Microscopic Transport and Degradation Behavior of CO2 in C-S-H with Varying Ca/Si Ratios during Carbonation
Abstract
1. Introduction
2. Modeling and Simulation Details
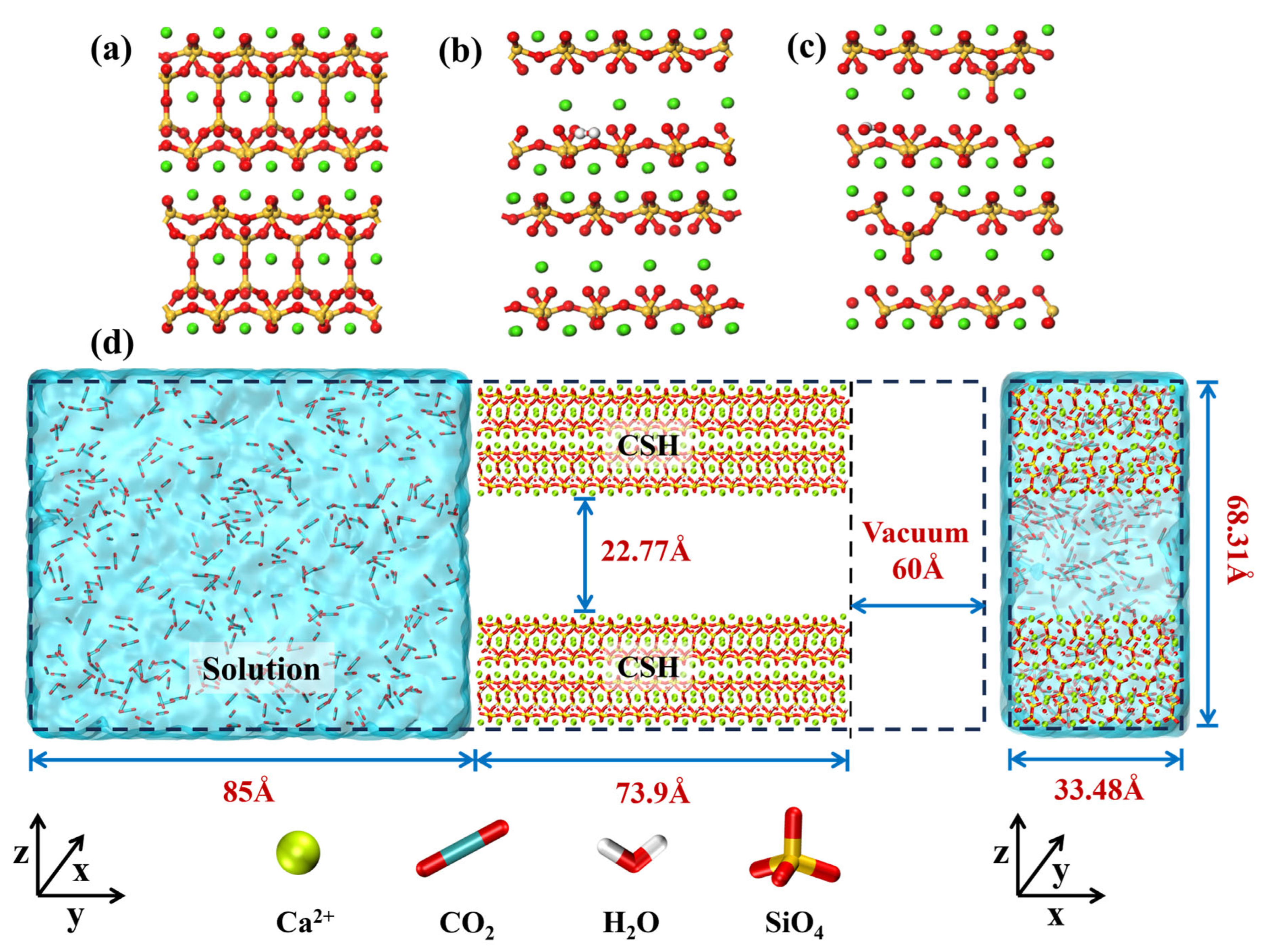
2.1. Model Construction
2.2. Force Field and Molecular Dynamics Programs
3. Results and Discussion
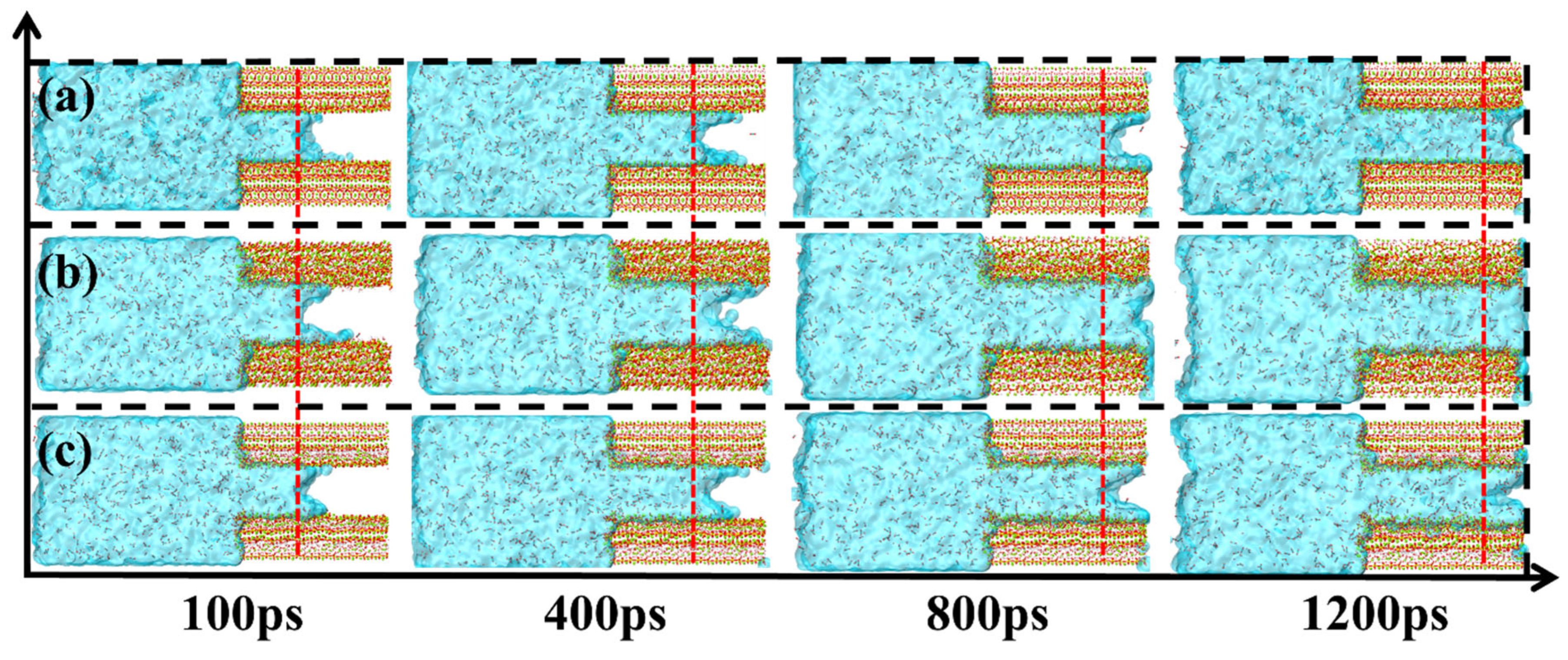
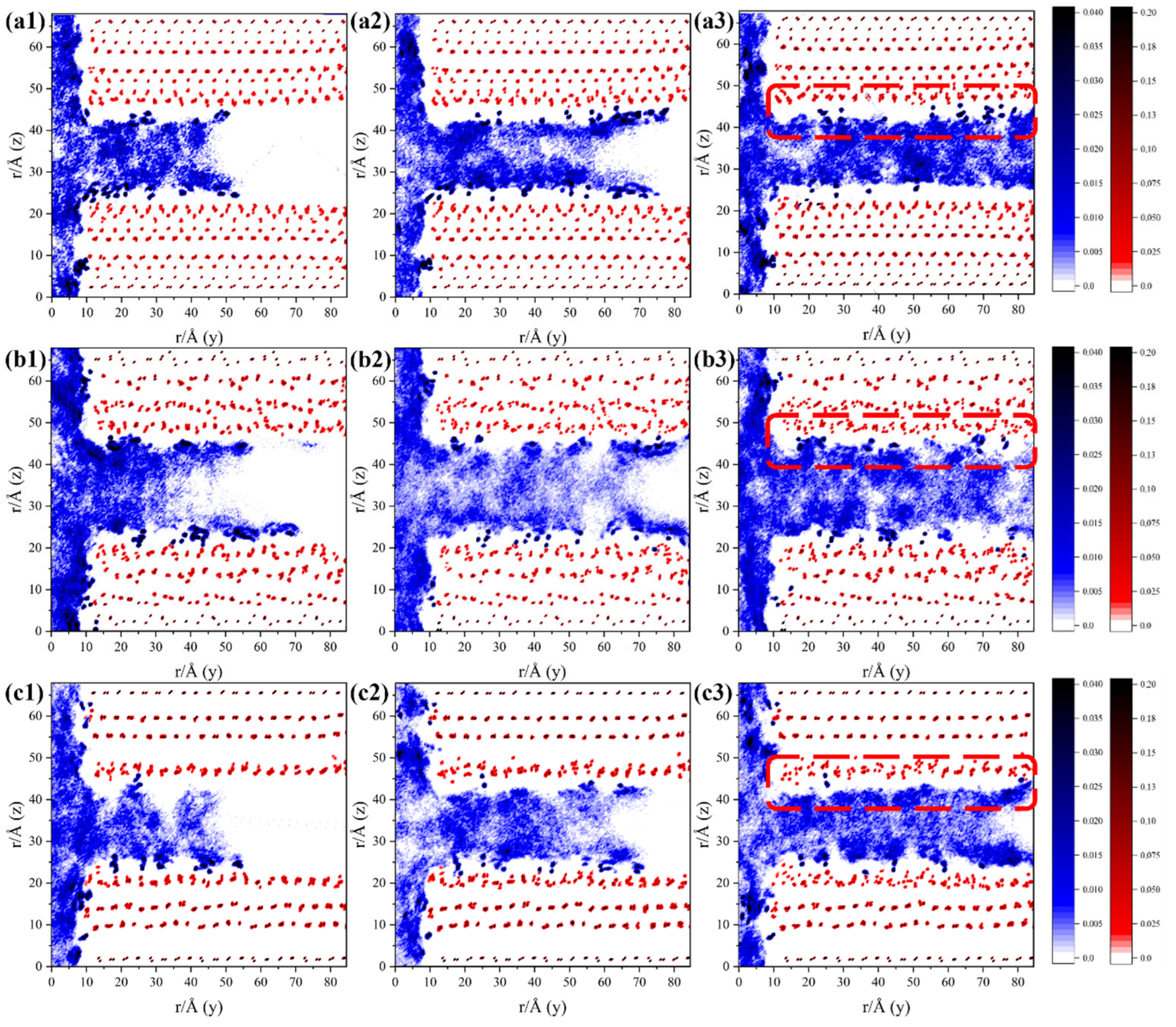
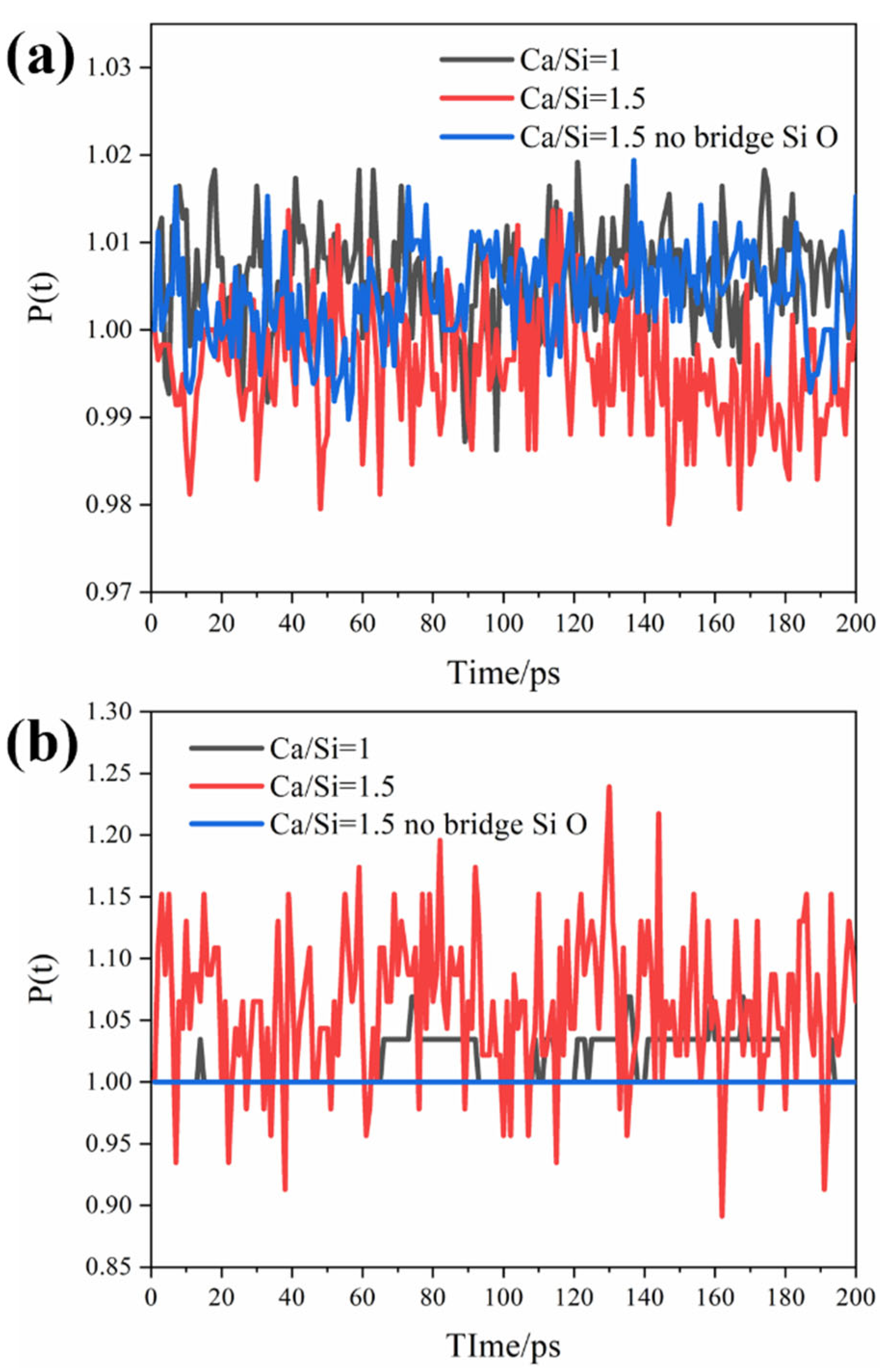
3.1. Solution and Ion Transport Processes
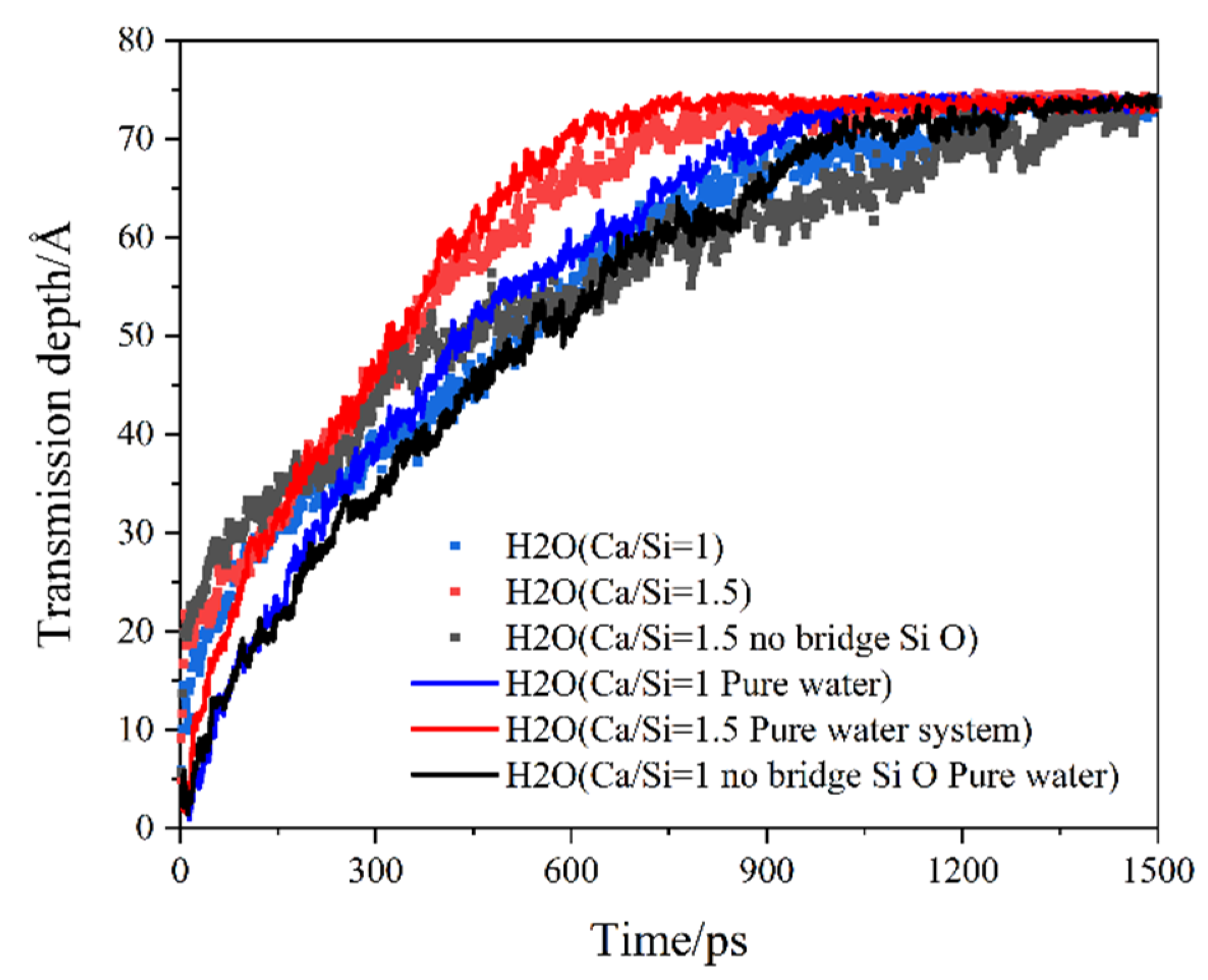
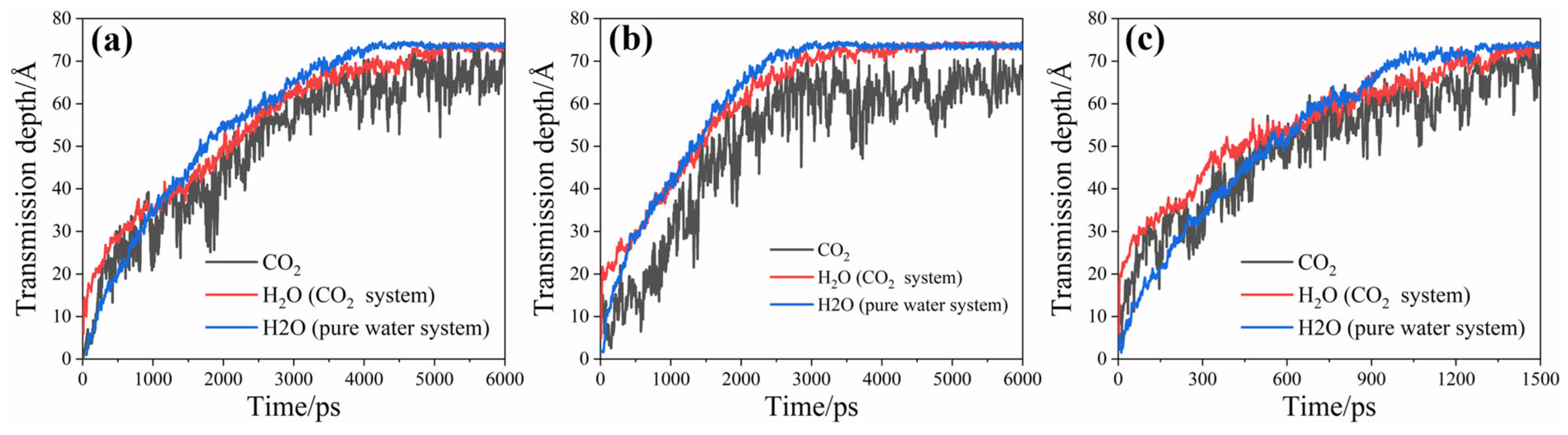
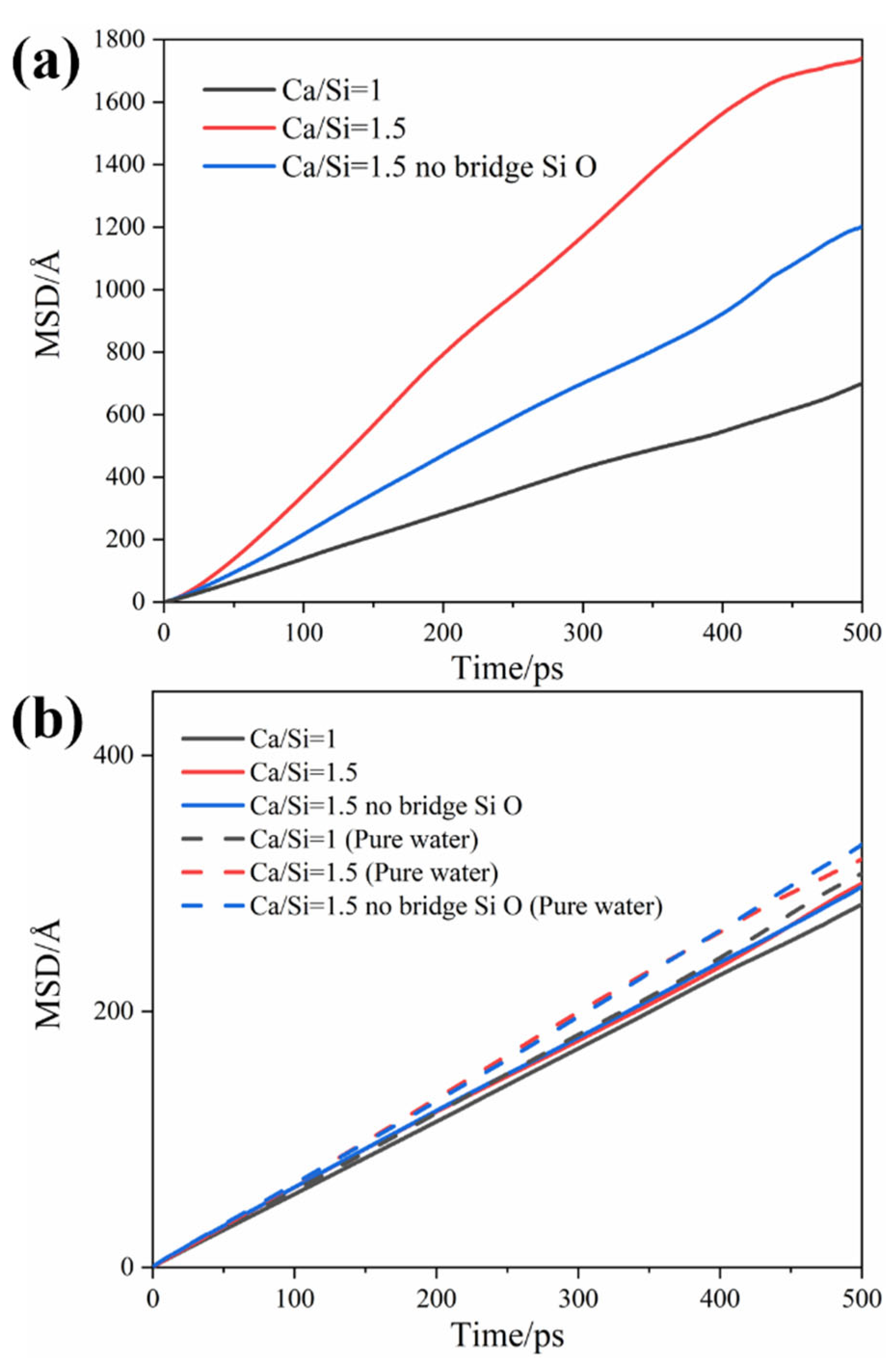
3.2. Distribution and Transmission Rate of H2O and CO2
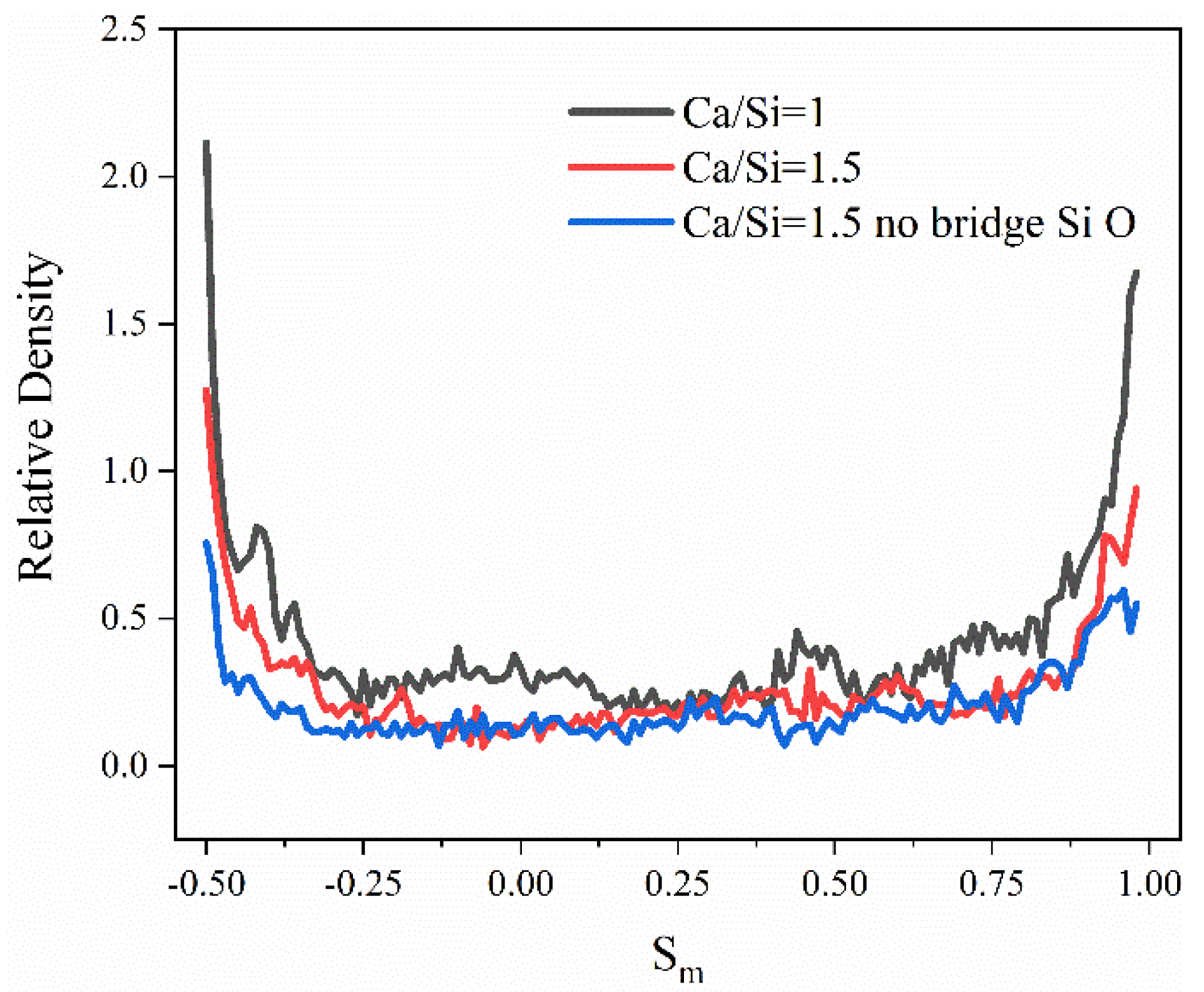
3.3. Reasons for Transmission Differences
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, C.-F.; Chen, J.-W. The Experimental Investigation of Concrete Carbonation Depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- Tang, B.; Fan, M.; Yang, Z.; Sun, Y.; Yuan, L. A Comparison Study of Aggregate Carbonation and Concrete Carbonation for the Enhancement of Recycled Aggregate Pervious Concrete. Constr. Build. Mater. 2023, 371, 130797. [Google Scholar] [CrossRef]
- Qian, R.; Li, Q.; Fu, C.; Zhang, Y.; Wang, Y.; Jin, N.; Jin, X. Investigations on Atmospheric Carbonation Corrosion of Concrete Structure Beam Exposed to Real Marine-Environment for 7 Years. J. Build. Eng. 2023, 71, 106517. [Google Scholar] [CrossRef]
- Kahyarian, A.; Brown, B.; Nesic, S. Electrochemistry of CO2 Corrosion of Mild Steel: Effect of CO2 on Iron Dissolution Reaction. Corros. Sci. 2017, 129, 146–151. [Google Scholar] [CrossRef]
