerzo en el hormigónarmadoa partir de técnicaselectroquímicas y convencionales. Rev. CENIC. Chem. Sci. 2010, 41, 1–9. [Google Scholar]
- Behera, P.K.; Misra, S.; Mondal, K. Corrosion Behavior of Strained Rebar in Simulated Concrete Pore Solution. J. Mater. Eng. Perform. 2020, 33, 1939–1954. [Google Scholar] [CrossRef]
- Poursaee, A. Corrosion of Steel Bars in Saturated Ca(OH)2 and Concrete Pore Solution. Concr. Res. Lett. 2010, 3, 90–97. [Google Scholar]
- Mundra, S.; Criado, M.; Bernal, S.A.; Provis, J.L. Chloride-Induced Corrosion of Steel Rebars in Simulated Pore Solutions of Alkali Activated Concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Hussain, R.R.; Singh, J.K.; Alhozaimy, A.; Al-Negheimish, A.; Bhattacharya, C.; Pathania, R.S.; Singh, D.D.N. Effect of Reinforcing Bar Microstructure on Passive Film Exposed to Simulated Concrete Pore Solution. ACI Mater. J. 2018, 115, 181. [Google Scholar] [CrossRef]
- Williamson, J.; Isgor, O.B. The Effect of Simulated Concrete Pore Solution Composition and Chlorides on the Electronic Properties of Passive Films on Carbon Steel Rebar. Corros. Sci. 2016, 106, 82–95. [Google Scholar] [CrossRef]
- De Rincón, O.T.; Castro, P.; Moreno, E.I.; Torres-Acosta, A.A.; De Bravo, O.M.; Arrieta, I.; Martínez-Madrid, M. Chlorideprofiles in two marine structures—Meaning and somepredictions. Build. Environ. 2004, 39, 1065–1070. [Google Scholar] [CrossRef]
- Pang, L.; Li, Q. Service life prediction of RC structures in marine environment using long term chloride ingress data: Comparison between exposure trials and real structure surveys. Constr. Build. Mater. 2016, 113, 979–987. [Google Scholar] [CrossRef]
- Cui, Z.; Alipour, A. Concrete cover cracking and service life prediction of reinforced concrete structures in corrosive environments. Constr. Build. Mater. 2018, 159, 652–671. [Google Scholar] [CrossRef]
- Vieira, D.R.; Moreira, A.L.R.; Calmon, J.L.; Dominicini, W.K. Service life modeling of a bridge in a tropical marine environment for durable design. Constr. Build. Mater. 2018, 163, 315–325. [Google Scholar] [CrossRef]
- Castañeda, A.; Corvo, F.; Marrero, R.; Fernández, A.; Del Angel-Meraz, E. The service life of reinforced concrete structures in an extremely aggressive coastal city. Influence of concrete quality. Mater. Struct. 2023, 56, 12. [Google Scholar] [CrossRef]
- De Vera, G.; Anton, C.; Lopez, M.P.; Climent, M.A. Depassivation time estimation in reinforced concrete structures exposed to chloride ingress: A probabilistic approach. Cem. Concr. Compos. 2017, 79, 21–33. [Google Scholar] [CrossRef]
- Samindi, S.M.; Samarakoon, M.; Jan Sælensminde, K. Condition assessment of reinforced concrete structures subject to chloride ingress: A case study of updating the model prediction considering inspection data. Cem. Concr. Compos. 2015, 60, 92–98. [Google Scholar]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, F. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Zacchei, E.; Bastidas-Arteaga, E. Multifactorial Chloride Ingress Model for Reinforced Concrete Structures Subjected to Unsaturated Conditions. Collection Advanced Concrete Structures in Civil Engineering. Buildings 2022, 12, 107. [Google Scholar] [CrossRef]
- Anwar Hossain, K.M.; Easa, S.M.; Lachemi, M. Evaluation of the effect of marine salts on urban built infrastructure. Build. Environ. 2009, 44, 713–722. [Google Scholar] [CrossRef]
- Trocónis, O.; Duracon Collaboration. Durability of concrete structures: DURACON, an Iberoamerican project. Preliminary results. Build. Environ. 2006, 41, 952–962. [Google Scholar] [CrossRef]
- ISO 12944-9:2018; Paints and Varnishes-Corrosion and Protection of Steel Structures by Protective Paint Systems. Part 9. Protective Paint Systems and Laboratory Performance Test Methods for Offshore and Related Structures. Beuth: Berlin, Germany, 2018.