- Ai, Z.; Jiang, J.; Sun, W.; Jiang, X.; Yu, B.; Wang, K.; Zhang, Z.; Song, D.; Ma, H.; Zhang, J. Enhanced Passivation of Alloy Corrosion-Resistant Steel Cr10Mo1 under Carbonation—Passive Film Formation, the Kinetics and Mechanism Analysis. Cem. Concr. Compos. 2018, 92, 178–187. [Google Scholar] [CrossRef]
- Ming, J.; Wu, M.; Shi, J. Passive Film Modification by Concrete Carbonation: Re-Visiting a Corrosion-Resistant Steel with Cr and Mo. Cem. Concr. Compos. 2021, 123, 104178. [Google Scholar] [CrossRef]
- Roventi, G.; Bellezze, T.; Giuliani, G.; Conti, C. Corrosion Resistance of Galvanized Steel Reinforcements in Carbonated Concrete: Effect of Wet—Dry Cycles in Tap Water and in Chloride Solution on the Passivating Layer. Cem. Concr. Res. 2014, 65, 76–84. [Google Scholar] [CrossRef]
- Shi, J.; Geng, Z.; Zhou, X. Reinforcing Steels in Low-Carbon Mortars Subjected to Chloride Attack and Natural Carbonation: Contradictory Trends in Passivation Ability and Corrosion Resistance. Cem. Concr. Compos. 2024, 152, 105666. [Google Scholar] [CrossRef]
- Mei, K.; He, Z.; Yi, B.; Lin, X.; Wang, J.; Wang, H.; Liu, J. Study on Electrochemical Characteristics of Reinforced Concrete Corrosion under the Action of Carbonation and Chloride. Case Stud. Constr. Mater. 2022, 17, e01351. [Google Scholar] [CrossRef]
- Al Fuhaid, A.F.; Niaz, A. Carbonation and Corrosion Problems in Reinforced Concrete Structures. Buildings 2022, 12, 586. [Google Scholar] [CrossRef]
- Ramirez, D.E.A.; Meira, G.R.; Quattrone, M.; John, V.M. A Review on Reinforcement Corrosion Propagation in Carbonated Concrete—Influence of Material and Environmental Characteristics. Cem. Concr. Compos. 2023, 140, 105085. [Google Scholar] [CrossRef]
- Angst, U.; Moro, F.; Geiker, M.; Kessler, S.; Beushausen, H.; Andrade, C.; Lahdensivu, J.; Köliö, A.; Imamoto, K.; von Greve-Dierfeld, S.; et al. Corrosion of Steel in Carbonated Concrete: Mechanisms, Practical Experience, and Research Priorities—A Critical Review by RILEM TC 281-CCC. RILEM Tech. Lett. 2020, 5, 85–100. [Google Scholar] [CrossRef]
- Rusheng, Q.; Yunsheng, Z.; Yu, Z.; Chuanqing, F.; Cheng, L.; Lin, Y.; Guojian, L. Various Gas Transport Properties in Concrete Considering Transporting Mechanisms and Testing Methods-A Review. Constr. Build. Mater. 2023, 389, 131636. [Google Scholar] [CrossRef]
- Holland, B.; Alapati, P.; Kurtis, K.E.; Kahn, L. Effect of Different Concrete Materials on the Corrosion of the Embedded Reinforcing Steel. In Corrosion of Steel in Concrete Structures; Elsevier: Amsterdam, The Netherlands, 2023; pp. 199–218. [Google Scholar]
- Hou, D.; Li, Z. Molecular Dynamics Study of Water and Ions Transport in Nano-Pore of Layered Structure: A Case Study of Tobermorite. Microporous Mesoporous Mater. 2014, 195, 9–20. [Google Scholar] [CrossRef]
- Kaddah, F.; Ranaivomanana, H.; Amiri, O.; Rozière, E. Accelerated Carbonation of Recycled Concrete Aggregates: Investigation on the Microstructure and Transport Properties at Cement Paste and Mortar Scales. J. CO2 Util. 2022, 57, 101885. [Google Scholar] [CrossRef]