- ISO 9223:2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. ISO: Geneva, Switzerland, 2012.
- Jiang, J.H.; Yuan, Y.S. Development and prediction strategy of steel corrosion rate in concrete under natural climate. Constr. Build. Mater. 2013, 44, 287–292. [Google Scholar] [CrossRef]
- Castañeda, A.; Rivero, C.; Corvo, F. Evaluación de sistemas de protección contra la corrosión en la rehabilitación de estructurasconstruidas en sitios de elevadaagresividadcorrosiva en Cuba. Rev. Construcción 2012, 11, 49–61. [Google Scholar] [CrossRef]
- ISO 9225: 2012; Corrosion of Metals and Alloys—Corrosivity of Atmosphere—Measurement of Environmental Parameters Affecting Corrosivity of Atmosphere. International Organization for Standardization: Geneva, Switzerland, 2012.
- Cuban Standard. 120:2014; Reinforced Concrete. Specifications. Cuban National Bureau of Standards: La Habana, Cuba, 2014.
- ISO 8407: 2009; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Tests Specimens. International Organization for Standardization: Geneva, Switzerland, 2009.
- Troconis de Rincon, O. Manual for Inspecting, Evaluating and Diagnosing Corrosion in Reinforced Concrete Structures; NACE: Houston, TX, USA, 2000. [Google Scholar]
- Cuban Standard 724:2009; Test on Concrete. Resistance of Hardened Concrete. Cuban National Bureau of Standards: La Habana, Cuba, 2009.
- Cuban Standard 231:2012; Test on Concrete. Determination, Interpretation and Application of Ultrasonic Pulse Velocity in Concrete. Cuban National Bureau of Standards: La Habana, Cuba, 2012.
- Fagerlund, G. On the Capillarity of Concrete. Nord. Concr. Res. 1986, 1. [Google Scholar]
- ISO 9224:2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Guiding Values for the Corrosivity Categories. ISO: Geneva, Switzerland, 2012.
- Castañeda, A.; Corvo, F.; Fernández, D.; Valdés, C. Outdoor-indoor atmospheric corrosion in a coastal wind farm located in a tropical island. Eng. J. 2017, 21, 43–62. [Google Scholar] [CrossRef]
- Corvo, F.; Pérez, T.; Dzib, L.; Martín, Y.; Castañeda, A.; González, E.; Pérez, J. Outdoor-Indoor corrosion of metals in tropical coastal atmospheres. Corros. Sci. 2008, 50, 220–230. [Google Scholar] [CrossRef]
- Castañeda, A.; Corvo, F.; Howland, J.J.; Marrero, R. Penetration of marine aerosol in a tropical coastal City: Havana. Atmósfera 2018, 31, 87–104. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Padaratz, I.J.; Borba, J.C. Modelling sea-salt transport and deposition in marine atmosphere environment—A tool for corrosion studies. Corros. Sci. 2008, 50, 2724–2731. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Borba, J.C., Jr.; Padilha, M., Jr. Durability of concrete structure in marine atmosphere environment. The use of chloride deposition rate on wet candle as an environment indicator. Cem. Concr. Compos. 2010, 32, 427–435. [Google Scholar] [CrossRef]
- Molina, L.; Alonso, F.J.; Alonso, M.C.; Sánchez, M.; Jarabo, R. Corrosion protection of galvanized rebars in ternary binder concrete exposed to chloride penetration. Constr. Build. Mater. 2017, 156, 468–475. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, J.G.; Li, W.; Poon, C.S. Influence of chloride ion on depassivation of passive film on galvanized steel bars in concrete pore solution. Constr. Build. Mater. 2018, 156, 572–580. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, G. Corrosion inhibition of galvanized steel by MnO4− ion as a soluble inhibitor in simulated fresh concrete environment. Constr. Build. Mater. 2020, 257, 119532. [Google Scholar] [CrossRef]