- Auroy, M.; Poyet, S.; Le Bescop, P.; Torrenti, J.-M.; Charpentier, T.; Moskura, M.; Bourbon, X. Impact of Carbonation on Unsaturated Water Transport Properties of Cement-Based Materials. Cem. Concr. Res. 2015, 74, 44–58. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; Jacques, D.; De Schutter, G.; Ye, G.; Perko, J. Modelling the Carbonation of Cement Pastes under a CO2 Pressure Gradient Considering Both Diffusive and Convective Transport. Constr. Build. Mater. 2016, 114, 333–351. [Google Scholar] [CrossRef]
- Kari, O.-P.; Puttonen, J.; Skantz, E. Reactive Transport Modelling of Long-Term Carbonation. Cem. Concr. Compos. 2014, 52, 42–53. [Google Scholar] [CrossRef]
- Sharmilan, S.; Stang, H.; Michel, A. A Multi-Species Reactive Transport Model Based on Gas-Ion-Solid Phase Interaction for the Carbonation of Cement-Based Materials. Cem. Concr. Res. 2024, 175, 107349. [Google Scholar] [CrossRef]
- Xie, M.; Dangla, P.; Li, K. Reactive Transport Modelling of Concurrent Chloride Ingress and Carbonation in Concrete. Mater. Struct. 2021, 54, 177. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhao, W.; Liu, Y. Chloride Transport Resistance of Alkali-Activated Concrete Exposed to Combined Chloride, Sulfate and Carbonation Environment. Constr. Build. Mater. 2023, 367, 130353. [Google Scholar] [CrossRef]
- Georget, F.; Soja, W.; Scrivener, K.L. Characteristic Lengths of the Carbonation Front in Naturally Carbonated Cement Pastes: Implications for Reactive Transport Models. Cem. Concr. Res. 2020, 134, 106080. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H Samples Studied by 13 C, 27 Al and 29 Si MAS NMR Spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Mi, T.; Li, Y.; Liu, W.; Dong, Z.; Gong, Q.; Min, C.; Xing, F.; Wang, Y.; Chu, S.H. The Effect of Carbonation on Chloride Redistribution and Corrosion of Steel Reinforcement. Constr. Build. Mater. 2023, 363, 129641. [Google Scholar] [CrossRef]
- Bui, H.; Delattre, F.; Levacher, D. Experimental Methods to Evaluate the Carbonation Degree in Concrete—State of the Art Review. Appl. Sci. 2023, 13, 2533. [Google Scholar] [CrossRef]
- Hou, D.; Zheng, H.; Wang, P.; Wan, X.; Yin, B.; Wang, M.; Zhang, J. Molecular Dynamics Study on Sodium Chloride Solution Transport through the Calcium-Silicate-Hydrate Nanocone Channel. Constr. Build. Mater. 2022, 342, 128068. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Jiang, J.; Yin, B.; Li, Z. Effect of Temperature on the Capillary Transport of Sodium Sulfate Solution in Calcium Silicate Hydrate Nanopore: A Molecular Dynamics Study. Constr. Build. Mater. 2020, 231, 117111. [Google Scholar] [CrossRef]
- Tu, Y.; Cao, J.; Wen, R.; Shi, P.; Yuan, L.; Ji, Y.; Das, O.; Försth, M.; Sas, G.; Elfgren, L. Molecular Dynamics Simulation Study of the Transport of Pairwise Coupled Ions Confined in C-S-H Gel Nanopores. Constr. Build. Mater. 2022, 318, 126172. [Google Scholar] [CrossRef]
- Pellenq, R.J.-M.; Kushima, A.; Shahsavari, R.; Van Vliet, K.J.; Buehler, M.J.; Yip, S.; Ulm, F.-J. A Realistic Molecular Model of Cement Hydrates. Proc. Natl. Acad. Sci. USA 2009, 106, 16102–16107. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Fedkin, M.V.; Shin, Y.K.; Dasgupta, N.; Yeon, J.; Zhang, W.; Van Duin, D.; Van Duin, A.C.T.; Mori, K.; Fujiwara, A.; Machida, M.; et al. Development of the ReaxFF Methodology for Electrolyte—Water Systems. J. Phys. Chem. A 2019, 123, 2125–2141. [Google Scholar] [CrossRef]