- Castañeda, A.; Howland, J.J.; Corvo, F.; Marrero, R. Concrete quality assessment before building structures submitting to environmental exposure conditions. Rev. Constr. 2017, 16, 374–387. [Google Scholar]
- Castañeda, A.; Howland, J.J.; Corvo, F.; Marrero, R. Concrete quality assessment before submitting to environmental exposure conditions in Cuba. Rev. Acta Microscópica 2017, 26. [Google Scholar] [CrossRef]
- Carvajal, M.; Vera, R.; Corvo, F.; Castañeda, A. Diagnosis and rehabilitation of real reinforced concrete structures in coastal areas. Corros. Eng. Sci. Technol. 2011, 47, 70–77. [Google Scholar] [CrossRef]
- Corvo, F.; Pérez, T.; Martin, Y.; Reyes, J.; Dzib, L.R.; González, J.A.; Castañeda, A. Corrosion research frontiers. Atmospheric corrosion in tropical climate. On the concept of Wetness and its interaction with contaminants deposition. In Electroanalytical Chemistry: New Research; Nova Science Publishers: New York, NY, USA, 2008; Chapter 2. [Google Scholar]
- Corvo, F.; Pérez, T.; Martin, Y.; Reyes, J.; Dzib, L.R.; González-Sánchez, J.; Castañeda, A. Atmosphericcorrosion in tropical humidclimates. Environmental Degradation of Infrastructure and Cultural Heritage in Coastal Tropical Climate; Transworld Research Network: Kerala, India, 2009. [Google Scholar]
- Chico, B.; De la Fuente, D.; Díaz, I.; Simancas, J.; Morcillo, M. Annual Atmospheric Corrosion of Carbon Steel Worldwide. An Integration of ISOCORRAG, ICP/UNECE and MICAT Databases. Materials 2017, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Mariaca, L.; Menchaca, C.; Sarmiento, E.; Sarmiento, O.; Ramírez, J.L.; Uruchurtu, J. Atmospheric Corrsion Dose/Response Functions from Statistical Data Analysis for Different Sites of Mexico. Innov. Corros. Mater. Sci. 2014, 4, 11–20. [Google Scholar]
- Alcántara, J.; Chico, B.; Diaz, I.; De la Fuente, D.; Morcillo, M. Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel. Corros. Sci. 2015, 97, 74–88. [Google Scholar] [CrossRef]
- Castañeda, A.; Valdés, C.; Corvo, F. Atmospheric corrosion study in a harbor located in a tropical island. Mater. Corros. 2018, 69, 1462–1477. [Google Scholar] [CrossRef]
- Guerra, J.C.; Castañeda, A.; Corvo, F.; Howland, J.J.; Rodríguez, J. Atmospheric corrosion of low carbon steel in a coastal environment of Ecuador: Anomalous behavior of chloride deposition versus distance from the sea. Mater. Corros. 2018, 69, 444–460. [Google Scholar]
- Valipour, M.; Shekarchi, M.; Ghods, P. Comparative studies of experimental and numerical techniques in measurement of corrosion rate and time-to-corrosion-initiation of rebar in concrete in marine environments. Cem. Concr. Compos. 2014, 48, 98–107. [Google Scholar] [CrossRef]
- Trocónis, O.; Duracon Collaboration. Effect of the marine environment on reinforced concrete durability in Iberoamerican countries: DURACON project/CYTED. Corros. Sci. 2007, 49, 2832–2843. [Google Scholar] [CrossRef]
- Siamphukdee, K.; Collins, F.; Zou, R. Sensitivity Analysis of Corrosion Rate Prediction Models Utilized for Reinforced Concrete Affected by Chloride. J. Mater. Eng. Perform. 2012, 22, 1530–1540. [Google Scholar] [CrossRef]
- Corvo, F.; Pérez, T.; Martin, Y.; Reyes, J.; Dzib, L.R.; González-Sánchez, J.; Castañeda, A. Time of wetness in tropical climate: Considerations on the estimation of TOW according to ISO 9223 standard. Corros. Sci. 2008, 50, 206–219. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Zhao, Y.-G.; Yu, K. Stochastic Modeling of Corrosion Propagation for Service Life Prediction of Chloride Contaminated RC Structures. In Proceedings of the 1st International Symposium on Life-Cycle Civil Engineering, Lake Como, Italy, 11–14 June 2008; pp. 195–201. [Google Scholar]