- Subbaraman, R.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S. Atomistic Insights into Early Stage Oxidation and Nanoscale Oxide Growth on Fe (100), Fe (111) and Fe (110) Surfaces. J. Phys. Chem. C 2013, 117, 5195–5207. [Google Scholar] [CrossRef]
- Nosé, S. A Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- William, H. VMD-Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar]
- Hou, D.; Zhang, K.; Hong, F.; Wu, S.; Wang, Z.; Li, M.; Wang, M. The Corrosion Deterioration of Reinforced Passivation Film: The Impact of Defects. Appl. Surf. Sci. 2022, 582, 152408. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Ji, X.; Wang, P.; Ma, H.; Zhang, J.; Hou, D. Molecular Insight into the Fluidity of Cement Pastes: Nano-Boundary Lubrication of Cementitious Materials. Constr. Build. Mater. 2022, 316, 125800. [Google Scholar] [CrossRef]
- Kirkwood, J.G.; Boggs, E.M. The Radial Distribution Function in Liquids. J. Chem. Phys. 1942, 10, 394–402. [Google Scholar] [CrossRef]
- Zimm, B.H. The Scattering of Light and the Radial Distribution Function of High Polymer Solutions. J. Chem. Phys. 1948, 16, 1093–1099. [Google Scholar] [CrossRef]
- Hou, D.; Wang, M.; Sun, H.; Wang, P.; Zhang, J.; Wang, M. Structural Deterioration of Calcium Aluminosilicate Hydrate by Sulfate Attack: Atomic Processes of Al-Si Chain Breaking. Constr. Build. Mater. 2023, 401, 132867. [Google Scholar] [CrossRef]
- Hong, F.; Yu, S.; Hou, D.; Li, Z.; Sun, H.; Wang, P.; Wang, M. Study on the Mechanical Properties, Gelling Products and Alkalization Process of Alkali-Activated Metakaolin: From Experiment to Molecular Dynamics Simulation. J. Build. Eng. 2023, 79, 107705. [Google Scholar] [CrossRef]
- Hou, D.; Yu, J.; Liu, Q.; Dong, B.; Wang, X.; Wang, P.; Wang, M. Nanoscale Insight on the Epoxy-Cement Interface in Salt Solution: A Molecular Dynamics Study. Appl. Surf. Sci. 2020, 509, 145322. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Zhou, X.; Wang, P.; Zhang, Y.; Wang, X.; Zhang, X.; Hou, D.; Wang, M. Atomistic Insights into the Deposition of Corrosion Products on the Surfaces of Steels and Passivation Films. Langmuir 2023, 39, 6812–6822. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wang, M.; Dong, B.; Diao, X.; Zhang, X.; Pang, K.; Zhang, Y.; Hou, D. Molecular Insight into the Pozzolanic Reaction of Metakaolin and Calcium Hydroxide. Langmuir 2023, 39, 3601–3609. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Ji, X.; Yang, X.; Hong, F.; Hou, D.; Wang, M. Nanoscale Insight into Fluidity Improvement of Supplementary Cementitious Materials on Concrete: The Nano-Lubrication Process. J. Build. Eng. 2024, 82, 108193. [Google Scholar] [CrossRef]
- Wang, M.; Ji, X.; Manzano, H.; Hou, D.; Li, Z. Unlocking Cementitious Performance: Nano-Lubrication via Polycarboxylate Superplasticizers. J. Am. Ceram. Soc. 2024, 107, 3055–3067. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Zhou, X.; Wang, P.; Li, Z.; Hou, D. Surface Engineering of Migratory Corrosion Inhibitors: Controlling the Wettability of Calcium Silicate Hydrate in the Nanoscale. Langmuir 2023, 39, 17110–17121. [Google Scholar] [CrossRef]