- Vu, K.A.T.; Stewart, M.G. Structural reliability of concrete bridges including improved chloride-induced corrosion models. Struct. Saf. 2000, 22, 313–333. [Google Scholar] [CrossRef]
- Costa, A.; Appleton, J. Case studies of concrete deterioration in a marine environment in Portugal. Cem. Concr. Compos. 2002, 24, 169–179. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; De la Fuente, D.; Simancas, J. Looking Back on Contributions in the Field of Atmospheric Corrosion Offered by the MICAT Ibero-American Testing Network. Int. J. Corros. 2012, 2012, 824365. [Google Scholar] [CrossRef]
- Castañeda, A.; Corvo, F.; Pech, I.; Valdés, C.; Marrero, R.; Del Angel-Meraz, E. Atmospheric Corrosion in an Oil Refinery Located on a Tropical Island under New Pollutant Situation. J. Mater. Eng. Perform. 2021, 30, 4529–4542. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. A micro-Raman spectroscopic study of marine atmospheric corrosion of carbon steel: The effect of Akaganeite. J. Electrochem. Soc. 2015, 162, C495. [Google Scholar] [CrossRef]
- Reguer, S.; Mirambet, F.; Dooryhee, E.; Hodeau, J.L.; Dillmann, P.; Lagarde, P. Structural evidence for the desalination of Akaganeite in the preservation of iron archaeological objects, using synchrotron X-ray powder diffraction and absorption spectroscopy. Corros. Sci. 2009, 51, 2795–2802. [Google Scholar] [CrossRef]
- Ståhl, K.; Nielsen, K.; Jiang, J.; Lebech, B.; Hanson, J.C.; Norby, P.; Van Lanschot, J. On the akaganéite crystal structure, phase transformations and possible role in post-excavational corrosion of iron artifacts. Corros. Sci. 2003, 45, 2563–2575. [Google Scholar] [CrossRef]
- Mazeina, L.; Deore, S.; Navrotsky, A. Energetics of bulk and nano-Akaganeite, β-FeOOH: Enthalpy of formation, surface enthalpy, and enthalpy of water adsorption. Chem. Mater. 2006, 18, 1830–1838. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses; VCH: Weinheim, Germany, 1996. [Google Scholar]
- de la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M.J. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- De la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of rust surfaces formed on mild steel exposed to marine atmospheres using XRD and SEM/Micro-Raman techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Morcillo, M.; González-Calbet, J.M.; Jiménez, J.A.; Díaz, I.; Alcántara, J.; Chico, B.; De la Fuente, D. Environmental conditions for Akaganeite formation in marine atmosphere mild steel corrosion products and its characterization. Corrosion 2015, 71, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Huet, B.; L’Hostis, V.; Miserque, F.; Idrissi, H. Electrochemical behavior of mild steel in concrete: Influence of pH and carbonate content of concrete pore solution. Electrochim. Acta 2005, 51, 172–180. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Lara, E. Role of Akaganeite (β-FeOOH) in the growth of hematite (α-Fe2O3) in an inorganic silica hydrogel. Cryst. Growth Des. 2015, 15, 3388–3398. [Google Scholar] [CrossRef]
- Naren, G.; Ohashi, H.; Okaue, Y.; Yokoyama, T. Adsorption kinetics of silicic acid on Akaganeite. J. Colloid Interface Sci. 2013, 399, 87–91. [Google Scholar] [CrossRef]



| Components (%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | L.O.I |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 20.64 | 4.83 | 3.27 | 63.64 | 1.26 | 1.94 | 0.56 | 0.45 | 3.41 |
| Gravel | 7.86 | 0.58 | 1.02 | 45.15 | 3.96 | 0.15 | 0.01 | 0.34 | 40.93 |