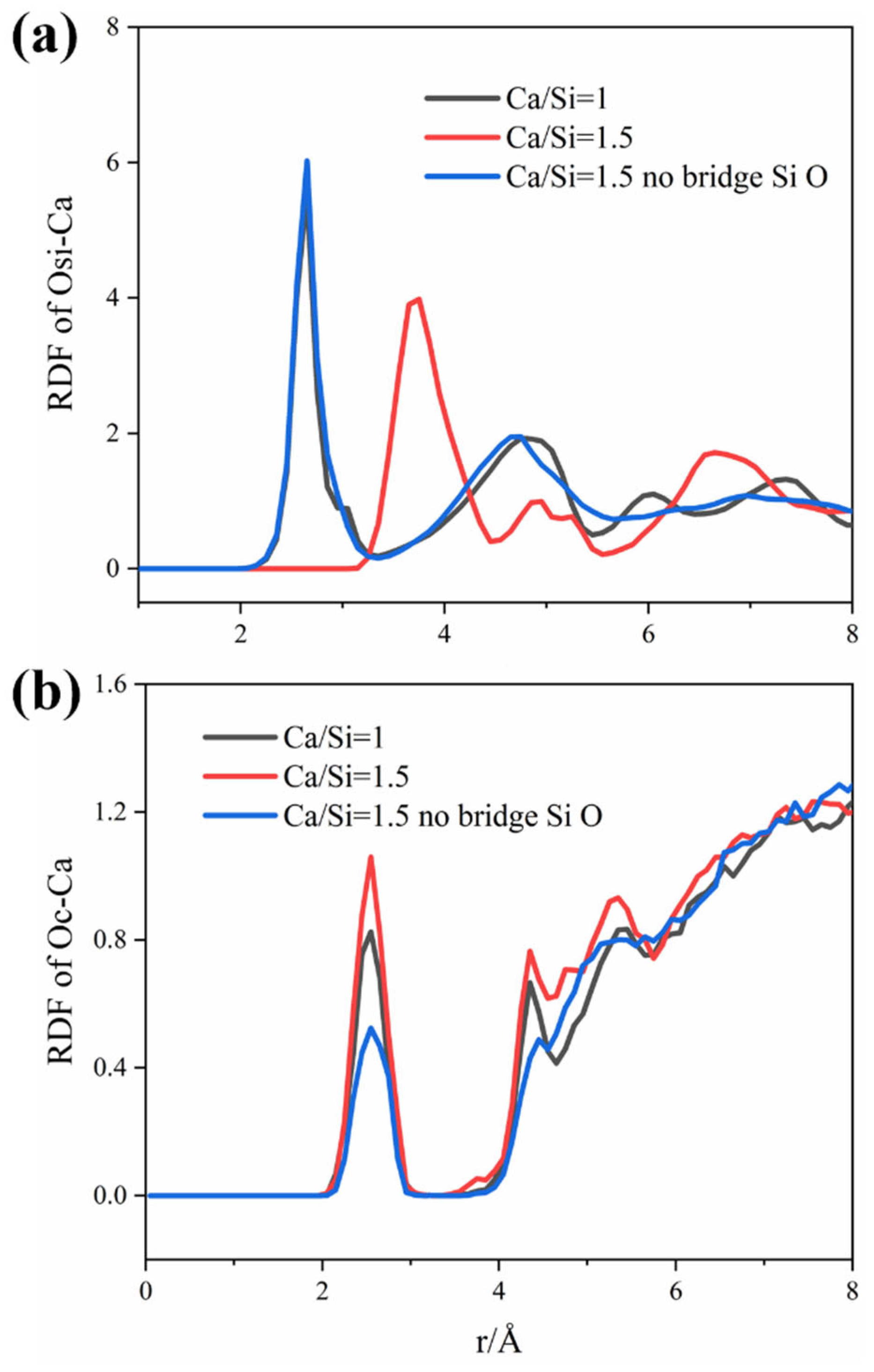

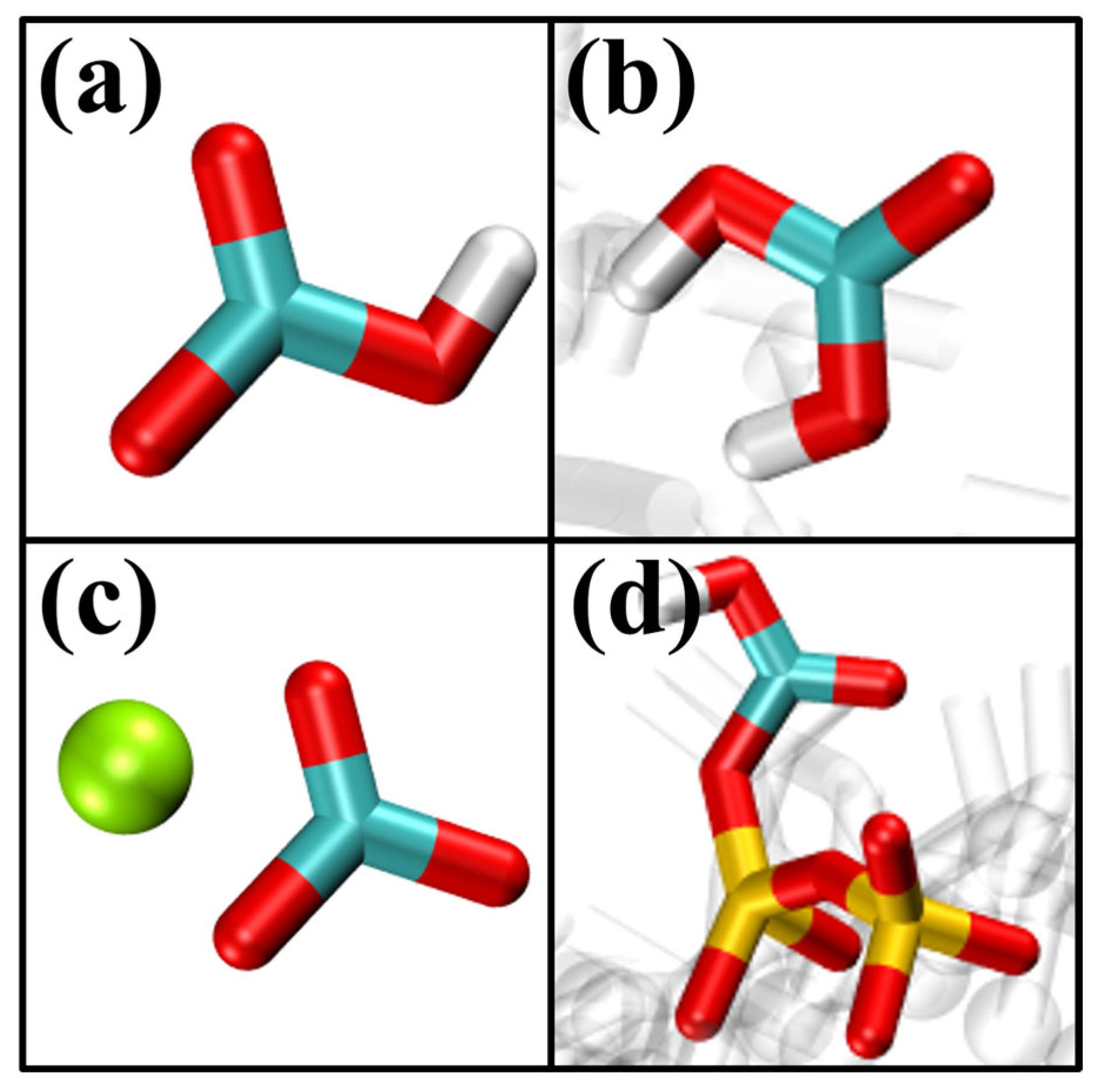
| Coordination Number | Osi-Ca | OC-Ca |
|---|---|---|
| Ca/Si = 1 | 0.110 | 0.019 |
| Ca/Si = 1.5 | 0.303 | 0.024 |
| Ca/Si = 1.5 (no bridging) | 0.118 | 0.013 |
| Number of Bonds | Osi-Ca | OC-Ca |
|---|---|---|
| Ca/Si = 1 | 1095 | 46 |
| Ca/Si = 1.5 | 980 | 29 |
| Ca/Si = 1.5 (no bridging) | 586 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Liu, C.; Hong, F.; Wang, P.; Zhang, Y.; Wang, X.; Hou, D.; Wang, M. Microscopic Transport and Degradation Behavior of CO2 in C-S-H with Varying Ca/Si Ratios during Carbonation. Buildings 2024, 14, 2808. https://doi.org/10.3390/buildings14092808
Sun W, Liu C, Hong F, Wang P, Zhang Y, Wang X, Hou D, Wang M. Microscopic Transport and Degradation Behavior of CO2 in C-S-H with Varying Ca/Si Ratios during Carbonation. Buildings. 2024; 14(9):2808. https://doi.org/10.3390/buildings14092808
Chicago/Turabian StyleSun, Wangzhe, Chengbo Liu, Fen Hong, Pan Wang, Yue Zhang, Xinpeng Wang, Dongshuai Hou, and Muhan Wang. 2024. "Microscopic Transport and Degradation Behavior of CO2 in C-S-H with Varying Ca/Si Ratios during Carbonation" Buildings 14, no. 9: 2808. https://doi.org/10.3390/buildings14092808
APA StyleSun, W., Liu, C., Hong, F., Wang, P., Zhang, Y., Wang, X., Hou, D., & Wang, M. (2024). Microscopic Transport and Degradation Behavior of CO2 in C-S-H with Varying Ca/Si Ratios during Carbonation. Buildings, 14(9), 2808. https://doi.org/10.3390/buildings14092808