| Sand | 1.42 | 0.68 | 0.30 | 45.46 | 3.52 | - | 0.07 | 0.05 | 48.50 |
| w/c | Water (kg m−3) | Superplasticiser (kg m−3) | Slump (cm) |
|---|---|---|---|
| 0.6 | 222 | 1.0 | 18 |
| 0.5 | 186 | 1.5 | 17 |
| 0.4 | 148 | 1.7 | 15 |
| Material | Corrosion Rate (µm y−1) | Range per Category (µm y−1) | Category |
|---|---|---|---|
| Carbon Steel | 452.45 | 200 > rcorr > 700 | CX |
| Zinc | 24.33 | 8.4 > rcorr > 25 | CX |
| w/c | Concrete Cover Thickness (mm) | fck (MPa) | εe (%) | UPV (m s−1) | D (kg m−3) | Ic (μA cm−2) | ti (Years) |
|---|---|---|---|---|---|---|---|
| 0.4 | 40 | [23,24,35] | [5–9] | [4220–4300] | [2340–2360] | [0.001–0.002] | 42 |
| Coastal City/Country | Cl−DR (mg m−2d−1) | Corrosivity Category (C) rcorr (μm y−1) |
|---|---|---|
| Coatzacoalcos/Mexico | 1040.37 | 320.24 |
| Minatitlán/Mexico | 763.54 | 294.58 |
| Veracruz/Mexico | 1483.00 | 294.58 |
| Tuxpan/Mexico | 671.37 | 263.84 |
| Laguna Verde/Mexico | 826.8 | 216.98 |
| Tampico/Mexico | 1307.69 | 327.49 |
| Tulúm/Mexico | 513.48 | 202.59 |
| Refinery/Irán | 1409.96 | 450.24 |
| Saudi Arabia | 608.00 | 472.0 |
| Choshi/Japan | 324.66 | 323.0 |
| Limón/Costa Rica | 220.00 | 371.5 |
| Sines/Portugal | 203.00 | 365.0 |
| Number of Samples (n) | Concrete Cover Thickness of (mm) | Relationship | R2 (%) | p (≤0.0000) |
|---|---|---|---|---|
| 36 | 20 | 94 | 0.0000 | |
| 36 | 40 | 99 | 0.0000 |
| Durability Requirements | CCTh of 20 mm | CCTh of 40 mm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Average | Homogeneity Group | Average | Homogeneity Group | |||||||
| X Column | X Column | ||||||||||
| Ic (μA-cm−2) | 36 | 1.260 | X | 0.486 | X | ||||||
| w/c ratio | 36 | 0.5 | X | 0.5 | X | ||||||
| εe (%) | 36 | 13.7 | X | X | 13.7 | X | X | ||||
| fck (MPa) | 36 | 30 | X | 30 | X | ||||||
| D (kg m−3) | 36 | 2333 | X | 2333 | X | ||||||
| UPV (m s−1) | 36 | 4097 | X | 4097 | X | ||||||
| Contrast | CCTh of 20 mm | CCTh of 40 mm | ||||
|---|---|---|---|---|---|---|
| Sig. | Difference | ±Limit | Sig. | Difference | ±Limit | |
| Ic-w/c | 0.760 | 24.863 | −0.013 | 24.863 | ||
| Ic-fck | * | −29.158 | * | −29.932 | ||
| Ic-εe | −12.499 | −13.273 | ||||
| Ic-UPV | * | −4095.8 | * | −4096.57 | ||
| Ic-D | * | −2331.43 | * | −2332.21 | ||
| w/c-fck | * | −29.9194 | * | −29.919 | ||
| w/c-εe | −13.260 | −13.260 | ||||
| w/c-UPV | * | −4096.56 | * | −4096.56 | ||
| w/c-D | * | −2332.19 | * | −2332.19 | ||
| fck-εe | 16.659 | 16.659 | ||||
| fck-UPV | * | −4066.64 | * | −4066.64 | ||
| fck-D | * | −2302.28 | * | −2302.28 | ||
| εe-UPV | * | −4083.3 | * | −4083.3 | ||
| εe-D | * | −2318.93 | * | −2318.93 | ||
| UPV-D | * | 1764.36 | * | 1764.36 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda Valdés, A.; Corvo Pérez, F.; Pech Pech, I.; Marrero Águila, R.; Bastidas-Arteaga, E. Durability Requirements for Reinforced Concrete Structures Placed in a Hostile Tropical Coastal Environment. Buildings 2024, 14, 2494. https://doi.org/10.3390/buildings14082494
Castañeda Valdés A, Corvo Pérez F, Pech Pech I, Marrero Águila R, Bastidas-Arteaga E. Durability Requirements for Reinforced Concrete Structures Placed in a Hostile Tropical Coastal Environment. Buildings. 2024; 14(8):2494. https://doi.org/10.3390/buildings14082494
Chicago/Turabian StyleCastañeda Valdés, Abel, Francisco Corvo Pérez, Ildefonso Pech Pech, Rigoberto Marrero Águila, and Emilio Bastidas-Arteaga. 2024. "Durability Requirements for Reinforced Concrete Structures Placed in a Hostile Tropical Coastal Environment" Buildings 14, no. 8: 2494. https://doi.org/10.3390/buildings14082494





